Abstract
Lung carcinoma is the most common cause of malignant death worldwide. CD8+ T cells, as critical elements in antitumor immunity, could function as good prognostic indicators in various kinds of cancers such as renal cell carcinoma and colorectal cancer, but its prognostic role in lung adenocarcinoma is still unclear. The objective of this study was to explore the prognostic role of CD8 expression in lung adenocarcinoma.
Paired tumor and adjacent noncancerous tissues were obtained from 102 patients with lung adenocarcinoma, and CD8 expression of these samples was examined by immunohistochemistry. We evaluated the relationships between the expression of CD8 and pathological grade, TNM stage, clinical stage, as well as overall survival (OS).
Expression of CD8 was significantly increased in lung adenocarcinoma compared with that in adjacent lung adenocarcinoma (P < .001). CD8 expression was negatively correlated with pathological grade (r = –0.216, P = .022) and N stage (r = –0.372, P < .001), while no statistical correlation with T stage, or clinical stage. Importantly, OS was numerically increased in patients with high expression of CD8 than the group of intermediate and low CD8 expression (P = .12). Furthermore, CD8 could significantly increase OS (P = .043, HR: .713, 95%CI: .515–.989) by univariate Cox's proportional hazards regression analysis.
Our data indicated that expression of CD8, as a protective factor, is correlated with the outcome of patients with lung adenocarcinoma. Furthermore, CD8 might be a novel prognostic biological marker for patients with lung adenocarcinoma.
Keywords: CD8, lung adenocarcinoma, OS, prognosis
1. Introduction
As the most commonly diagnosed cancer and the leading cause of cancer-related death, lung cancer leads to the great cancer burden worldwide.[1,2] Nonsmall-cell lung cancer (NSCLC), which accounts for approximately 85% primary lung cancers, consists of adenocarcinoma, squamous cell carcinoma, large cell carcinoma, sarcomatoid carcinoma, and adenosquamous cell carcinoma.[3,4] Meanwhile, adenocarcinoma, the main subtype of NSCLC, is the most aggressive histological type in lung cancer.
Since symptoms and signs are often absent in early stage, most lung cancers are usually diagnosed at an advanced stage. Furthermore, although our understanding in the pathogenesis of lung cancer is rapidly evolving, the major clinical treatment is still surgical resection combined with chemotherapy and radiation therapy, with poor survival prognosis.[1,4–7] Therefore, it is necessary and urgent to investigate some biomarkers that function as predictive predictors for lung adenocarcinoma.
It is well known that lung cancer is a multifactorial disease, which is caused by the combination of smoke exposure, environmental factor, and immune response.[3,8] As immune cells are extensively involved in the pathogenesis of cancers, exploring how immune cells affect the development and progression of cancers has received broad attention globally. Among immune cells, CD8+ T cells, also known as cytotoxic T cells, are critical element in antitumor immunity by killing cancer cells.[9]
In our study, the expression of CD8 in lung adenocarcinoma was measured, and clinical, pathological characteristics of patients were collected to analyze their correlation. We aimed to measure the expression of CD8 in lung adenocarcinoma, and analyze its correlation with pathological grade, TNM stage, clinical stage, as well as OS.
2. Materials and methods
2.1. Participants and tissue samples
All patients with lung adenocarcinoma were recruited at department of respiratory medicine and department of thoracic surgery, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Hubei Province, China, from May 2005 to Apr 2009. In total, 102 patients with lung adenocarcinoma, who received no preoperative adjuvant therapy and underwent pulmonary section, provided their written consent to be enrolled in this study. This study was approved by the Ethics Review Board of Wuhan Central Hospital. The diagnosis of lung adenocarcinoma was based on clinical characteristics, radiological examination, and pathological confirmation. Tissue samples were obtained from the tumoral area and the adjacent noncancerous area of the surgically removed lung tissue. All tumors were staged on the basis of the 7th edition of the American Joint Committee on Cancer (AJCC) cancer staging manual.[10] Besides, clinical data were collected, including patients characteristics (age, gender), tumor features (pathological classification, tumor size, pathological grade, TNM stage), and OS.
2.2. Immunohistochemistry
Lung tissues were taken from patients with lung adenocarcinoma, fixed, embedded in paraffin and cut into 4 μm sections. Subsequently, immunohistochemical staining was then performed using anti-CD8 antibody (Abcam). Briefly, tissue sections were deparaffinized and rehydrated, followed by antigen retrieval in Tris-EDTA (pH=9.0). After blocking endogenous peroxidase using 3% H2O2, sections were immersed with 4% bovine serum albumin for blocking nonspecific endogenous antigens. Then, these sections were incubated with anti-CD8 antibody as primary antibody at 4°C overnight. Negative controls were incubated with phosphate-buffered saline (PBS) only. After washing, the sections were incubated with horseradish peroxidase (HRP)-conjugated anti-IgG (Abcam) as secondary antibody at room temperature for 30 minutes. Afterward, tissue sections were washed and treated with DAB, followed by counterstaining with hematoxylin. Then, sections were dehydrated and mounted. Finally, the positive cells were observed and counted under a light microscopy by 2 specialists without knowing any information about patients. In each slide, 100 cells in 5 high-power fields (HPF, ×400) were counted to evaluate the intensity of positive cells. Staining intensity was scored as 0 (negative), 1 (weak), 2 (moderate), to 3 (strong), whereas the grading scale for labeling frequency ranged from 0 (0%), 1 (1–25%), 2 (26–50%), 3 (51–75%), and 4 (76–100%) on the basis of the percentage of positively stained cells. Finally, multiplying the score of staining intensity by the labeling frequency score was used to divide sections into 3 groups: low (final score ≤3, intermediate (final score >3, ≤ 6); high (final score>6).
2.3. Statistical methods
Comparison of CD8 expression between 2 groups was determined by the χ2 test. The Spearman test was used to determine the correlation of CD8 expression with clinical and pathological stages. Kaplan–Meier curves were used to analyze overall survival of 3 groups. OS was calculated in months from the initial diagnosis to the time of death for any causes. Univariate and multivariate Cox's proportional hazards regression analyses were performed to identify independent factors of OS in patients with lung adenocarcinoma. Statistical significance was set at P < .05. All statistical analysis performed using IBM SPSS software (Version 19).
3. Results
3.1. Patient characteristics
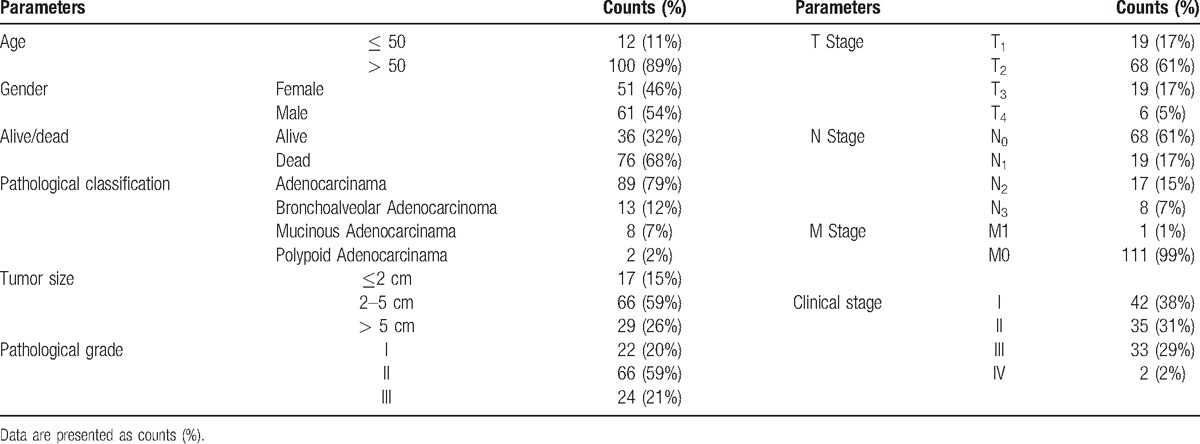
Demographic, clinical, and pathological characteristics of the enrolled patients with lung adenocarcinoma were elaborated in Table 1. Among 102 subjects, there were 12 (11%) patients in age ≤ 50 and 100 (89%) patients in age > 50, with 51 (46%) males and 61 (54%) females. The number of alive patients was 36 (32%), and dead patients 76 (68%). As to pathological classification, 89 (79%), 13 (12%), 8 (7%), and 2 (2%) cases were identified as adenocarcinoma, bronchoalveolar adenocarcinoma, mucinous adenocarcinoma, and polypoid adenocarcinoma, respectively. Patients with tumor size > 5 cm accounted for 26% total subjects, whereas the number of patients with tumor size ranging from 2 to 5 cm were 66 (59%). According to pathological grade, 22 (20%), 66 (59%), and 24 (21%) cases were classified into 1, 2, 3 grade, whereas 42 (38%), 35 (31%), 33 (29%), and 2 (2%) subjects were ranked as 1, 2, 3, 4 on the basis of clinical stage.
Table 1.
Demographic, clinical, and pathological characteristics of lung adenocarcinoma patients.
3.2. Expression of CD8 in tumoral regions and the adjacent noncancerous regions of patients with lung adenocarcinoma
To determine the difference of CD8 expression between lung adenocarcinoma and adjacent lung adenocarcinoma, immunohistochemistry assay was performed. We found that a higher expression of CD8+ cells in lung adenocarcinoma as compared with the paired adjacent lung adenocarcinoma (P < 0.001, Table 2, Fig. 1). As illustrated in Table 2, the numbers of tissues with high, intermediate, and low CD8 staining are 45, 52, 15 in lung adenocarcinoma, and 8, 69, 35 in adjacent lung adenocarcinoma. Collectively, these data indicated that expression of CD8 was significantly increased in lung adenocarcinoma compared with that in paired adjacent lung adenocarcinoma.
Table 2.
Expression of CD8 in lung adenocarcinoma and adjacent lung adenocarcinoma.
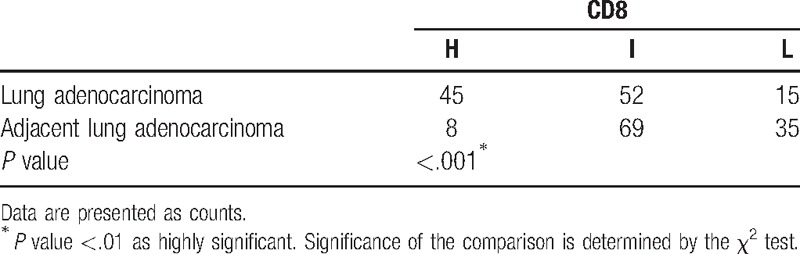
Figure 1.
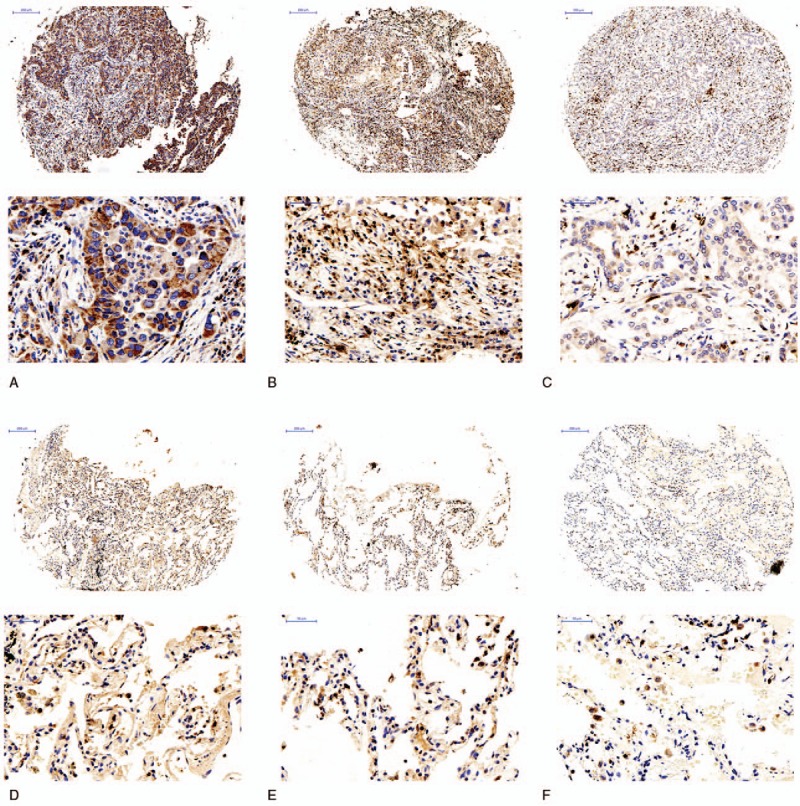
Expression of CD8 in lung adenocarcinoma and adjacent lung adenocarcinoma. High expression of CD8 in lung adenocarcinoma, intermediate expression of CD8 in lung adenocarcinoma, low expression of CD8 in lung adenocarcinoma, high expression of CD8 in adjacent lung adenocarcinoma, intermediate expression of CD8 in adjacent lung adenocarcinoma, low expression of CD8 in adjacent lung adenocarcinoma.
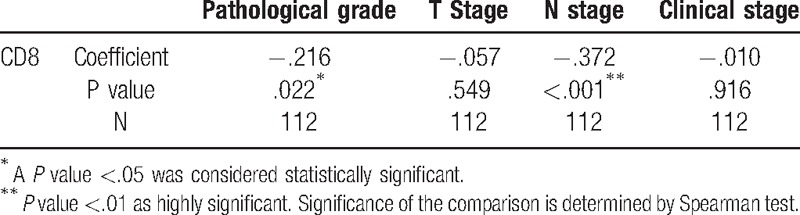
3.3. Correlation of CD8 expression with clinical and pathological stage
The Spearman test was next performed to explore whether CD8 expression was associated with clinical and pathological stages. We found that the levels of CD8 expression were negatively correlated with pathological (r = –0.216, P = .022) and N stage (r = –0.372, P < .001), whereas there was no statistical correlation between CD8 expression and T stage, or clinical stage (Fig. 2, Table 3).
Figure 2.
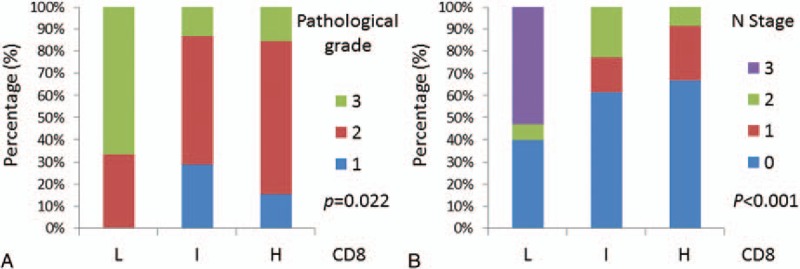
The negative correlation of CD8 expression with pathological stage (A) and N stage (B).
Table 3.
Correlation of CD8 expression with clinical and pathological stages.
3.4. Association between CD8 expression and overall survival
Kaplan–Meier survival curve was plotted to analyze the association of CD8 expression with OS. As shown in Fig. 3, although there was no statistically significant association between CD8 expression and OS (P = .12), patients with high-CD8 staining presented increased OS compared with the groups with intermediate-CD8 and low-CD8 staining, indicating that CD8, which may influence prognosis of patients with lung adenocarcinoma, might be a protective factor for lung adenocarcinoma.
Figure 3.
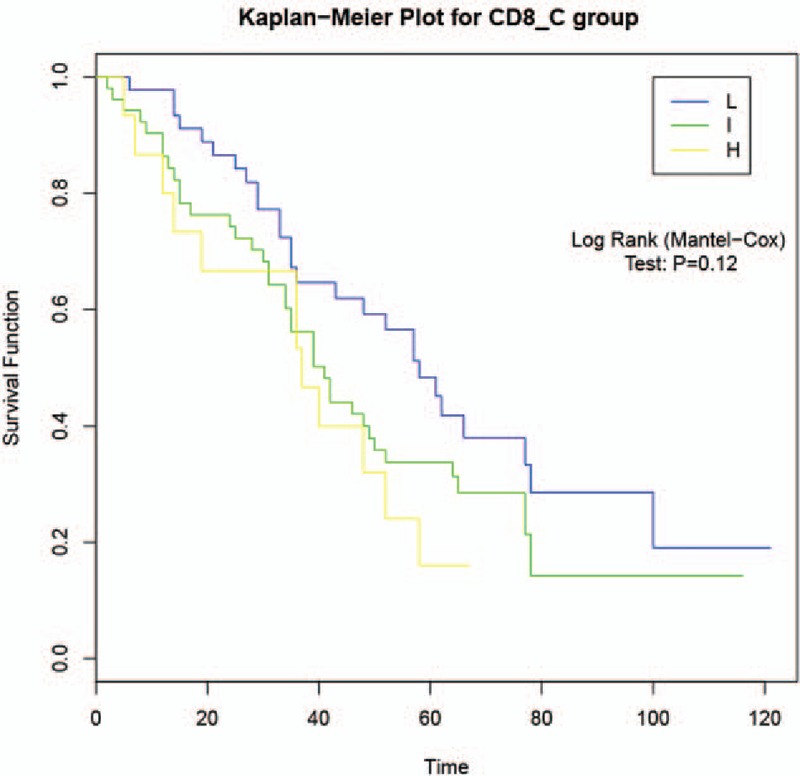
Association of CD8 expression with overall survival by the Kaplan–Meier method.
As to further investigate the association between CD8 and OS, univariate Cox's and multivariate Cox's proportional hazards regression analyses were performed. As illustrated in Table 4, univariate analysis with a Cox proportional hazards regression model revealed that CD8 could significantly increase OS (P = .043, HR: .713, 95%CI: .515–.989), whereas pathological grade (P = .042, HR: 1.994, 95%CI: 1.455–2.732), T stage (P < .001, HR: 1.994, 95%CI: 1.455–2.732), N stage (P < .001, HR: 1.829, 95%CI: 1.472–2.274) and clinical stage (P < .001, HR: 2.010, 95%CI: 1.549–2.608) were also negatively correlated with OS.
Table 4.
Association between CD8 expression and overall survival by univariate Cox's and multivariate Cox's proportional hazards regression analysis.
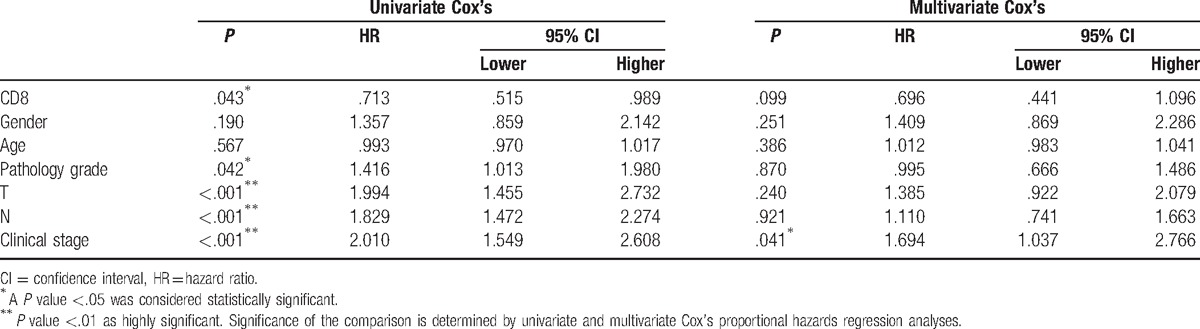
Next, multivariate Cox's proportional hazards regression analysis was carried out to explore whether CD8 was an independent protective factor of OS. Unexpectedly, there was no significant association between CD8 expression and OS (P = .099, HR: .696, 95%CI: .441–1.096) (Table 4), although there was a tendency that CD8 could increase OS. However, the clinical stage was an independent risk factor of OS in lung adenocarcinoma (P = .042, HR: 1.694, 95%CI: 1.037–2.766, Table 4).
4. Discussion
During the progression of cancers, tumor cells interact with their microenvironment, mainly including endothelial cells, tumor-associated macrophages, T cells, B cells, dendritic cells (DCs), and neutrophils.[11–13] Currently, accumulated data have shown that inflammatory responses promote the development of cancers, whereas high infiltrated number of lymphocytes, especially CD8+ T cells, could function as good prognostic indicators in various kinds of cancers such as renal cell carcinoma, and colorectal cancer.[14,15] However, the association between CD8 expression and clinical, pathological characteristics, clinical outcome in lung adenocarcinoma still remains to be explored. Therefore, identification of CD8+ T cells in lung adenocarcinoma might be useful for prognosis of lung adenocarcinoma. Based on all the information related to CD8 and cancer, we assumed that CD8 functioned as a helpful biomarker to predict outcome of patients with lung adenocarcinoma.
In our study, we assessed the expression of CD8 in lung adenocarcinoma and adjacent lung adenocarcinoma determined by immunohistochemistry. The expression of CD8 was found to be significantly upregulated in tumor tissues compared with that in adjacent noncancerous tissues in patients with lung adenocarcinoma, which consistent with other types of cancers and NSCLC.[14–18]
CD8+ T cells, also known as cytotoxic T cells, are the central elements in antitumor immunity and have been investigated widely to examine the association of CD8 with various clinical and pathological features in many cancers.[19,20] Therefore, we next analyze the relationship between expression of CD8 and clinical and pathological stage using the Spearman test. We found that the levels of CD8 expression were negatively correlated with pathological and N stage, which may be explained by that CD8+ T cells can eradicate tumor cells and destroy large tumor masses.[21] However, there was no statistical correlation between CD8 expression and T stage, or clinical stage.
The increased presence of infiltrating CD8+ T cells has been reported to an independent favorable prognostic factor for patients with NSCLC and other cancers.[11,14,16–18,22,23] Subsequently, the Kaplan–Meier survival curve was plotted to analyze the association of CD8 expression with OS in this study. No statistically significant association between CD8 expression and OS was demonstrated in lung adenocarcinoma, which was not the same as above-mentioned studies. The discrepancy might be caused by the complexity of inadequate cases, different crowds, and varied methods for grouping. However, there was a tendency that patients with high-CD8 staining presented increased OS compared with the groups with intermediate-CD8 and low-CD8 staining, indicating that CD8 might be a protective element for outcome of patients with lung adenocarcinoma. Therefore, univariate Cox's and multivariate Cox's proportional hazards regression analyses were then performed to further investigate the association between CD8 and OS. However, multivariate Cox's proportional hazards regression analyses revealed that there was no significant association between CD8 expression and OS, whereas the expression of CD8 was positively correlated with OS when analyzed by univariate Cox's proportional hazards regression. Interaction effect among other factors, such as gender and age, might be the main reason accounting for this different result.
There were some limitations in this study: First, medications during the observational period were not analyzed due to data missing, which would affect the OS. However, the therapies after surgery were almost similar that might not distract the prognosis analysis. Second, it was obscure whether the CD8+ T cells activity is for pathogens or for tumors, since lung shares common microenvironment space for infectious pathogens as well as adenocarcinomas. Nevertheless, the samples collected from the patients in this study were confirmed to be cancer tissues which might reduce the influence. Moreover, in our subsequent study, we would determine the pathogens when tissue was collected.
In summary, the expression of CD8 was significantly increased in lung adenocarcinoma. High-CD8+ T cells infiltration was negatively correlated with pathological grade and T stage. Most importantly, OS was increased in patients with high expression of CD8 than the group of low CD8 expression. All the evidence indicated that CD8 is a protective factor for progression of lung adenocarcinoma and might be a potential therapeutic target for the treatment of lung adenocarcinoma.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CI = confidence interval, DCs = dendritic cells, HPF = high-power fields, HRP = horseradish peroxidase, NSCLC = non-small-cell lung cancer, OS = overall survival, PBS = phosphate-buffered saline.
S-LY and X-YL contributed equally to this study and share first authorship.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [2].Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [3].Gridelli C, Rossi A, Carbone DP, et al. Non-small-cell lung cancer. Nat Rev Dis Primers 2015;1:15009. [DOI] [PubMed] [Google Scholar]
- [4].Cheng L, Alexander RE, Maclennan GT, et al. Molecular pathology of lung cancer: key to personalized medicine. Mod Pathol 2012;25:347–69. [DOI] [PubMed] [Google Scholar]
- [5].Reck M, Heigener DF, Mok T, et al. Management of non-small-cell lung cancer: recent developments. Lancet 2013;382:709–19. [DOI] [PubMed] [Google Scholar]
- [6].Greenlee RT, Hill-Harmon MB, Murray T, et al. Cancer statistics, 2001. CA Cancer J Clin 2001;51:15–36. [DOI] [PubMed] [Google Scholar]
- [7].Ettinger DS. Overview and state of the art in the management of lung cancer. Oncology (Williston Park) 2004;18:3–9. [PubMed] [Google Scholar]
- [8].Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 2011;331:1565–70. [DOI] [PubMed] [Google Scholar]
- [9].Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016;16:275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706–14. [DOI] [PubMed] [Google Scholar]
- [11].Pages F, Galon J, Dieu-Nosjean MC, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 2010;29:1093–102. [DOI] [PubMed] [Google Scholar]
- [12].Yang L, Wang L, Zhang Y. Immunotherapy for lung cancer: advances and prospects. Am J Clin Exp Immunol 2016;5:1–20. [PMC free article] [PubMed] [Google Scholar]
- [13].Liotta LA, Kohn EC. The microenvironment of the tumour–host interface. Nature 2001;411:375–9. [DOI] [PubMed] [Google Scholar]
- [14].Naito Y, Saito K, Shiiba K, et al. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res 1998;58:3491–4. [PubMed] [Google Scholar]
- [15].Nakano O, Sato M, Naito Y, et al. Proliferative activity of intratumoral CD8(+) T-lymphocytes as a prognostic factor in human renal cell carcinoma: clinicopathologic demonstration of antitumor immunity. Cancer Res 2001;61:5132–6. [PubMed] [Google Scholar]
- [16].Al-Shibli KI, Donnem T, Al-Saad S, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res 2008;14:5220–7. [DOI] [PubMed] [Google Scholar]
- [17].Hiraoka K, Miyamoto M, Cho Y, et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br J Cancer 2006;94:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kawai O, Ishii G, Kubota K, et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 2008;113:1387–95. [DOI] [PubMed] [Google Scholar]
- [19].Koch M, Beckhove P, Op den Winkel J, et al. Tumor infiltrating T lymphocytes in colorectal cancer: tumor-selective activation and cytotoxic activity in situ. Ann Surg 2006;244:986–92. discussion 992-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol 2015;26:259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kershaw MH, Teng MW, Smyth MJ, et al. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol 2005;5:928–40. [DOI] [PubMed] [Google Scholar]
- [22].Cho Y, Miyamoto M, Kato K, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 2003;63:1555–9. [PubMed] [Google Scholar]
- [23].Fukunaga A, Miyamoto M, Cho Y, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas 2004;28:e26–31. [DOI] [PubMed] [Google Scholar]


