Abstract
Rationale:
Osteosarcoma is the most common malignant bone tumor in children and adolescents. Pulmonary metastases lead to a significantly increased risk of death. Apatinib, a new potent oral small-molecule tyrosine kinase inhibitor targeting the intracellular domain of vascular endothelial growth factor receptor 2 (VEGFR-2), shows survival benefits in treating advanced or metastatic gastric adenocarcinoma, non-squamous non-small cell lung cancer and metastatic breast cancer. However, its efficacy in metastatic osteosarcoma has not been reported yet.
Patient concerns:
Herein, we presented a 50-year-old man patient who visited hospital due to local bone pain in the left leg.
Diagnoses:
He was initially diagnosed with osteoblastic osteosarcoma.
Interventions:
The patient suffered repeated resection surgeries but developed multiple lung metastases. Positive staining for CD31, CD34, and VEGFR-2 were detected in the tumor section. As he refused to receive chemotherapy due to concerns regarding the chemotherapy toxicities and sorafenib due to high cost, apatinib was given at a dose of 500 mg daily.
Outcomes:
Eleven months following apatinib administration, the patient achieved a partial response according to the RECIST 1.1 standard. No severe toxicity or drug-related side effect was observed during the treatment.
Lessons:
Therefore, apatinib could be a new option for the treatment of metastatic osteosarcoma. Clinical trials are required to further confirm the efficacy and safety of apatinib in treating pulmonary metastases from osteosarcoma.
Keywords: angiogenesis inhibitor, apatinib, case report, osteosarcoma, pulmonary metastases, targeted therapy
1. Introduction
Osteosarcoma is the most common malignant bone tumor in children and adolescents with a high tendency of pulmonary metastases.[1] Although the 5-year survival rate for patients with osteosarcoma has reached 60% to 70% which is dramatically improved by multidisciplinary treatment (surgical resection in conjunction with perioperative multiagent chemotherapy), patients exhibiting metastasis or disease recurrence still have a low long-term survival rate of <20%.[2–5] Thus, it is highly desired in developing novel therapeutic strategies for osteosarcoma patients with pulmonary metastases.
Sorafenib, an orally active multikinase inhibitor targeting mitogen-activated protein kinase (MAPK), vascular endothelial growth factor receptors (VEGFRs), platelet-derived growth factor receptors (PDGFRs) and KIT,[6] is recommended for metastatic high-grade relapsed and unresectable osteosarcoma by National Comprehensive Cancer Network (NCCN) Guidelines[7] based on a phase II clinical trial.[8] Like sorafenib, apatinib is an oral tyrosine kinase inhibitor (TKI) targeting VEGFR-2.[9] The phase II[10] and III[11] studies have shown that apatinib improved overall survival and progression-free survival in patients with advanced or metastatic gastric or gastroesophageal junction adenocarcinoma who failed at least two lines of previous systemic chemotherapies. Additionally, apatinib exerts anti-cancer effects against a broad range of malignancies,[12] including advanced non-squamous and non-small-cell lung cancer,[13] metastatic breast cancer,[14,15] intrahepatic cholangiocarcinoma,[16] hepatocellular carcinoma,[17] and advanced malignant fibrous histiocytoma.[18] However, the efficacy of apatinib in metastatic osteosarcoma patients has not been reported yet. Herein, we firstly reported a case of osteosarcoma patient with pulmonary metastases who responded to apatinib (Fig. 1).
Figure 1.
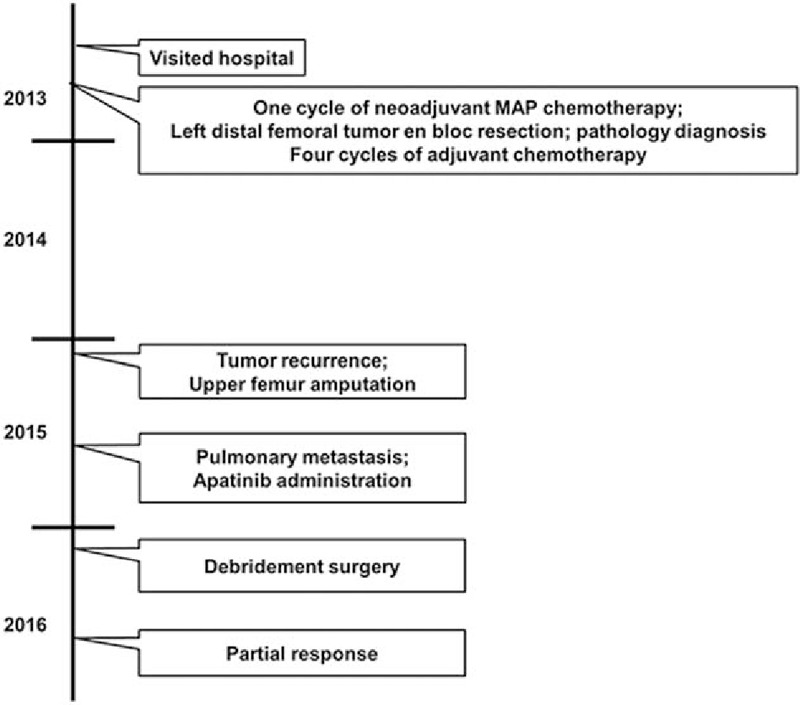
Timeline of patient's management.
2. Case presentation
In 2013, a 50-year-old man patient visited hospital complaining of local bone pain in the left leg. On August 14, 2013, the patient underwent a left distal femoral tumor en bloc resection and reconstruction with a modular femoral prosthetic system. Pathology diagnosis confirmed osteoblastic osteosarcoma (Fig. 2). One cycle of neoadjuvant chemotherapy and 4 cycles of adjuvant chemotherapy with MAP regimen (high-dose methotrexate, cisplatin, and doxorubicin) were administered.
Figure 2.
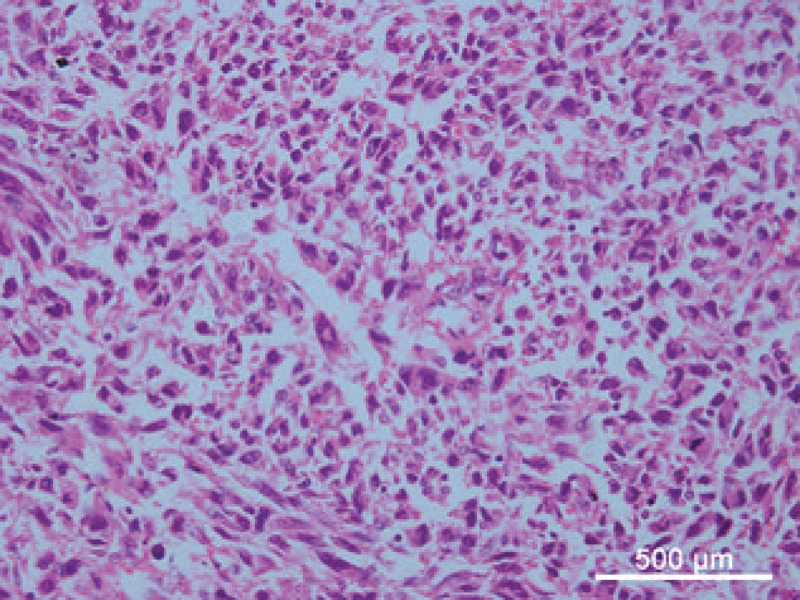
The patient was diagnosed with osteoblastic osteosarcoma (Hematoxylin and eosin stain, 400× magnification).
In January 2015, a mass was found on the left upper crus area. Tumor recurrence was confirmed by biopsy on January 23, 2015. However, only an upper femur amputation was carried out, as the patient refused hip joint replacement. After wound healing, the patient went back to his normal life in the help of artificial limb, but refused to receive further chemotherapy due to concerns regarding the chemotherapy toxicities such as nausea, vomiting, leucopenia, deadlimb, and headache.
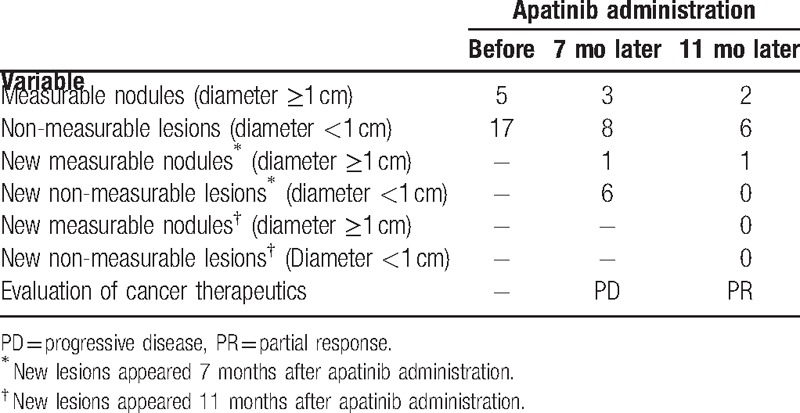
About half a year after the amputation, the patient got occasional cough and chest tightness. Then a thin chest computed tomography (CT) was performed on July 15, 2015. The CT results revealed multiple pulmonary nodules (Table 1), considering the possibility of metastases. The patient still rejected further chemotherapy.
Table 1.
Number of nodules detected by thin chest computed tomography and efficacy evaluation according to the RECIST 1.1 standard.
Immunophenotype was suggestive of CD31+ and CD34+ tumor cells (Fig. 3). Furthermore, most cells showed strong positive staining for VEGFR-2 (Fig. 3). Apatinib was administered at a dose of 500 mg daily. Half a month later, the symptoms disappeared, but a progressive wound necrosis appeared. A debridement surgery was finally conducted and an enlarged lymph node near iliac vessels was resected on February 24, 2016 (Fig. 4). The result of pathological examination revealed an inflammatory hyperplasia lymph node. Apatinib administration was stopped during the 3 weeks of wound healing period.
Figure 3.
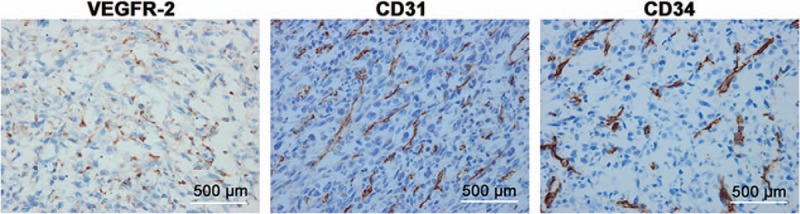
Expressions of VEGFR-2, CD31, and CD34 in a tumor section. Strong positive staining for VEGFR-2, CD31, and CD34 was found in cancer cells (Immunohistochemical staining, 400× magnification). VEGFR-2 = vascular endothelial growth factor receptor 2; CD31 = cluster of differentiation 31; CD34 = cluster of differentiation 34.
Figure 4.
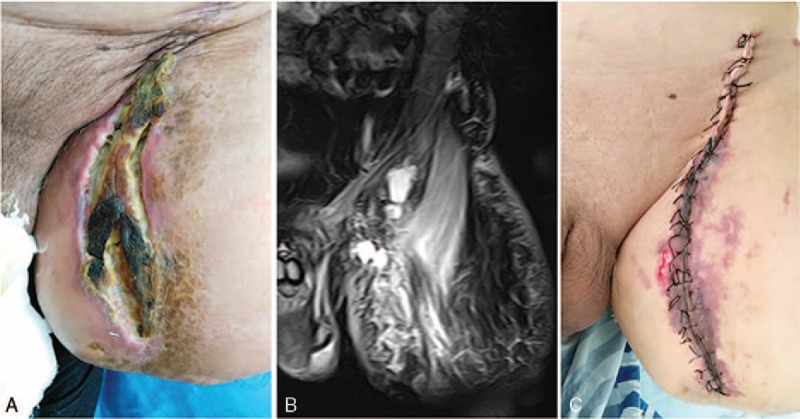
Treatment for wound necrosis. (A) Wound necrosis at half a month following apatinib administration. (B) Wound necrosis and the enlarged lymph node shown by magnetic resonance imaging. (C) Wound healing after debridement surgery.
The thin chest CT was performed 7 and 11 months following apatinib administration. At the 7-month follow-up time point, 2 out of 5 measurable and 9 out of 17 non-measurable lesions disappeared, but 1 new measurable nodule and 6 new non-measurable lesions were observed (Table 1, Fig. 5), which considered PD according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 standard. However, at the 11-month follow-up time point, a total of 9 lesions disappeared, including 1 measurable nodule and 2 non-measurable lesions presented before apatinib treatment as well as 6 non-measureable lesions presented 7 months after apatinib treatment. No new lesion was raised. After target lesion measurement according to the RECIST 1.1 standard, it was narrowly considered PR.
Figure 5.
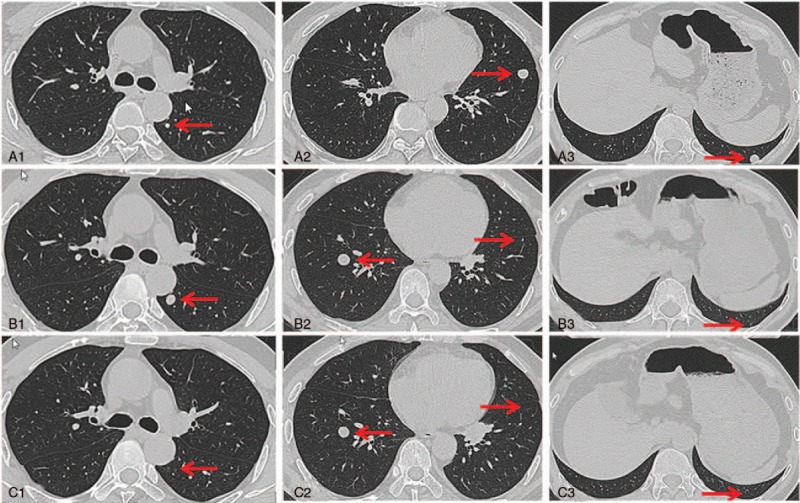
Nodules detected by thin chest CT. A1 to A3, images of CT performed on July 15, 2015 (before apatinib treatment). B1 to B3, images of CT performed on Feb 18, 2016 (7 months after apatinib treatment). C1 to C3, images of CT performed on June 21, 2016 (11 months after apatinib treatment). The nodule was located on left lung (A1) and grew to 1 cm in diameter (B1). But the nodule disappeared 4 months later (C1). The two nodules located on left lung (A2 and A3) were both disappeared after using apatinib for 7 months (B2, B3, C2, and C3). There was a new nodule that arose on right lung (B2), but the nodule did not get bigger (C2). CT = computed tomography.
The toxicities the patient experienced included mild hand-foot syndrome and slight high blood pressure. Both were well controlled after appropriate treatment. No severe toxicities and other treatment-related adverse events were observed. The patient continued to use apatinib as maintenance therapy without major toxic effects, and went back to normal life, even driving an automatic car.
3. Discussion
The patient in this case report was initially diagnosed as osteoblastic osteosarcoma. Preoperative and postoperative MAP regimen are recommended by the NCCN Guidelines.[7] Based on the clinical experiences in China, 1 to 2 cycles of neoadjuvant MAP chemotherapy and 4 to 5 cycles of adjuvant MAP chemotherapy are recommended.[19] Considering his old age, low weight and poor performance status, 1 cycle and 4 cycles of chemotherapy with MAP regimen were administered before and after first resection, respectively.
Osteosarcoma is highly malignant and has a high tendency to metastasize to the lung. Pulmonary metastasis occurs in approximately 40% to 50% of patients with osteosarcoma and remains a major cause of fatal outcome.[20] The patient in this case report also presented with pulmonary metastases 2 years after first tumor resection. As he refused to receive chemotherapy, therapies molecularly targeted at angiogenesis were considered.
Vascular endothelial growth factor (VEGF) is overexpressed by the vast majority of solid tumors.[21] Serum VEGF levels are elevated in many primary tumors, including osteosarcoma.[22–25] A study also showed the overexpression of VEGF mRNA in a murine model of highly metastatic osteosarcoma.[26] Moreover, blocking VEGF significantly inhibits angiogenesis in a murine model of osteosarcoma.[24] Some studies also demonstrated that VEGF-A/VEGFR expression in human osteosarcoma is associated with an aggressive clinical course, and inhibition of VEGF effectively suppresses osteosarcoma-associated angiogenesis.[24,27,28]
VEGFRs, including VEGFR-1 (flt-1), VEGFR-2 (KDR, flk-1), and neuropilin1 (NRP1), act to promote cell proliferation, survival, adhesion, migration, and capillary morphogenesis. VEGFR-2 is the primary receptor of VEGF on endothelial cells.[25,29,30] A study showed that osteosarcoma-associated neoangiogenesis could be attenuated and endothelial cell proliferation and migration could be inhibited when inhibiting VEGFR-2 expression in endothelial cells.[28] Another study also demonstrated that it is the VEGFR-2, not VEGFR-1, that mediates the effect of VEGF.[16]
Considering the high expression level of VEGFR-2 in the tumor section of present patient and the NCCN Guidelines,[7] sorafenib was firstly recommended. However, he did not afford the cost of sorafenib (approximately 50,000 RMB per month). Apatinib is a highly selective VEGFR-2 inhibitor,[31] and exerts a stronger inhibitory effect on VEGFR-2 compared with sorafenib (IC50 = 2.43 nm vs. 90 nm).[6] Besides, sorafenib is an inhibitor of multiple tyrosine kinases, which may lead to more off-target adverse events. Moreover, the cost of apatinib is one-third of that of sorafenib. Thus, the patient chose apatinib after careful consideration. At the 7-month time point following apatinib treatment, a progressive disease (PD) was met according to the RECIST 1.1 standard. However, apatinib was continued to be given as the symptoms were remised. Fortunately, a partial response (PR) to apatinib was achieved 11 months after therapy, suggesting a long-term efficacy of apatinib. The positive staining for CD31 and CD34, as well as high expression of VEGFR-2, were responsible for the good efficacy in this patient. Additionally, the patient was alive without severe toxicity or drug-related side effect.
In conclusion, apatinib may provide an additional option for the treatment of osteosarcoma patient with pulmonary metastases. Also, apatinib significantly inhibited the tumor volume in the sarcoma 180 (S180) tumor mice models (data not shown). Combined with our findings, it is necessary to further assess the efficacy and safety of apatinib in osteosarcoma patient with pulmonary metastases by large-scale prospective studies.
Acknowledgments
The authors thank the patient for his agreement to publication of this report.
Footnotes
Abbreviations: CT = computed tomography, MAPK = mitogen-activated protein kinase, PDGFR = platelet-derived growth factor receptor, TKI = tyrosine kinase inhibitor, VEGF = vascular endothelial growth factor, VEGFR = vascular endothelial growth factor receptor.
Informed Consent: Written informed consent with regard to publication of this case report and accompanying images was obtained from the patient.
The authors report no conflicts of interest.
References
- [1].Ta HT, Dass CR, Choong PF, et al. Osteosarcoma treatment: state of the art. Cancer Metastasis Rev 2009;28:247–63. [DOI] [PubMed] [Google Scholar]
- [2].Bacci G, Ferrari S, Lari S, et al. Osteosarcoma of the limb. Amputation or limb salvage in patients treated by neoadjuvant chemotherapy. J Bone Joint Surg Br 2002;84:88–92. [DOI] [PubMed] [Google Scholar]
- [3].Gelderblom H, Jinks RC, Sydes M, et al. Survival after recurrent osteosarcoma: data from 3 European Osteosarcoma Intergroup (EOI) randomized controlled trials. Eur J Cancer 2011;47:895–902. [DOI] [PubMed] [Google Scholar]
- [4].Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer 2003;98:2447–56. [DOI] [PubMed] [Google Scholar]
- [5].Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J Clin Oncol 2005;23:2004–11. [DOI] [PubMed] [Google Scholar]
- [6].Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099–109. [DOI] [PubMed] [Google Scholar]
- [7].National Comprehensive Cancer Network. The NCCN Clinical Practice Guidences in Oncology (NCCN Guidelines): Bone Cancer. Version 2; 2016. [Google Scholar]
- [8].Grignani G, Palmerini E, Dileo P, et al. A phase II trial of sorafenib in relapsed and unresectable high-grade osteosarcoma after failure of standard multimodal therapy: an Italian Sarcoma Group study. Ann Oncol 2012;23:508–16. [DOI] [PubMed] [Google Scholar]
- [9].Mi YJ, Liang YJ, Huang HB, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res 2010;70:7981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [11].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled Phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- [12].Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther 2015;9:6075–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang L, SHI M, Huang C, et al. A phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimens. Paper presented at: ASCO Annual Meeting Proceedings 2012. [Google Scholar]
- [14].Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961–9. [DOI] [PubMed] [Google Scholar]
- [16].Peng H, Zhang Q, Li J, et al. Apatinib inhibits VEGF signaling and promotes apoptosis in intrahepatic cholangiocarcinoma. Oncotarget 2016;7:17220–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lin Y, Wang K, Hu C, et al. Elemene injection induced autophagy protects human hepatoma cancer cells from starvation and undergoing apoptosis. Evid Based Complement Alternat Med 2014;2014:637528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ji G, Hong L, Yang P. Successful treatment of advanced malignant fibrous histiocytoma of the right forearm with apatinib: a case report. Onco Targets Ther 2016;9:643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Guo W. Guidelines for Diagnosis and Treatment of Osteosarcoma (in Chinese). Beijing:Peking University People's Hospital; 2011. [Google Scholar]
- [20].Kaya M, Wada T, Akatsuka T, et al. Vascular endothelial growth factor expression in untreated osteosarcoma is predictive of pulmonary metastasis and poor prognosis. Clin Cancer Res 2000;6:572–7. [PubMed] [Google Scholar]
- [21].Yu XW, Wu TY, Yi X, et al. Prognostic significance of VEGF expression in osteosarcoma: a meta-analysis. Tumour Biol 2014;35:155–60. [DOI] [PubMed] [Google Scholar]
- [22].Savitskaya YA, Rico-Martinez G, Linares-Gonzalez LM, et al. Serum tumor markers in pediatric osteosarcoma: a summary review. Clin Sarcoma Res 2012;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rastogi S, Kumar R, Sankineani SR, et al. Role of vascular endothelial growth factor as a tumour marker in osteosarcoma: a prospective study. Int Orthop 2012;36:2315–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yin D, Jia T, Gong W, et al. VEGF blockade decelerates the growth of a murine experimental osteosarcoma. Int J Oncol 2008;33:253–9. [PubMed] [Google Scholar]
- [25].Ohba T, Cates JM, Cole HA, et al. Autocrine VEGF/VEGFR1 signaling in a subpopulation of cells associates with aggressive osteosarcoma. Mol Cancer Res 2014;12:1100–11. [DOI] [PubMed] [Google Scholar]
- [26].Asai T, Ueda T, Itoh K, et al. Establishment and characterization of a murine osteosarcoma cell line (LM8) with high metastatic potential to the lung. Int J Cancer 1998;76:418–22. [DOI] [PubMed] [Google Scholar]
- [27].Hassan SE, Bekarev M, Kim MY, et al. Cell surface receptor expression patterns in osteosarcoma. Cancer 2012;118:740–9. [DOI] [PubMed] [Google Scholar]
- [28].Ohba T, Cates JM, Cole HA, et al. Pleiotropic effects of bisphosphonates on osteosarcoma. Bone 2014;63:110–20. [DOI] [PubMed] [Google Scholar]
- [29].Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–76. [DOI] [PubMed] [Google Scholar]
- [30].Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol 2005;23:1011–27. [DOI] [PubMed] [Google Scholar]
- [31].Li J, Zhao X, Chen L, et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010;10:529. [DOI] [PMC free article] [PubMed] [Google Scholar]


