These strains have heightened pathogenic potential for rapid dissemination to multiple tissues, including the central nervous system.
Keywords: melioidosis, Burkholderia pseudomallei, virulence, neurologic, neurotropism, route of infection, actin-based motility, intracellular, bimA, bacteria, bacterial infection
Abstract
Neurologic melioidosis is a serious, potentially fatal form of Burkholderia pseudomallei infection. Recently, we reported that a subset of clinical isolates of B. pseudomallei from Australia have heightened virulence and potential for dissemination to the central nervous system. In this study, we demonstrate that this subset has a B. mallei–like sequence variation of the actin-based motility gene, bimA. Compared with B. pseudomallei isolates having typical bimA alleles, isolates that contain the B. mallei–like variation demonstrate increased persistence in phagocytic cells and increased virulence with rapid systemic dissemination and replication within multiple tissues, including the brain and spinal cord, in an experimental model. These findings highlight the implications of bimA variation on disease progression of B. pseudomallei infection and have considerable clinical and public health implications with respect to the degree of neurotropic threat posed to human health.
Burkholderia mallei, the etiologic agent of glanders, is thought to have evolved from a single strain of B. pseudomallei, becoming highly specialized for intracellular persistence (1). B. mallei and B. pseudomallei share sequence similarity and are highly pathogenic through the respiratory route, often initiating rapid disease progression resulting in high mortality (2). Unlike B. pseudomallei, B. mallei has a narrower host range and is less capable of extended persistence in the environment.
Knowledge of the virulence factors responsible for inducing the diverse spectrum of clinical manifestations of B. pseudomallei infection remains limited (3). Similar to bacteria of other genera, such as Listeria, Rickettsia, Mycobacterium, and Shigella, intercellular and intracellular movement of Burkholderia are facilitated by actin polymerization at 1 pole of the bacterium (4). The putative autotransporter protein Burkholderia intracellular motility A (BimA) has been shown to mediate actin-based motility in B. pseudomallei and B. mallei, promoting bacterial dissemination while shielding the pathogen from immune surveillance and autophagy (5). Differences in the structure of the bimA gene in B. mallei and B. pseudomallei (6–8) suggest that actin assembly might occur through distinct mechanisms in these 2 Burkholderia species. B. mallei–like bimA variants (bimBm) have been identified in a subset of B. pseudomallei isolates from Australia and 2 B. pseudomallei isolates from India (9,10). This allele has not yet been identified in isolates from Southeast Asia.
Neurologic melioidosis is a serious, potentially fatal form of B. pseudomallei infection. Recently, we reported that although B. pseudomallei isolates from patients with neurologic melioidosis do not demonstrate selective neurotropism in an experimental model, a distinct subset of B. pseudomallei isolates appeared equipped for rapid dissemination to multiple tissues, including the central nervous system (CNS), after infection (11). Correlation of virulence genes of B. pseudomallei with clinical presentations of melioidosis identified the bimBm allele as a risk factor for neurologic melioidosis (12). Given the importance of BimA in intercellular and intracellular spread of Burkholderia spp. and the recognition of bimBm variants of B. pseudomallei in northern Australia, we hypothesized that bimBm variants of B. pseudomallei would have an increased advantage for establishment of infection and dissemination compared with typical bimBp strains. Therefore, we used a well-characterized animal model of melioidosis to compare virulence and disease progression after infection with clinical isolates of B. pseudomallei collected in the Northern Territory of Australia during October 1989–October 2012 and identified as having either the bimBm or bimBp allele (13).
Methods
B. pseudomallei Isolates
B. pseudomallei strains were isolated from patients with melioidosis. Clinical details and the sequence type determined from multilocus sequence typing of the B. pseudomallei strains investigated are noted (Table). Additional details are described elsewhere (11,12,14,15). These isolates were chosen to represent B. pseudomallei strains previously identified as having bimBm (n = 7) and bimBp (n = 8) alleles within the bimA gene (10,12).
Animal Infection
We used 8- to 12-week-old C57BL/6 and BALB/c mice purchased from the Small Animal Breeding Facility at James Cook University. Experiments were approved by the Institutional Animal Ethics committee (A1500). To mimic natural routes of infection, intranasal or subcutaneous routes were used for inoculation by using methods described previously (16). B. pseudomallei isolates were cultured to logarithmic phase and prepared for inoculations as previously described (11).
Virulence Determination
Virulence of bimBm (n = 7) and bimBp (n = 6) isolates were compared in mice as described previously (11). The 50% infectious dose (ID50) was determined by using a modified version of the Reed and Meunch method (17). Virulence, as defined by the ID50 values for B. pseudomallei strains, were compared in BALB/c and C57BL/6 mice after intranasal and subcutaneous infection. Data for bimBm and bimBp strains are expressed as mean log10 ID50 +SD
Bacterial Dissemination and Disease Progression
We selected bimBm (MSHR543) and bimBp (MSHR305) strains of comparable virulence (determined by intranasal ID50 values as 2.6 × 102 CFU and 2.9 × 102 CFU, respectively) for comparison of bacterial dissemination after intranasal infection of C57BL/6 mice. C57BL/6 mice provide a more accurate model for neurologic melioidosis because this form of the disease tends to occur in otherwise healthy persons without known risk factors (13). MSHR543 (bimBm) was isolated from a localized skin infection in a healthy 22-year-old with a cut on her hand that was exposed to muddy water. Blood cultures were negative, and she remained systemically well with no evidence of dissemination of B. pseudomallei. The bimBp (MSHR305) strain was isolated from a patient with a fatal case of neurologic melioidosis. The 64-year-old patient had a history of excessive alcohol consumption and had had onset of flaccid paralysis after a period of influenza-like illness (14). An equivalent dose of MSHR543 (1.4 × 104 CFU) or MSHR305 (1.1 × 104 CFU) was used to inoculate mice. Survival rates and signs of disease were monitored daily for a period of 21 days (n = 10 mice per isolate). Mice that became moribund during the experimental period were euthanized, and bacterial loads were determined in organs and pathology of CNS investigated. Parallel groups of mice were inoculated with MSHR543 (bimBm) (n = 15) and MSHR305 (bimBp) (n = 15) for assessment of bacterial loads within blood, liver, spleen, lung, cervical lymph node , brain, and nasal-associated lymphoid tissue (NALT) at 2 hours, 1 day, and 3 days postinfection (n = 5 mice per time point) by using methods described previously (11). The detection limit of bacteria in blood and organs was 2 CFU. Data are expressed as the mean log10 CFU +SD.
Bacterial Growth Rate
The growth of B. pseudomallei isolates in trypticase soy broth (TSB) was compared. Overnight broth cultures of B. pseudomallei isolates were diluted 1:10 in fresh TSB and incubated in triplicate at 37°C with shaking at 120 rpm. Absorbance (600 nm) was measured hourly for 10 hours with a microplate reader (Fluostar Omega; BMG Labtech, Mornington, VIC, Australia) and the exponential growth rate for each isolate determined. Data are presented as the mean gradient (μhr–1) +SD for bimBm and bimBp strains.
Internalization and Persistence of Bacteria in Phagocytic Cells
We determined internalization and intracellular persistence of B. pseudomallei isolates (n = 7 bimBm; n = 8 bimBp) in mononuclear phagocytes after co-culture with murine leukocytes. Leukocytes were isolated from spleen and peripheral lymph nodes (cervical, mediastinal, axillary, inguinal, and popliteal) of uninfected female C57BL/6 mice (18). B. pseudomallei isolates were grown to logarithmic phase, washed then added to leukocyte cultures at a multiplicity of infection of 1 (mononuclear cell): 5 (bacteria) (19). After 2 hours of co-culture, kanamycin (250 μg/mL) was added to wells to limit extracellular bacterial growth (18). Internalization (2 h) and persistence (8 and 24 h) of B. pseudomallei isolates in leukocytes was determined by flow cytometry. Uninfected and B. pseudomallei–infected leukocytes were fluorescently stained with a combination of anti-mouse fluorescein isothiocyanate–conjugated CD45 and F4/80 (BD Biosciences, North Ryde, NSW, Australia) and peridinin chlorophyll-cyanine 5.5 (PerCP-Cy5.5)–conjugated CD11c (eBioscience, San Diego, CA, USA) by using methods described previously (18). After fixation and permeabilization, leukocytes were stained with polyclonal rabbit anti–B. pseudomallei outer membrane protein antibody (BpOMP). A secondary biotinylated goat anti-rabbit IgG (Vector Labs, Burlingame, CA, USA) monoclonal antibody and streptavidin–phycoerythrin conjugate (eBioscience) was used for detection of the primary antibody. Acquisition (2 × 105 leukocytes) was performed by using a FACSCalibur with Cell Quest software (BD Biosciences) and FlowJo software (Tree Star, Inc., San Carlos, CA, USA) was used for postacquisition analysis. The fluorescence of extracellular bacteria was quenched with Trypan blue (0.2%). Data are expressed as the percentage or total number of leukocytes (CD45+), macrophages (F4/80+), or dendritic cells (CD11c+) positive for intracellular BpOMP staining. Two independent experiments were conducted, and the mean +SD of data from both experiments is shown. Microbiologic culture was used to confirm intracellular B. pseudomallei numbers estimated by BpOMP staining (20).
Statistical Analysis
We performed statistical analysis by using Graphpad Prism Version 6 (Graphpad Software, La Jolla, CA, USA) and used Kaplan–Meier survival curves to compare susceptibility to infection with B. pseudomallei isolates. Virulence parameters (ID50 values, time for development of neurologic symptoms, and intracellular bacterial loads within leukocytes) for bimBm and bimBp strains were compared by using the Mann-Whitney U test. Bacterial load kinetics in organs after infection with MSHR543 (bimBm) and MSHR305 (bimBp) were tested for significance using 2-way analysis of variance with Sidak’s post hoc analysis. We considered comparisons significant at p<0.05.
Results
High Virulence of BimBm Variants in Murine Models of Melioidosis
We compared virulence, as defined by ID50, for bimBm and bimBp strains in B. pseudomallei–susceptible (BALB/c) and B. pseudomallei–partially resistant (C57BL/6) mice after intranasal and subcutaneous infection (16,21). B. pseudomallei bimBm strains were significantly more virulent for BALB/c and C57BL/6 (Figure 1, panels A and B) mice than bimBp strains, regardless of route of infection. These findings are consistent with the BALB/c–C57BL/6 model of contrasting resistance to B. pseudomallei (21).
Figure 1.
Virulence of bimBm and bimBp Burkholderia pseudomallei isolates. Day 21 50% infectious dose values after intranasal and subcutaneous infection of BALB/c (A) and C57BL/6 (B) mice with bimBm (n = 7) and bimBp (n = 6) B. pseudomallei isolates. Groups of 5 mice were inoculated via intranasal and subcutaneous routes at 10-fold increasing doses of B. pseudomallei, ranging from 100 CFU to 107 CFU. Virulence of bimBm isolates was significantly greater for both mouse strains, regardless of the infection route. Data are expressed as mean log10 CFU +SD. C57BL/6 mice (n = 10) were infected with bimBm (n = 7) and bimBp (n = 6) B. pseudomallei isolates at equivalent doses (104 CFU) and monitored for 21 days postinfection. The percentage of mice for a given bacterial strain for which evidence indicated establishment of B. pseudomallei infection (culture-positive growth from tissues) (C) and signs of neurologic involvement (e.g., head tilt, spinning behavior, and hind leg paresis) (D) was increased for animals exposed to bimBm compared with bimBp isolates. *p<0.05; **p<0.01.
When equivalent inoculating doses of B. pseudomallei strains were compared (104 CFU), bimBm strains were more likely to establish persistent infection with bacteria recoverable from multiple organs at 21 days postinfection after intranasal infection of C57BL/6 mice (p = 0.077) (Figure 1, panel C). Additionally, neurologic involvement occurred with more frequency in animals infected through the intranasal route with bimBm compared with those infected with bimBp strains when an equivalent inoculating dose (104 CFU; n = 10 mice/B. pseudomallei strain) was used (p = 0.046) (Figure 1, panel D). Most B. pseudomallei strains tested were capable of CNS infection; however, neurologic involvement tended to occur at comparatively lower inoculating doses for bimBm than bimBp strains. The mean number of bacteria required to infect C57BL/6 mice through the respiratory tract and result in the development of neurologic signs in >20% of mice was 9 × 103 CFU (range 5.3 × 101 to 2 × 104 CFU) for bimBm and 3.7 × 105 CFU (range 2.6 × 104 to 6.6 × 105) for bimBp (p = 0.048). Despite infection of C57BL/6 mice with doses as high as 108 CFU, neurologic symptoms were never observed after infection with 2 strains (MSHR3709 and MSHR1655), both of which are type bimBp.
The mean number of bacteria required to infect susceptible BALB/c mice via the respiratory route and manifest neurologic signs in >20% of mice was 8.6 × 103 CFU (range 4 × 101 to 3 × 104 CFU) for bimBm and 1.5 × 105 CFU (range 2.6 × 104 to 4.2 × 105 CFU) for bimBp (p = 0.03). For C57BL/6 mice, the mean number of days postinfection for onset of neurologic symptoms was 9 (range 5–16) days; for BALB/c mice, it was 11 (range 4–18) days. These findings indicate that bimBm variants are significantly more virulent than bimBp strains in murine models of melioidosis and suggest that fewer inoculating bacteria are required to establish CNS infection.
Differing Disease Progression for bimBm and bimBp Strains after Intranasal Infection
We selected a bimBm (MSHR543) and bimBp (MSHR305) strain of comparable virulence to compare organ tropism after intranasal infection (intranasal ID50 values of 2.6 × 102 and 2.9 × 102 CFU, respectively). Twenty-one day mortality rates were comparable after intranasal infection with either MSHR543 (bimBm) or MSHR305 (40% and 50%, respectively). However, of the animals monitored for survival, 2 of the 5 mice that succumbed to infection with MSHR305 (bimBp) had neurologic symptoms (1 with head tilt on day 7, another with hind limb paresis on day 14). In contrast, all of the 4 mice that succumbed to infection with MSHR543 (bimBm) had symptoms of neurologic melioidosis (3 with head tilt on day 5 and day 7, the other with hind leg paresis on day 7). Moribund mice were euthanized and tissues processed for bacterial load determination. Bacterial loads were high in brains of moribund mice (Figure 2). B. pseudomallei was typically recovered from all tissues investigated, although levels tended to be low or undetectable in the blood of moribund mice that had signs of neurologic infection in the first week postinfection. Compared with moribund animals infected with MSHR543 (bimBm), bacterial loads were significantly higher in NALT of moribund mice infected with MSHR305 (bimBp, p = 0.025), with a similar trend observed in lung. Abscessation was observed in the nasal epithelium, with extensive suppurative inflammation in the olfactory submucosa extending to the olfactory bulb and moderate infiltration in the trigeminal nerve branches (Figure 3, panels A and B) in mice that had signs of neurologic involvement at day 5 postinfection with MSHR543 (bimBm). Leptomeningitis and encephalomyelitis were cardinal features in these animals (Figure 3, panels C and D). We also observed cranial microabscesses were in animals that succumbed to infection, although the area affected varied and included the cerebellum, brainstem, and cerebral cortex (Figure 3, panel E).
Figure 2.
Brain bacterial loads in mice that had signs of neurologic involvement and succumbed to infection with MSHR543 (bimBm) and MSHR305 (bimBp) Burkholderia pseudomallei isolates. Bacterial loads in brains of C57BL/6 mice (MSHR543, n = 4; MSHR305, n = 5) that had become moribund and required euthanasia within the 21-day experimental period after intranasal infection with MSHR543 (1.4 × 104 CFU; white bars) and MSHR305 (1.1 × 104 CFU; black bars). N indicates mice that displayed symptoms of neurologic involvement. Data are expressed as log10 CFU. Mice exposed to MSHR543 (bimBm) had signs of neurologic involvement and became moribund within 7 days of exposure.
Figure 3.
Central nervous system pathology in mice that had signs of neurologic involvement and succumbed to infection with bimBm and bimBp Burkholderia pseudomallei isolates. Evidence of central nervous system pathology was demonstrated in these mice. Inflammatory infiltrates were prominent in trigeminal nerve branches and ganglion (original magnification ×400) (A) and in the olfactory bulb (original magnification ×200) (B). Cranial meningitis (C) and spinal (D) meningitis were observed, often with involvement of underlying parenchyma (original magnification ×400). Microabscesses were frequently observed in cerebral cortex (original magnification ×100) (E), brainstem (not shown) and cerebellum (not shown) of mice that had neurologic symptoms and succumbed to infection.
Systemic dissemination occurred rapidly for MSHR543 (bimBm) and MSHR305 (bimBp); bacteria were recovered from multiple sites by day 1 postinfection (Figure 4). At 2 hours postinfection, NALT was the only tissue that bacteria were cultured from, with levels comparable for mice infected with MSHR543 (bimBm) and MSHR305 (bimBp) (log10 CFU of 0.9 +1.1 and 0.3 +1.1, respectively). Compared with MSHR305 (bimBp), replication of MSHR543 (bimBm) was significantly higher in cervical lymph nodes and spleen (Figure 4). Bacterial loads were low in brains of mice infected with MSHR543 (bimBm) and MSHR305 (bimBp) within 3 days of infection despite signs of neurologic involvement by day 5 postinfection in 4 mice infected with MSHR543 (bimBm), corresponding to bacterial loads in the brain in excess of 102 CFU (Figure 2). In comparison, only 1 animal infected with MSHR305 (bimBp) had symptoms of neurologic melioidosis and required euthanasia within 7 days.
Figure 4.
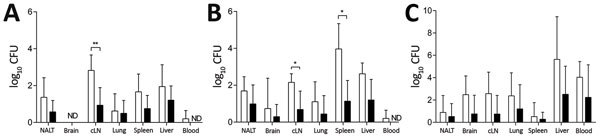
Comparison of early bacterial dissemination and persistence after intranasal infection of C57BL/6 mice with MSHR543 (bimBm) and MSHR305 (bimBp) Burkholderia pseudomallei isolates. A, B) Bacterial load at day 1 (A) and day 3 (B) postinfection in nasal-associated lymphoid tissue, brain, cervical lymph nodes, lung, spleen, liver, and blood after intranasal infection of C57BL/6 mice (n = 5/time point) with MSHR543 (1.4 × 104 CFU; white bars) and MSHR305 (1.1 × 104 CFU; black bars). C) Bacterial organ loads in mice that survived the 21-day experimental period (MSHR543, n = 6; MSHR305, n = 5). Data are expressed as mean log10 CFU + SD (upper bars only). cLN, cervical lymph nodes; NALT, nasal-associated lymphoid tissue; ND, not detected. *p<0.05; **p<0.01.
Five mice (50%) survived to 21 days after intranasal infection with MSHR305 (bimBp), of which 4 had evidence of persistent B. pseudomallei infection, with bacteria recovered from the brain of 1 mouse (Figure 4, panel C). Six mice (60%) survived after intranasal infection with MSHR543 (bimBm), and all had evidence of persistent infection, with bacteria recovered from the brains of 5 mice (Figure 4, panel C).
These findings demonstrate that despite equivalent inoculating doses and similar 21-day mortality rates, the pattern and kinetics of dissemination differ for MSHR543 (bimBm) and MSHR305 (bimBp) after intranasal infection, with neurologic involvement occurring with more frequency after infection with MSHR543 (bimBm).
Increased Persistence of bimBm Strains in Mononuclear Phagocytic Cells
To investigate whether differences observe in systemic dissemination in vivo might be attributable to inherent differences in multiplication of bimBm and bimBp strains, we compared the in vitro growth rate of isolates in broth culture. No significant differences were observed for the exponential growth of bimBm and bimBp variants in TSB (slope, μhr-1, 0.105 +0.02 and 0.092 +0.02, respectively). Having demonstrated that bimBm and bimBp strains multiply at the same rate in cell-free media, we next investigated whether intracellular growth rates were comparable for the 2 groups of isolates. Because macrophages and dendritic cells play a pivotal role in protection against B. pseudomallei infection (3), we compared the uptake and persistence of bimBm (n = 7) and bimBp (n = 8) isolates in ex vivo cultures of murine spleen and lymph node–derived macrophages and DC. Absolute numbers of leukocytes were comparable for bimBm- and bimBp-infected cultures at 2, 8, and 24 hours postinfection (Figure 5, panel A). The percentage of leukocytes positive for BpOMP staining was also comparable in cultures infected with bimBm and bimBp strains at 2 and 8 hours postinfection (Figure 5, panel B). However, by 24 hours, the proportion of BpOMP+ leukocytes was significantly higher in cultures infected with bimBm than bimBp strains (p = 0.002), and persistence of bimBm isolates was greater in CD11c+ dendritic cells (p = 0.012) and F4/80+ macrophages (p = 0.006) than bimBp strains (Figure 5, panel C). Overall, these data suggest that bimBm strains of B. pseudomallei might possess mechanisms to facilitate their internalization and intracellular persistence within professional phagocytes.
Figure 5.
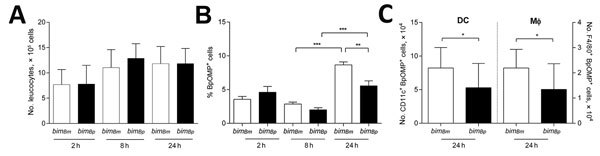
Internalization and persistence of bimBm and bimBp Burkholderia pseudomallei isolates within murine leukocytes. Spleen and lymph node–derived leukocytes were co-cultured with B. pseudomallei isolates at multiplicity of infection 1:5. A) At 2, 8, and 24 hours postinfection, absolute numbers of CD45+ leukocytes were comparable in cultures infected with bimBm and bimBp strains. B) Bacterial uptake (2 h) and persistence (8 h and 24 h) was compared by assessing the percentage of CD45+ leukocytes that were positive for intracellular B. pseudomallei outer membrane protein antibody (BpOMP) staining using flow cytometry. BpOMP staining increased within leukocytes between 8 hours and 24 hours of cultures. Compared with bimBp, the percentage of leukocytes positive for intracellular BpOMP was significantly higher in cultures stimulated with bimBm isolates at 24 hours postinfection. Internalization of bimBm or bimBp isolates by CD11c+ dendritic cells and F4/80+ macrophages was comparable (not shown). C) However, persistence of bimBm strains was significantly higher in dendritic cells and macrophages after 24 hours of culture. Data reflect the mean +SD of 2 independent experiments. BpOMP, B. pseudomallei outer membrane protein antibody; DC, dendritic cells; Mϕ, macrophages. *p<0.05; **p<0.01; ***p<0.001.
Discussion
Although uncommon, neurologic melioidosis is a severe and debilitating form of B. pseudomallei infection, primarily affecting healthy persons with no recognizable risk factors and occurring with increased frequency in Australia (13,14,22). Diagnosis and management of neurologic melioidosis is challenging because of nonspecific clinical presentation, poor diagnostics, and intrinsic resistance to antibiotics. Similar to other intracellular bacteria, B. pseudomallei and B. mallei are able to spread to adjacent host cells and evade immune surveillance through the formation of actin tails in a process that involves polymerization of host actin monomers (5,10,23,24). Polymorphisms in machinery used for actin assembly in other obligate intracellular bacteria have been reported to influence virulence and tissue tropism (25–27). Recently, isolates possessing a B. mallei–like bimA allele (bimBm) were shown to be associated with neurologic involvement in human melioidosis (12). Our study provides in vivo evidence of the implications of the bimBm sequence variation on disease progression and severity of experimental melioidosis. Compared with B. pseudomallei isolates with typical BimA motifs, bimBm variants were more virulent in an animal model of melioidosis when delivered intranasally or subcutaneously. This subset of strains was associated with increased persistence within phagocytic cells and increased likelihood of establishing CNS infection compared with bimBp strains of B. pseudomallei.
Although no evidence from our study indicates preferential seeding of the CNS compared with other tissues, CNS infection did occur with increased frequency and at lower inoculating doses after infection of mice with bimBm than bimBp strains of B. pseudomallei. Neurologic involvement was observed after intranasal and subcutaneous inoculation with B. pseudomallei isolates, although the frequency of CNS infection increased after intranasal infection. Neurologic involvement, as evidenced by bacterial colonization of the brain and neutrophil infiltration to the cranial and spinal meninges, occurred with more frequency in animals infected with MSHR543 (bimBm) than those exposed to MSHR305 (bimBp). Although we observed considerable variability in the sites of abscessation in the CNS, leptomeningitis, meningoencephalitis, and encephalomyelitis were common features in animals that succumbed to infection. Similar neuropathology has been reported in experimental models using intravenous (rather than intranasal) challenge of mice with B. pseudomallei (28). Furthermore, the neuropathology observed in our study is consistent with the only published histopathologic study of human CNS from patients with melioidosis encephalomyelitis (14).
Clinical and experimental data suggest B. pseudomallei is capable of using >1 mechanism for entry into the brain and spinal cord (28–37). B. pseudomallei has been shown to take advantage of olfactory and trigeminal nerve branches to gain direct access to the brain after respiratory infection of mice (29–32), and St. John et al. (32) recently demonstrated a role for bimA in direct CNS invasion by B. pseudomallei. Clinical reports also support progression of sinusitis or upper respiratory tract infection with B. pseudomallei to neurologic melioidosis (33–35). Additionally, cortical brain abscesses, a clinical presentation commonly reported for neurologic melioidosis in Southeast Asia (33), were observed and are consistent with bacteremic spread of B. pseudomallei, directly or through transmigration of infected leukocytes, to the CNS (28). In addition to direct infection through the upper respiratory tract, cases of neurologic melioidosis from the Darwin Prospective Melioidosis Study have recently provided strong support for direct brainstem or spinal cord infection occurring through nerve root translocation of bacteria secondary to skin inoculation with B. pseudomallei on the face/scalp or limbs (36,37). The observation of hind leg paraparesis in some animals after B. pseudomallei infection in our study provides additional support for this postulated mechanism of CNS entry.
In our study, rapid systemic dissemination to secondary lymphoid tissues was observed for B. pseudomallei bimBm and bimBp variants, with significantly higher bacterial loads observed earlier in these tissues after infection with the bimBm variant. Moreover, despite significant reduction in intracellular bacterial loads, persistence of B. pseudomallei was evident in vitro in dendritic cells and macrophages, tissue phagocytic cells that B. pseudomallei would be exposed to in the early stages of subcutaneous and intranasal infection. We acknowledge that other leukocyte subsets might support intracellular infection with B. pseudomallei and therefore potentially contribute to rapid dissemination of this bacterium in vivo. We limited our assessment to dendritic cells and macrophages because these cells are among the earliest responders to infection and are critical for controlling B. pseudomallei infection (3,20,21). Skin dendritic cells also migrate to secondary lymphoid tissues, facilitating the trafficking and systemic dissemination of live intracellular B. pseudomallei (18). Our data support a potential role for professional phagocytic cells in rapid systemic dissemination of B. pseudomallei to distant sites such as the CNS.
As an increasing number of clinically derived strains are genotyped, it is becoming apparent that the manifestations of melioidosis are likely to be influenced by the infecting strain, as well as the route of infection, infecting dose, and host risk factors for melioidosis. Our findings from this current study provide strong support to our clinical observations (12) that bimBm variation is a predictor for severe forms of melioidosis, including neurologic involvement. Despite comparative interrogation of genomes between B. pseudomallei strains of contrasting virulence (38,39), to date bimA has been identified as the only gene with a strong association with neurologic melioidosis. However, our observation that bimBp strains have the potential to invade the CNS, albeit typically at higher inoculating doses than bimBm strains, suggest that genes other than bimA also contribute to B. pseudomallei invasion and dissemination in vivo. Under favorable circumstances, avirulent B. pseudomallei strains and even the closely related but avirulent bacterium, B. thailandensis, can initiate systemic and lethal infection (40,41). Identifying and characterizing bacterial effector proteins involved in the intracellular and intercellular spread and persistence of B. pseudomallei and B. mallei will be critical for identification of novel agents to manipulate these processes with therapeutic application.
Table. Clinical and patient characteristics and sequence type diversity of bimBm and bimBp Burkholderia pseudomallei isolates, Australia.
| Isolate no. | Age, y/sex | Risk factors | Clinical presentation | Outcome | MLST genotype |
|---|---|---|---|---|---|
| bimBm | |||||
| MSHR62 | 23/M | None | Brainstem encephalitis | Survived | 148 |
| MSHR435 | 37/M | None | Brainstem encephalitis | Survived | 126 |
| MSHR543 | 22/F | None | Skin ulcer | Survived | 294 |
| MSHR668 | 53/M | None | Diffuse encephalitis | Survived | 129 |
| MSHR1153 | 59/M | DBT | Brainstem encephalitis | Died | 117 |
| MSHR2138 | 49/F | DBT | Bacteremia | Survived | 456 |
| NCTC13178 |
6/M |
None |
Brainstem encephalitis |
Died |
286 |
| bimBp | |||||
| MSHR305 | 64/M | ALC | Encephalitis, myelitis | Died | 36 |
| MSHR346 | 49/M | ALC, COPD | Pneumonia | Survived | 243 |
| MSHR465 | 67/M | DBT, COPD | Pneumonia, septic shock | Died | 132 |
| MSHR1655 | 61/F | COPD | Pneumonia | Survived | 131 |
| MSHR3709 | 14/M | None | Brainstem encephalitis | Survived | 132 |
| MSHR974* | 16/F | None | Skin ulcer | Survived | 554 |
| MSHR4237* | 45/F | None | Pneumonia | Survived | 868 |
| NCTC13179 | 54/M | DBT | Skin ulcer | Survived | 613 |
*Additional isolates included for internalization and persistence assays. ALC, hazardous alcohol use; COPD, chronic obstructive pulmonary disease; DBT, diabetes; MLST, multilocus sequence typing.
Acknowledgment
We thank Christopher Davis and Ifor Beacham of Griffith University for their helpful discussions and contribution to the work described in this article.
Biography
Dr. Morris is a postdoctoral researcher in the Australian Institute of Tropical Health and Medicine at James Cook University. Her research interests include the immunopathogenesis of Burkholderia pseudomallei infection.
Footnotes
Suggested citation for this article: Morris JL, Fane A, Sarovich DS, Price EP, Rush CM, Govan BL, et al. Increased neurotropic threat from Burkholderia pseudomallei strains with a B. mallei–like variation in the bimA motility gene, Australia. Emerg Infect Dis. 2017 May [date cited]. http://dx.doi.org/10.3201/eid2305.151417
References
- 1.Holden MT, Titball RW, Peacock SJ, Cerdeño-Tárraga AM, Atkins T, Crossman LC, et al. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci U S A. 2004;101:14240–5. 10.1073/pnas.0403302101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres AG, Steinmetz I. Recent progress in melioidosis and glanders. Front Microbiol. 2012;3:149. 10.3389/fmicb.2012.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ketheesan N. Melioidosis: a century of observation and research. Amsterdam: Elsevier Press; 2012. [Google Scholar]
- 4.Stevens JM, Galyov EE, Stevens MP. Actin-dependent movement of bacterial pathogens. Nat Rev Microbiol. 2006;4:91–101. 10.1038/nrmicro1320 [DOI] [PubMed] [Google Scholar]
- 5.Stevens MP, Stevens JM, Jeng RL, Taylor LA, Wood MW, Hawes P, et al. Identification of a bacterial factor required for actin-based motility of Burkholderia pseudomallei. Mol Microbiol. 2005;56:40–53. 10.1111/j.1365-2958.2004.04528.x [DOI] [PubMed] [Google Scholar]
- 6.Schell MA, Ulrich RL, Ribot WJ, Brueggemann EE, Hines HB, Chen D, et al. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol Microbiol. 2007;64:1466–85. 10.1111/j.1365-2958.2007.05734.x [DOI] [PubMed] [Google Scholar]
- 7.Stevens JM, Ulrich RL, Taylor LA, Wood MW, Deshazer D, Stevens MP, et al. Actin-binding proteins from Burkholderia mallei and Burkholderia thailandensis can functionally compensate for the actin-based motility defect of a Burkholderia pseudomallei bimA mutant. J Bacteriol. 2005;187:7857–62. 10.1128/JB.187.22.7857-7862.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suwannasaen D, Mahawantung J, Chaowagul W, Limmathurotsakul D, Felgner PL, Davies H, et al. Human immune responses to Burkholderia pseudomallei characterized by protein microarray analysis. J Infect Dis. 2011;203:1002–11. 10.1093/infdis/jiq142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhopadhyay C, Kaestli M, Vandana KE, Sushma K, Mayo M, Richardson L, et al. Molecular characterization of clinical Burkholderia pseudomallei isolates from India. Am J Trop Med Hyg. 2011;85:121–3. 10.4269/ajtmh.2011.11-0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sitthidet C, Stevens JM, Chantratita N, Currie BJ, Peacock SJ, Korbsrisate S, et al. Prevalence and sequence diversity of a factor required for actin-based motility in natural populations of Burkholderia species. J Clin Microbiol. 2008;46:2418–22. 10.1128/JCM.00368-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris J, Fane A, Rush C, Govan B, Mayo M, Currie BJ, et al. Neurotropic threat characterization of Burkholderia pseudomallei strains. Emerg Infect Dis. 2015;21:58–63. 10.3201/eid2101.131570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarovich DS, Price EP, Webb JR, Ward LM, Voutsinos MY, Tuanyok A, et al. Variable virulence factors in Burkholderia pseudomallei (melioidosis) associated with human disease. PLoS One. 2014;9:e91682. 10.1371/journal.pone.0091682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Currie BJ, Ward L, Cheng AC. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis. 2010;4:e900. 10.1371/journal.pntd.0000900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koszyca B, Currie BJ, Blumbergs PC. The neuropathology of melioidosis: two cases and a review of the literature. Clin Neuropathol. 2004;23:195–203. [PubMed] [Google Scholar]
- 15.Ulett GC, Currie BJ, Clair TW, Mayo M, Ketheesan N, Labrooy J, et al. Burkholderia pseudomallei virulence: definition, stability and association with clonality. Microbes Infect. 2001;3:621–31. 10.1016/S1286-4579(01)01417-4 [DOI] [PubMed] [Google Scholar]
- 16.Barnes JL, Ketheesan N. Route of infection in melioidosis. Emerg Infect Dis. 2005;11:638–9. 10.3201/eid1104.041051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 18.Williams NL, Morris JL, Rush CM, Ketheesan N. Migration of dendritic cells facilitates systemic dissemination of Burkholderia pseudomallei. Infect Immun. 2014;82:4233–40. 10.1128/IAI.01880-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes JL, Ketheesan N. Development of protective immunity in a murine model of melioidosis is influenced by the source of Burkholderia pseudomallei antigens. Immunol Cell Biol. 2007;85:551–7. 10.1038/sj.icb.7100084 [DOI] [PubMed] [Google Scholar]
- 20.Williams NL, Morris JL, Rush C, Govan BL, Ketheesan N. Impact of streptozotocin-induced diabetes on functional responses of dendritic cells and macrophages towards Burkholderia pseudomallei. FEMS Immunol Med Microbiol. 2011;61:218–27. 10.1111/j.1574-695X.2010.00767.x [DOI] [PubMed] [Google Scholar]
- 21.Leakey AK, Ulett GC, Hirst RG. BALB/c and C57Bl/6 mice infected with virulent Burkholderia pseudomallei provide contrasting animal models for the acute and chronic forms of human melioidosis. Microb Pathog. 1998;24:269–75. 10.1006/mpat.1997.0179 [DOI] [PubMed] [Google Scholar]
- 22.Kandasamy Y, Norton R. Paediatric melioidosis in North Queensland, Australia. J Paediatr Child Health. 2008;44:706–8. 10.1111/j.1440-1754.2008.01410.x [DOI] [PubMed] [Google Scholar]
- 23.Kespichayawattana W, Rattanachetkul S, Wanun T, Utaisincharoen P, Sirisinha S. Burkholderia pseudomallei induces cell fusion and actin-associated membrane protrusion: a possible mechanism for cell-to-cell spreading. Infect Immun. 2000;68:5377–84. 10.1128/IAI.68.9.5377-5384.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sitthidet C, Korbsrisate S, Layton AN, Field TR, Stevens MP, Stevens JM. Identification of motifs of Burkholderia pseudomallei BimA required for intracellular motility, actin binding, and actin polymerization. J Bacteriol. 2011;193:1901–10. 10.1128/JB.01455-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balandyté L, Brodard I, Frey J, Oevermann A, Abril C. Ruminant rhombencephalitis-associated Listeria monocytogenes alleles linked to a multilocus variable-number tandem-repeat analysis complex. Appl Environ Microbiol. 2011;77:8325–35. 10.1128/AEM.06507-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacquet C, Gouin E, Jeannel D, Cossart P, Rocourt J. Expression of ActA, Ami, InlB, and listeriolysin O in Listeria monocytogenes of human and food origin. Appl Environ Microbiol. 2002;68:616–22. 10.1128/AEM.68.2.616-622.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lutter EI, Bonner C, Holland MJ, Suchland RJ, Stamm WE, Jewett TJ, et al. Phylogenetic analysis of Chlamydia trachomatis Tarp and correlation with clinical phenotype. Infect Immun. 2010;78:3678–88. 10.1128/IAI.00515-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu PJ, Chen YS, Lin HH, Ni WF, Hsieh TH, Chen HT, et al. Induction of mouse melioidosis with meningitis by CD11b+ phagocytic cells harboring intracellular B. pseudomallei as a Trojan horse. PLoS Negl Trop Dis. 2013;7:e2363. 10.1371/journal.pntd.0002363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massey S, Johnston K, Mott TM, Judy BM, Kvitko BH, Schweizer HP, et al. In vivo bioluminescence imaging of Burkholderia mallei respiratory infection and treatment in the mouse model. Front Microbiol. 2011;2:174. 10.3389/fmicb.2011.00174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Owen SJ, Batzloff M, Chehrehasa F, Meedeniya A, Casart Y, Logue CA, et al. Nasal-associated lymphoid tissue and olfactory epithelium as portals of entry for Burkholderia pseudomallei in murine melioidosis. J Infect Dis. 2009;199:1761–70. 10.1086/599210 [DOI] [PubMed] [Google Scholar]
- 31.St John JA, Ekberg JA, Dando SJ, Meedeniya AC, Horton RE, Batzloff M, et al. Burkholderia pseudomallei penetrates the brain via destruction of the olfactory and trigeminal nerves: implications for the pathogenesis of neurological melioidosis. MBio. 2014;5:e00025. 10.1128/mBio.00025-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St John JA, Walkden H, Nazareth L, Beagley KW, Ulett GC, Batzloff MR, et al. Burkholderia pseuodomallei rapidly infects the brainstem and spinal cord via the trigeminal nerve after intranasal inoculation. Infect Immun. 2016;84:2681–8. 10.1128/IAI.00361-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chadwick DR, Ang B, Sitoh YY, Lee CC. Cerebral melioidosis in Singapore: a review of five cases. Trans R Soc Trop Med Hyg. 2002;96:72–6. 10.1016/S0035-9203(02)90248-8 [DOI] [PubMed] [Google Scholar]
- 34.Lim RS, Flatman S, Dahm MC. Sinonasal melioidosis in a returned traveller presenting with nasal cellulitis and sinusitis. Case Rep Otolaryngol. 2013;2013:920352. [DOI] [PMC free article] [PubMed]
- 35.Lumbiganon P, Kosalaraksa P. Uncommon clinical presentations of melioidosis in children: 2 cases with sore throat and 1 case with urticarial rash. Southeast Asian J Trop Med Public Health. 2013;44:862–5. [PubMed] [Google Scholar]
- 36.Currie BJ. Melioidosis: evolving concepts in epidemiology, pathogenesis, and treatment. Semin Respir Crit Care Med. 2015;36:111–25. 10.1055/s-0034-1398389 [DOI] [PubMed] [Google Scholar]
- 37.McLeod C, Morris PS, Bauert PA, Kilburn CJ, Ward LM, Baird RW, et al. Clinical presentation and medical management of melioidosis in children: a 24-year prospective study in the Northern Territory of Australia and review of the literature. Clin Infect Dis. 2015;60:21–6. 10.1093/cid/ciu733 [DOI] [PubMed] [Google Scholar]
- 38.Challacombe JF, Stubben CJ, Klimko CP, Welkos SL, Kern SJ, Bozue JA, et al. Interrogation of the Burkholderia pseudomallei genome to address differential virulence among isolates. PLoS One. 2014;9:e115951. 10.1371/journal.pone.0115951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sahl JW, Allender CJ, Colman RE, Califf KJ, Schupp JM, Currie BJ, et al. Genomic characterization of Burkholderia pseudomallei isolates selected for medical countermeasures testing: comparative genomics associated with differential virulence. PLoS One. 2015;10:e0121052. 10.1371/journal.pone.0121052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulett GC, Currie BJ, Clair TW, Mayo M, Ketheesan N, Labrooy J, et al. Burkholderia pseudomallei virulence: definition, stability and association with clonality. Microbes Infect. 2001;3:621–31. 10.1016/S1286-4579(01)01417-4 [DOI] [PubMed] [Google Scholar]
- 41.Deshazer D. Virulence of clinical and environmental isolates of Burkholderia oklahomensis and Burkholderia thailandensis in hamsters and mice. FEMS Microbiol Lett. 2007;277:64–9. 10.1111/j.1574-6968.2007.00946.x [DOI] [PubMed] [Google Scholar]


