Abstract
To investigate and evaluate the clinicopathological characteristics and treatment strategies for patients with low-grade endometrial stromal sarcoma (LG-ESS).
The medical records of LG-ESS patients who were treated at 2 cancer referral centers from January 2005 to December 2015 were retrospectively reviewed.
Twenty patients with LG-ESS met the inclusion criteria and were included in this analysis. Hysterectomy with bilateral salpingo-oophorectomy was the mainstay of surgery. Lymphadenectomy was performed in 12 (60%) cases, and no positive nodes were identified. CD10 was the most commonly used immunohistochemistry marker, followed by smooth muscle actin (SMA), estrogen receptor (ER), desmin, progesterone receptor (PR), and S-100; the positivity rates of these markers were 88.2%, 66.7%, 75.0%, 16.7%, 88.9%, and 0, respectively. Postoperative chemotherapy, radiotherapy, and hormonal treatment were provided alone or in combination in 10 (50%) patients, 4 (20%) patients, and 1 (5%) patient, respectively. One patient developed lung metastasis at initial diagnosis, and 2 (10%) patients had recurrence with distant metastasis. They all underwent complete or incomplete resection followed by hormonal treatment. The overall survival time of these patients was 66, 89, and 109 months at last contact, respectively. The 5-year and 10-year disease-free survival rates for the entire cohort were 90% and 72%, respectively. No patients died of the disease.
CD10+/SMA+/ER+/PR+ in combination with desmin−/S-100− might improve the diagnostic accuracy. Surgical resection is the foremost treatment for LG-ESS patients with recurrence or distant metastasis. Hormonal treatment may be beneficial for unresectable or residual tumors.
Keywords: distant metastasis, hormonal treatment, immunohistochemistry marker, low-grade endometrial stromal sarcoma, recurrence, treatment strategies
1. Introduction
Endometrial stromal sarcoma (ESS) is a rare uterine mesenchymal neoplasm that represents approximately 21% of all uterine sarcomas.[1] According to the WHO Classification of Tumors of Female Reproductive Organs, ESS is subdivided into distinct low- and high-grade entities based on histopathology. Because of its rarity and heterogeneous morphological appearance, low-grade ESS (LG-ESS) is often misdiagnosed as a different uterine neoplasm.[2–4] Accurate diagnoses are not established in some cases until the disease relapse.[3,5] Although immunohistochemistry (IHC) often plays an adjunct role in the differential diagnosis of different uterine mesenchymal lesions,[6] sensitive and specific IHC markers have not been identified for LG-ESS, and valid data are lacking.
LG-ESS usually exhibits indolent behavior and is associated with a favorable prognosis. The reported overall disease-specific 5-year and 10-year survival rates are 80%–90% and 70%, respectively.[7,8] However, the recurrence risk is as high as 1 in 3 to 1 in 2.[9–11] Chang et al[7] reported that the median time to recurrence was 65 months for stage I disease. The current mainstay of treatment for LG-ESS is hysterectomy with bilateral salpingo-oophorectomy (BSO).[12] However, there is no consensus on the role of ovarian preservation, lymphadenectomy, and postoperative adjuvant treatment. The treatment modality for recurrent or distant metastatic LG-ESS remains unclear. In the present study, we retrospectively investigated the clinicopathological characteristics and prognosis of patients with LG-ESS, and explored the effective treatment strategies, especially for those with disease recurrence or distant metastases. A review of the related literature is also included.
2. Materials and methods
2.1. Patients
After obtaining approval from the Local Ethical Committee, the medical records of patients with LG-ESS who were diagnosed and treated in 2 cancer referral centers including Beijing Chao-Yang Hospital, Affiliated China Capital Medical University, and the affiliated hospital Qingdao University from January 2005 to December 2015 were collected and retrospectively reviewed. The inclusion criteria were as follows: pathological slides were reexamined by 2 independent gynecologic pathologists to confirm the diagnosis that met the criteria for LG-ESS according to the 2014 WHO Classification; complete pathological and surgical records, and acceptance of regular follow-up after surgery. Patients with a diagnosis of HG-ESS were excluded. The following information was retrieved through a search of hospital charts or telephone interviews: demographic characteristics, surgical procedures, pathologic features, and recurrence and survival follow-up information. Staging was retrospectively revised based on surgical records and pathological results and according to the 2009 International Federation of Gynecology and Obstetrics (FIGO) system.[13]
2.2. Treatment protocol
In our series, hysterectomy with BSO was the mainstay of surgical procedures for patients with LG-ESS. Tumor debulking was performed if there was extra-uterine spread. Pelvic and/or para-aortic lymphadenectomy was performed based on clinicopathological characteristics and the physician's preference. Postoperative adjuvant treatment was administered based on the extent of the disease, medical comorbidities, and the physician's recommendation. Adjuvant treatments included chemotherapy, radiotherapy, and hormonal treatment, which were administered alone or in combination. The chemotherapy protocols included the following: PEI (70 mg/m2 cisplatin, d1–3; 60 mg/m2 epirubicin, d1; 1.5 g/m2 ifosfamide, d1–3; intravenous [iv]), PAC (60 mg/m2 cisplatin, d1; 50 mg/m2 adriamycin, d1–2; 500 mg/m2 cyclophosphamide, d1-2; [iv]), or TC (175 mg/m2, paclitaxel d1; area under the curve = 5, carboplatin, d2; [iv]). Three to 6 courses of chemotherapy were given at interval of 3 to 4 weeks. Adjuvant radiotherapy was defined as postoperative pelvic radiotherapy, with or without a vaginal boost. Gonadotropin-releasing hormone analog (GnRH-a) (3.75 mg, intramuscular, q28 days, 6 times) and megestrol acetate (160 mg, p.o. qd) were provided as hormonal treatment for patients with positive estrogen receptor (ER) and/or progesterone receptor (PR) status. In addition, observation management, defined as no adjuvant treatment after initial surgery, was used for some patients.
2.3. Follow-up
After the completion of the initial treatment, regular follow-up was performed every 3 months for the first 2 years and then every 6 to 12 months thereafter. Pelvic examinations, measurements of serum levels of tumor markers, such as CA125 and Ca 19-9, and pelvic and abdominal ultrasounds were routinely checked at each visit. Computed tomography (CT), and/or magnetic resonance imaging (MRI) were also performed when necessary. Relapse was defined as the occurrence of new measurable lesion(s) by clinical or imaging evidence and confirmed pathologically. Disease-free survival (DFS) was defined in months as the time from the date of initial surgery to the date of disease relapse. Patients living without evidence of disease at the time of their last visit were censored. Survival after relapse (SAR) was defined in months as the time from relapse to the date that the patient died from the disease. Overall survival (OS) was calculated in months as the time from the date of initial surgery to the date of death from the disease. Women who died of other diseases and survivors at the time of last contact were censored.
2.4. Statistical analysis
SPSS (IBM Corp, Armonk, NY) for Windows 22.0 was used for statistical analyses. Data from the present study were analyzed using the mean, standard deviation, median, ratio, and/or frequency. The Kaplan–Meier method was used to generate survival curves and rates.
3. Results
During the study period, a total of 20 consecutive patients with LG-ESS met the inclusion criteria. Their clinical characteristics are shown in Table 1. The median age at initial diagnosis was 46.5 (range: 26–77) years. Thirteen (60%) patients were premenopausal, and 1 (5%) patient was non-nulliparous. No patient in our series had received hormonal treatment at diagnosis. Abnormal uterine bleeding (45%) was the most common clinical presentation, followed by palpable mass (20%), and rapid growth of leiomyoma (10%). Five (25%) patients were asymptomatic. Abdominal or pelvic masses were identified in 17 (85%) women through physical and/or imaging examination. Additionally, a single, solitary, round asymptomatic lesion of approximately 1.2 cm in diameter was detected by pulmonary CT scan as a suspicious pulmonary metastasis. Preoperative diagnostic curettage was performed in 7 (35%) patients; 5 (71.4%) were diagnosed with ESS, and the remaining 2 were considered to have benign disease. One patient underwent cervical conization, and the tumor was revealed as ESS through pathological examination. Intraoperative frozen pathological examination was performed for 10 (50%) patients. The potential for uterine malignancy was high in all of these patients, including LG-ESS (5 cases) and other uncertain histological types (5 cases).
Table 1.
Clinical profile of the 20 patients with LG-ESS.
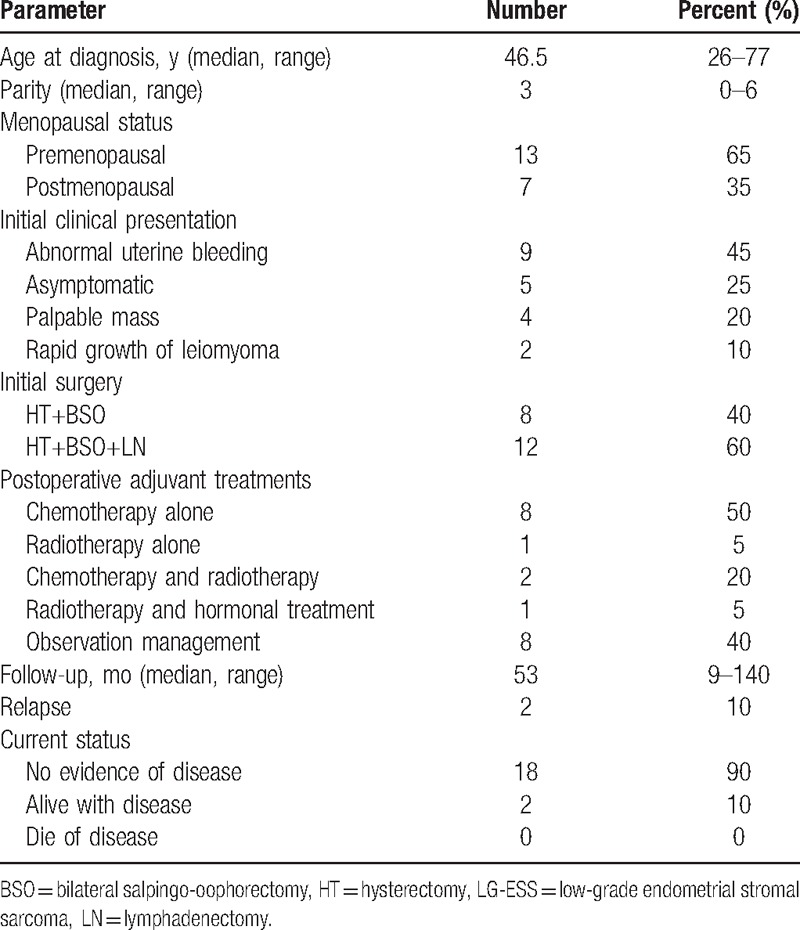
The surgery details are listed in Table 1. All 20 patients underwent hysterectomy and BSO as the mainstay of surgery. Radical hysterectomy was performed in 1 (5%) patient due to cervical involvement. Pelvic lymphadenectomy was performed in 12 (60%) patients, including 2 (10%) who underwent simultaneous para-aortic lymphadenectomy. Complete resection of the macroscopic lesions within the abdominopelvic cavity was achieved in all 20 patients through the initial surgical treatment. LG-ESS diagnosis was confirmed in all 20 patients through hematoxylin and eosin (HE) staining and histological examination.
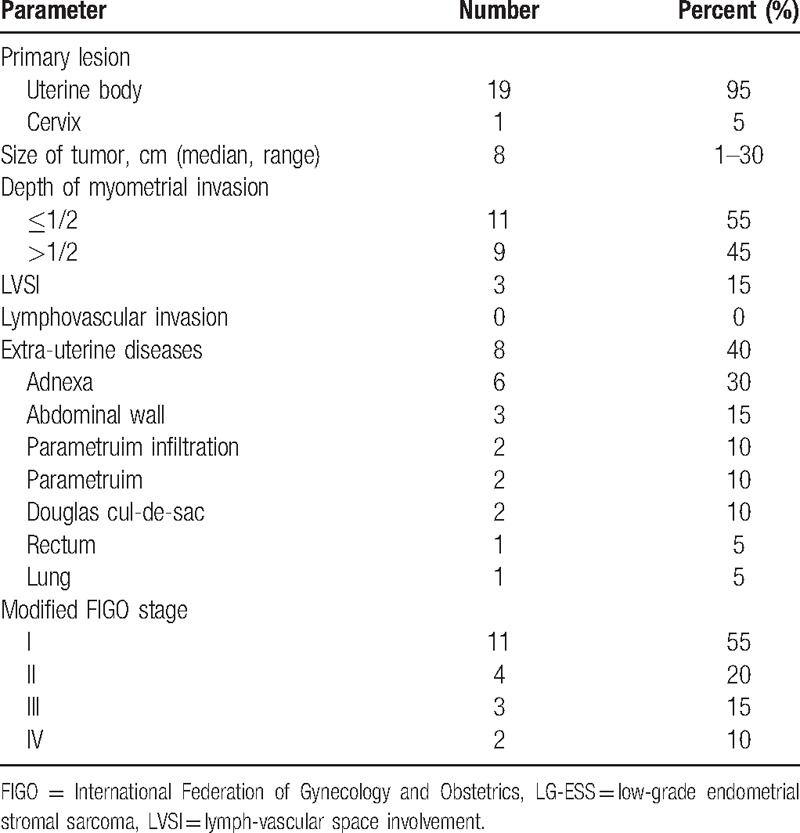
Table 2 shows the pathological characteristics of the 20 patients. Primary tumors had originated from the corpus uteri (19 cases) and cervix (1 cases). The median tumor size was 8 (range: 1–30) cm. Myometrial invasion (>1/2) and lymph-vascular space involvement (LVSI) were identified in 9 (45%) and 3 (15%) patients, respectively. The median number of removed lymph nodes (LNs) was 20 (range: 9–36) per patient, and no positive pelvic nodes were detected. Extrauterine disease was present in 8 (40%) patients, including parametrial infiltration (2 cases), and disease in the adnexa (6 cases), abdominal wall (3 case), Douglas cul-de-sac (2 cases), rectum (1 case), and lung (1 case). By FIGO 2009 staging, 11 patients (60%) were assessed as stage I, 4 (15%) as stage II, 3 (15%) as stage III, and 2 (10%) as stage IV.
Table 2.
Pathological characteristics of the 20 patients with LG-ESS.
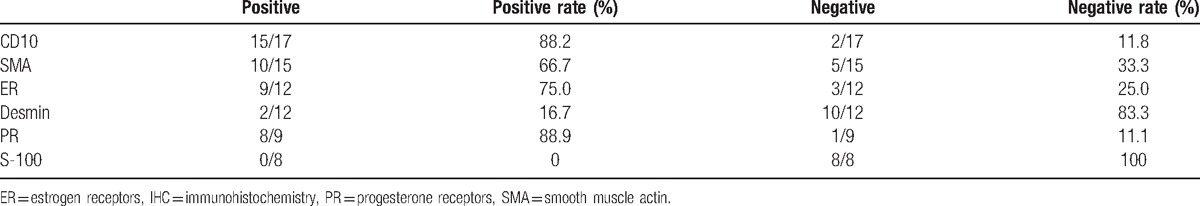
Twenty-seven IHC markers were used to distinguish LG-ESS from other uterine malignancies. CD10 was the most commonly used IHC markers, followed by smooth muscle actin (SMA), ER, desmin, PR, S-100 (Table 3). Tumors were predominantly positive for CD10 and PR, with a positivity rate of 88.2% and 88.7%, respectively. SMA and ER had moderately positivity rates of 66.7% and 75.0%, respectively. Tumors were largely negative for desmin and S-100 with a positivity rate of 16.7% and 0, respectively. Postoperative adjuvant treatments were administered in 12 (60%) patients, along or in combination, and included chemotherapy (10 cases), radiotherapy (4 cases), and hormonal treatment (1 case). Observation management was performed in the remaining 8 (40%) patients.
Table 3.
The expression patterns of the 6 commonly used IHC markers.
The median follow-up duration for the entire series was 53 (range: 9–140) months. During the follow-up period, the patient with pulmonary metastasis showed persistent and stable disease in repeat CT scan images about 1 year after initial surgery. This woman underwent a partial pulmonary lobectomy, and the histological examination confirmed the diagnosis of metastatic LG-ESS. After the second surgery, GnRH-a injections (6 times) was administered and was well tolerated. At last contact, she is alive without any evidence of disease, with a DFS of 89 months.
Disease recurrence was observed in 2 patients (10%). They both developed local relapse (pelvic cavity) and distant metastasis involving the iliac vein, inferior vena cava, and right atrium (1 case) or the bilateral lungs (1 case). The recurrence intervals were 57 and 76 months. Surgery was performed in both patients, and complete resection of the macroscopic lesions within the abdominopelvic cavity was achieved. During immunohistochemical staining, the tumors of both patients showed positive immunoreactivity for CD10, SMA, ER, and PR, and negative immunoreactivity for desmin and S-100. For the patient with metastases of the iliac vein, inferior vena cava, and right atrium, most of the tumor thrombi were removed with the assistance of cardiovascular surgeons. Residual disease was not forcibly resected considering the predicted operation-related injury. For the woman with pulmonary metastasis, CT images showed multiple scattered lesions spread through all lobes of the bilateral lungs, making complete resection impossible. Both women received GnRH-a injections (6 times), followed by long-term oral megestrol acetate treatment. Hormonal treatment was well tolerated and led to stable disease. At last contact, the SAR time of the 2 patients had reached 9 and 33 months, respectively.
The 5-year and 10-year DFS rates were 90% and 72%, respectively (Fig. 1). No one died of the disease in this study. As a result, at 5 and 10 years, OS was 100%.
Figure 1.
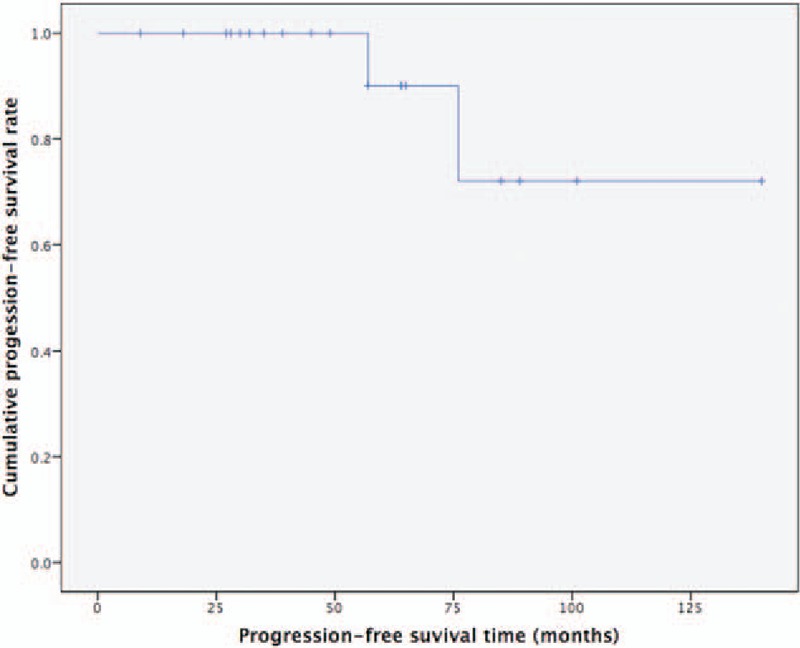
Disease-free survival (DFS). The 5-year and 10-year DFS rates for the entire cohort were 90% and 72%, respectively.
4. Discussion
Difficulties have persisted in the accurate diagnosis of LG-ESS due to its rarity and histological differences.[2–4] LG-ESS is often misdiagnosed as leiomyoma before tumor resection.[11] Imaging modalities have not been reliable in achieving an accurate preoperative diagnosis of LG-ESS.[12] However, the majority of patients with LG-ESS are symptomatic, and the main symptom of ESS is abnormal uterine bleeding,[12] which was confirmed by this study. Consequently, preoperative diagnostic curettage and pathological examination can reasonably be applied. In our series, the sensitivity of diagnostic curettage in identifying LG-ESS was 71.4% (5/7), which was relatively satisfactory. Uterine malignancy was suspected in all (10/10) of patients who received a pathological examination of frozen specimens. Therefore, preoperative diagnostic curettage and frozen pathological sectioning during the operation were helpful for making an accurate preoperative diagnosis of LG-ESS and for choosing the appropriate surgical procedure.
Even when surgical specimens are examined by HE staining and histopathology, misdiagnosis is common for LG-ESS.[14] IHC often plays an important role in the differential diagnosis of uterine mesenchymal lesions.[6] The usually used IHC markers include ER, PR, desmin, SMA, h-caldesmon, and CD10.[6,15] In the present study, tumors were predominantly positive for CD10, SMA, ER, and PR and negative for desmin and S-100, which is consistent with several previously published studies.[6,15–18] In addition, the expression of these 6 markers in the recurrent tumors was coincident with that in the primary tumors. CD10 is expressed in the endometrial stroma and endometrial stromal neoplasms, and it had been regarded as a useful marker for the diagnosis of ESS.[15,17] SMA is expressed in both stromal and smooth muscle cells, but desmin is usually absent from endometrial stromal cells but expressed smooth muscle cells.[15] S-100 was usually negative in LG-ESS.[4] LG-ESS tumors partly show some ER and/or PR activity,[19] which suggests a potential role for hormonal treatment in the treatment of LG-ESS. Although none of the existing IHC markers is sufficiently sensitive and specific to ensure an accurate diagnosis of LG-ESS, a combination of several IHC markers might improve the diagnostic accuracy rate for LG-ESS.[6,15] Hwang et al[6] suggested that the combination of CD10+/ER+/PR+ and h-caldesmon−/transgelin− might be useful in distinguishing LG-ESS from uterine leiomyosarcoma. Based on our data, CD10+/SMA+/ER+/PR+ in combination with desmin−/S-100− might improve the diagnostic accuracy of this rare tumor. The efficiency of IHC markers in the diagnosis of LG-ESS deserves further investigation.
In the current clinical practice, hysterectomy with BSO is the mainstay of surgical treatments for LG-ESS,[12] and it was the predominant surgery performed on patients with LG-ESS in our series. The role of BSO in treating LG-ESS is debated. BSO was shown to reduce the risk of recurrence in several previous studies.[11,14,20] In contrast, several other researchers have argued that ovary-sparing procedures have no significant effect on OS.[12,21] In our opinion, ovary-sparing procedures might be considered in younger patients with early-stage disease. However, the feasibility of this procedure must be further evaluated. Additionally, long-term follow-up is obligatory because late recurrence and distant metastases may occur.[11]
The role of lymphadenectomy in LG-ESS treatment is clinically important but is not conclusive. Over the last 10 years, lymphadenectomy has been performed for patients with LG-ESS in these 2 cancer referral centers with a purpose either to provide prognostic information or to guide postoperative adjuvant therapy. However, lymph nodes involvement was not common. There was no regional lymph node involvement in all the present 20 cases. The LNs metastasis rate was 9.9% (28/282)[22] and 7% (7/100)[21] which were reported in the recent literature. These results suggested that ESS was not a disease characterized with lymphatic metastasis. Additionally, lymphadenectomy was shown to have no demonstrable effect on OS.[21–23] Thus, lymphadenectomy omission may be feasible for this patient group.
There is also no consensus regarding the role of adjuvant treatment, including chemotherapy, radiation, and hormonal treatment. Chemotherapy was never shown to be definitively effective for the treatment of LG-ESS based on the previous studies [11,24] and the present data. However, adjuvant treatments were performed with a greater likelihood in patients with adverse factors of recurrence and death. The tendentiousness of selection and retrospective nature of these studies might bias the conclusions to some extent. We thus cannot arbitrarily claim that adjuvant chemotherapy could be safely omitted in all LG-ESS patients. Based on our data, as well as those in previous studies,[23,25] LG-ESS tends to express ER and PR, and hormonal treatment appears to be effective at reducing the recurrence of this disease. Chu et al[25] suggested that hormonal treatment could be regarded as a routine adjuvant treatment and could be used to treat recurrent disease. However, the optimal dose, regimen, and duration of hormonal treatment are not well established. In the present study, GnRH-a and megestrol acetate were administered at doses of 3.75 mg (q28 days, 6 times) and 160 mg (qd, long-term oral), respectively. Both medications were well tolerated. From our point of view, hormonal treatment, as a promising treatment modality, should be recommended with priority. Definitive conclusions about the efficacy of these adjuvant therapies in treating LG-ESS could not be made due to the retrospective nature of this study and analysis.
Despite the indolent nature of LG-ESS, recurrence is common. Multiple recurrences may occur,[12,26] with a predilection for the abdomen and lungs.[12] The recurrence rate in the present study was 10%, but rates as high as 1 in 3 to 1 in 2 have been reported in several previous studies.[9–11] For recurrence or distant metastasis of LG-ESS, even single cases or reports of very small series have demonstrated that this patient group may benefit from complete surgical removal, which should be the primary treatment.[12,27] In the present study, both for local recurrence and distant metastasis, surgical removal was performed by multidisciplinary team when the tumors were resectable. However, complete excision is not feasible in every case. Hormonal treatment could be used for residual or unresectable LG-ESS.[28] In the present study, patients with residual or unresectable tumors received GnRH-a injections followed by megestrol acetate, which were both well tolerated. Hormonal treatment was effective and led to stable disease. SAR reached as long as 9 and 33 months. Consequently, surgery should be considered the foremost treatment if feasible. Hormonal treatment may be beneficial for patients with unresectable or residual tumors that are positive for ER and/or PR. However, these treatment strategies must be further evaluated due to the current absence of valid data.
Due to the retrospective nature of this analysis, the results and conclusions should be interpreted cautiously. A large randomized trial is warranted to obtain a reliable conclusion, but it is difficult to run such trials on this rare disease. Despite this limitation, the present study has discussed and analyzed the management strategy for patients with LG-ESS in detail. In addition, this analysis spans the past 11 years, reflecting the latest treatment strategies for this disease.
Our preliminary results suggest that CD10+/SMA+/ER+/PR+ in combination with desmin−/S-100− might improve the diagnostic accuracy of this rare disease. For patients with LG-ESS recurrence or distant metastasis, surgery should be considered the foremost treatment. Hormonal treatment may be beneficial for patients with unresectable or residual tumors that are positive for ER and/or PR.
Footnotes
Abbreviations: BSO = bilateral salpingo-oophorectomy, CT = computed tomography, DFS = disease-free survival, ER = estrogen receptor, ESS = endometrial stromal sarcoma, FIGO = International Federation of Gynecology and Obstetrics, GnRH-a = gonadotropin-releasing hormone analog, HE = hematoxylin and eosin, IHC = immunohistochemistry, LG-ESS = low-grade endometrial stromal sarcoma, LNs = lymph nodes, LVSI = lymph-vascular space involvement, MRI = magnetic resonance imaging, OS = overall survival, PR = progesterone receptor, SAR = survival after relapse, SMA = smooth muscle actin.
RC and FY contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Trope CG, Abeler VM, Kristensen GB. Diagnosis and treatment of sarcoma of the uterus. A review. Acta Oncol 2012;51:694–705. [DOI] [PubMed] [Google Scholar]
- [2].Adegboyega PA, Qiu S. Immunohistochemical profiling of cytokeratin expression by endometrial stroma sarcoma. Hum Pathol 2008;39:1459–64. [DOI] [PubMed] [Google Scholar]
- [3].Bakker IS, Hoven-Gondrie ML, Moll FC, et al. A very late recurrence of a formerly misdiagnosed low grade endometrial stromal sarcoma metastasized to the colon. Int J Surg Case Rep 2013;4:1113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].He L, Li JD, Xiong Y, et al. Clinicopathological and molecular markers associated with prognosis and treatment effectiveness of endometrial stromal sarcoma: a retrospective study in China. Arch Gynecol Obstet 2014;289:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim MH, Jung CK, Hwang JK, et al. Low-grade endometrial stromal sarcoma with inferior vena cava extension: first report in Korea. Vasc Specialist Int 2014;30:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hwang H, Matsuo K, Duncan K, et al. Immunohistochemical panel to differentiate endometrial stromal sarcoma, uterine leiomyosarcoma and leiomyoma: something old and something new. J Clin Pathol 2015;68:710–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chang KL, Crabtree GS, Lim-Tan SK, et al. Primary uterine endometrial stromal neoplasms. A clinicopathologic study of 117 cases. Am J Surg Pathol 1990;14:415–38. [DOI] [PubMed] [Google Scholar]
- [8].Barney B, Tward JD, Skidmore T, et al. Does radiotherapy or lymphadenectomy improve survival in endometrial stromal sarcoma? Int J Gynecol Cancer 2009;19:1232–8. [DOI] [PubMed] [Google Scholar]
- [9].Leath CA, 3rd, Huh WK, Hyde J, Jr, et al. A multi-institutional review of outcomes of endometrial stromal sarcoma. Gynecol Oncol 2007;105:630–4. [DOI] [PubMed] [Google Scholar]
- [10].Garavaglia E, Pella F, Montoli S, et al. Treatment of recurrent or metastatic low-grade endometrial stromal sarcoma. Int J Gynecol Cancer 2010;20:1197–200. [DOI] [PubMed] [Google Scholar]
- [11].Bai H, Yang J, Cao D, et al. Ovary and uterus-sparing procedures for low-grade endometrial stromal sarcoma: a retrospective study of 153 cases. Gynecol Oncol 2014;132:654–60. [DOI] [PubMed] [Google Scholar]
- [12].Amant F, Floquet A, Friedlander M, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for endometrial stromal sarcoma. Int J Gynecol Cancer 2014;24:S67–72. [DOI] [PubMed] [Google Scholar]
- [13].Prat J. FIGO staging for uterine sarcomas. Int J Gynaecol Obstet 2009;104:177–8. [DOI] [PubMed] [Google Scholar]
- [14].Li N, Wu LY, Zhang HT, et al. Treatment options in stage I endometrial stromal sarcoma: a retrospective analysis of 53 cases. Gynecol Oncol 2008;108:306–11. [DOI] [PubMed] [Google Scholar]
- [15].Mittal K, Soslow R, McCluggage WG. Application of immunohistochemistry to gynecologic pathology. Arch Pathol Lab Med 2008;132:402–23. [DOI] [PubMed] [Google Scholar]
- [16].Reich O, Regauer S, Urdl W, et al. Expression of oestrogen and progesterone receptors in low-grade endometrial stromal sarcomas. Br J Cancer 2000;82:1030–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Agoff SN, Grieco VS, Garcia R, et al. Immunohistochemical distinction of endometrial stromal sarcoma and cellular leiomyoma. Appl Immunohistochem Mol Morphol 2001;9:164–9. [DOI] [PubMed] [Google Scholar]
- [18].McCluggage WG, Sumathi VP, Maxwell P. CD10 is a sensitive and diagnostically useful immunohistochemical marker of normal endometrial stroma and of endometrial stromal neoplasms. Histopathology 2001;39:273–8. [DOI] [PubMed] [Google Scholar]
- [19].Amant F, Coosemans A, Debiec-Rychter M, et al. Clinical management of uterine sarcomas. Lancet Oncol 2009;10:1188–98. [DOI] [PubMed] [Google Scholar]
- [20].Feng W, Hua K, Malpica A, et al. Stages I to II WHO 2003-defined low-grade endometrial stromal sarcoma: how much primary therapy is needed and how little is enough? Int J Gynecol Cancer 2013;23:488–93. [DOI] [PubMed] [Google Scholar]
- [21].Shah JP, Bryant CS, Kumar S, et al. Lymphadenectomy and ovarian preservation in low-grade endometrial stromal sarcoma. Obstet Gynecol 2008;112:1102–8. [DOI] [PubMed] [Google Scholar]
- [22].Chan JK, Kawar NM, Shin JY, et al. Endometrial stromal sarcoma: a population-based analysis. Br J Cancer 2008;99:1210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Amant F, De Knijf A, Van Calster B, et al. Clinical study investigating the role of lymphadenectomy, surgical castration and adjuvant hormonal treatment in endometrial stromal sarcoma. Br J Cancer 2007;97:1194–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou J, Zheng H, Wu SG, et al. Influence of different treatment modalities on survival of patients with low-grade endometrial stromal sarcoma: a retrospective cohort study. Int J Surg 2015;23:147–51. [DOI] [PubMed] [Google Scholar]
- [25].Chu MC, Mor G, Lim C, et al. Low-grade endometrial stromal sarcoma: hormonal aspects. Gynecol Oncol 2003;90:170–6. [DOI] [PubMed] [Google Scholar]
- [26].Yamazaki H, Todo Y, Mitsube K, et al. Long-term survival of patients with recurrent endometrial stromal sarcoma: a multicenter, observational study. J Gynecol Oncol 2015;26:214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Renzulli P, Weimann R, Barras JP, et al. Low-grade endometrial stromal sarcoma with inferior vena cava tumor thrombus and intracardiac extension: radical resection may improve recurrence free survival. Surg Oncol 2009;18:57–64. [DOI] [PubMed] [Google Scholar]
- [28].Dahhan T, Fons G, Buist MR, et al. The efficacy of hormonal treatment for residual or recurrent low-grade endometrial stromal sarcoma. A retrospective study. Eur J Obstet Gynecol Reprod Biol 2009;144:80–4. [DOI] [PubMed] [Google Scholar]


