Abstract
To investigate the relationship among anemia, physiological serum bilirubin levels, and cardiovascular autonomic neuropathy (CAN) in subjects with type 2 diabetes. In total, 2230 subjects with type 2 diabetes were evaluated in this cross-sectional study. CAN was diagnosed with a cardiovascular reflex test. The prevalence of anemia was greater in subjects with CAN. In multivariable analysis, the relationship between anemia and CAN remained statistically significant after adjusting for the risk factors (odds ratio [OR] 1.39; 95% confidence interval [CI] 1.07–1.80, P = .015). Additional adjustment for serum bilirubin concentrations abolished this relationship (OR 1.20, 95% CI 0.91–1.58, P = .189). Anemia is positively associated with the prevalence of CAN in subjects with type 2 diabetes. In addition, our results suggest that the putative increased CAN risk associated with anemia might be mediated by a correlated decrease in serum bilirubin levels.
Keywords: anemia, bilirubin, diabetic neuropathies, type 2 diabetes mellitus
1. Introduction
Anemia is commonly observed in subjects with diabetes mellitus.[1] Many studies have demonstrated that anemia is linked to an increased risk for hypoxia-induced organ damage including cardiovascular events and mortality.[2] In addition, there is a growing body of evidence indicating that anemia is a risk factor for diabetes-associated organ damage.[3] Endoneural hypoxia has been suggested to be related with nerve injury in diabetes.[2,4]
Bilirubin is a metabolite of heme and has been shown as a potent endogenous antioxidant.[5] Normal to moderately elevated range of bilirubin levels has been demonstrated to be cytoprotective, while severe hyperbilirubinemia might be linked to kernicterus in newborns.[5] Previous clinical studies have shown the inverse association between serum bilirubin concentrations and the risk of diabetes and diabetic microangiopathy.[6] Recent research has reported the inverse relationship between serum bilirubin levels and cardiovascular autonomic neuropathy (CAN) which is a significant contributor to cardiovascular morbidity and mortality in subjects with type 2 diabetes.[6,7]
Because most serum bilirubin is formed from the breakdown of hemoglobin (Hb),[8] the impact of anemia on nerve injury might be related to the decline in the levels of serum bilirubin with antioxidant properties. However, the relationship among anemia, bilirubin, and CAN has not been fully understood.
Thus, the purpose of this study was to investigate the relationship among anemia, serum bilirubin levels within the physiologic range, and CAN in subjects with type 2 diabetes.
2. Participants and methods
2.1. Study population
This cross-sectional study was performed from 2250 randomly selected subjects with type 2 diabetes who visited the diabetes clinic of our hospital between January 2014 and June 2015. The diagnosis of type 2 diabetes was based on the “Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus.”[9] The presence of anemia was defined as a Hb level <130 g/L in men and <120 g/L in women.[10] Hyperlipidemia was considered if the subject had serum levels of total cholesterol ≥6.5 mmol/L and/or triglyceride ≥2.3 mmol/L, or used lipid-lowering drugs. Hypertension was defined as a blood pressure ≥140/90 mm Hg or taking antihypertensive agents. The information on smoking status, diabetes duration, and other parameters related to health were collected through standardized questionnaires. Subjects with a history of malignancy, advanced renal dysfunction (serum creatinine more than 176 μmol/L), pancreatitis, chronic liver disease including hepatitis B or C, liver cirrhosis, heart failure, arrhythmia, infection, respiratory distress, thyrotoxicosis, hypothyroidism, alcoholism, acute or chronic blood loss, hemolysis, or red blood cell transfusion were excluded from the study. Subjects with serum bilirubin level greater than the upper limit of normal (ULN) (>22.2 μmol/L) and those with serum alanine aminotransferase (ALT) or aspartate aminotransferase (AST) level above twice the ULN (>74 U/L) were also excluded from the study. Informed consents were provided by all subjects participating in the study, which was approved by an ethics committee of Chonnam National University Hospital.
2.2. Methods
Venous blood samples were drawn after an overnight fast. Glycated Hb (HbA1c) level was measured using ion exchange liquid chromatography with a model HLC-723-GHbV apparatus (Tosoh, Tokyo, Japan). Hb levels were measured using cyanmethemoglobin spectrophotometry (Beckman-Coulter Inc., Miami, FL). Serum concentrations of ALT and AST were measured using the AU5407 analyzer (Olympus, Tokyo, Japan). Serum bilirubin concentration was measured with an enzymatic method using bilirubin oxidase on a model AU5407 automatic analyzer (Olympus). Urinary albumin excretion was assessed using the urinary albumin: creatinine ratio (UACR) in random urine samples. The glomerular filtration rate (GFR) was estimated from the Chronic Kidney Disease Epidemiology Collaboration equation.[11] Nephropathy was considered as a UACR ≥ 300 mg/gCr or estimated GFR (eGFR) < 60 mL/min/1.73 m2. Fundoscopy was performed after pupillary dilation to evaluate diabetic retinopathy. CAN was investigated by analyzing heart rate (HR) responses during the Valsalva maneuver, lying-to-standing, and deep breathing using Ewing's method, as described previously.[12–14] The beat-to-beat variability in HR during controlled deep breathing was estimated as ratio of the shortest R-R interval during inspiration to the longest R-R interval during expiration based on age-normative values.[14,15] The HR responses to standing were determined by using the ratio of the 15th R-R interval to the 30th R-R interval; if a value was below 1.00, the result was defined as abnormal.[14] The HR responses to the Valsalva maneuver were performed by forcefully blowing out through a mouthpiece of a manometer at 40 mm Hg for 15 seconds and determined by using the ratio of the longest R-R interval to the shortest R-R interval during the test; the abnormal test was defined as a value below 1.10.[14] CAN was defined as 2 or more abnormal HR tests according to the criteria recommended by The Toronto Diabetic Neuropathy Expert Group.[13] Patients were asked to avoid smoking, or consumption of caffeinated beverages or alcohol for 12 hours before testing, and to abstain from the use of medication including diuretic, beta-blocker, and antihistamine for 2 days before the test. A total of 20 patients were excluded as they could not perform 1 or more autonomic function tests. In total, 2230 subjects were analyzed.
2.3. Statistical analysis
Statistical analyses were conducted with SPSS version 20.0 (SPSS, Chicago, IL). For variables with a skewed distribution, the log (base 10) transformation was performed before analysis, and these parameters are shown as geometric mean (95% confidence interval [CI]). Categorical variables were analyzed using the chi-squared test, and Mann–Whitney U test or the Student t test was used to analyze continuous variables. To evaluate the association between anemia and CAN, multivariable analysis was conducted using logistic regression models with identified covariates and previously known risk factors. As covariates, Model 1 included sex, age, smoking habits, body mass index (BMI), ALT, hyperlipidemia, and hypertension. Model 2 was adjusted by Model 1 variables plus diabetes duration, HbA1c, retinopathy, and nephropathy. In addition, bilirubin was included as a covariate (Model 2a). P value <.05 was considered to indicate statistical significance.
3. Results
The characteristics of the subjects with type 2 diabetes are summarized in Table 1. Patients with CAN were older and had longer diabetes duration, lower eGFR, and lower levels of serum bilirubin, ALT, and Hb than those without CAN. The prevalence of anemia was significantly higher in subjects with CAN than in those without CAN. In addition, the patients with CAN were associated with a higher prevalence of retinopathy, nephropathy, and hypertension than those without CAN.
Table 1.
Clinical characteristics of subjects with type 2 diabetes according to CAN.
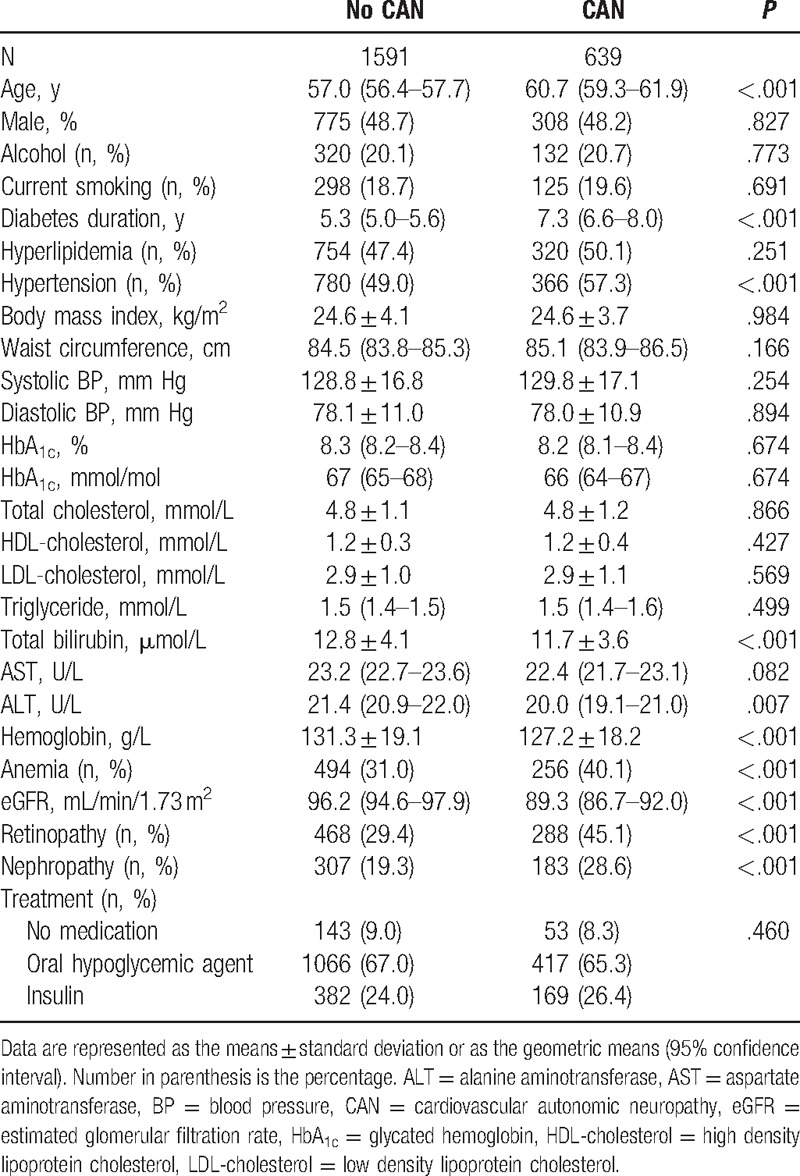
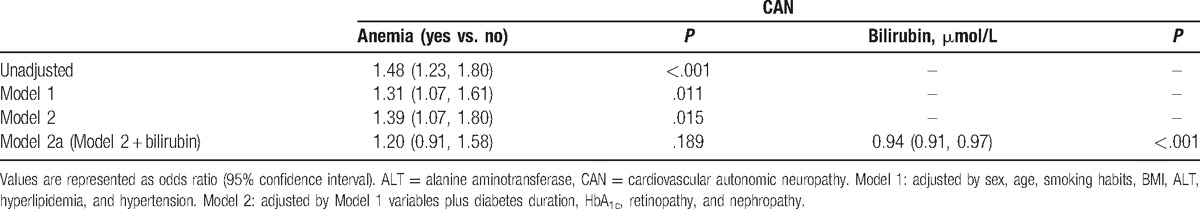
Table 2 shows the results of multivariable analyses performed for CAN using logistic regression models. The relationship between anemia and CAN remained statistically significant after adjusting for sex, age, smoking habits, BMI, ALT, hyperlipidemia, hypertension, diabetes duration, HbA1c, retinopathy, and nephropathy (odds ratio [OR] 1.39; 95% CI 1.07–1.8, P = .015) (Model 2). When serum bilirubin levels were entered into the same model, additional adjustment for bilirubin abolished this relationship (OR 1.20, 95% CI 0.91–1.58, P = .189) (Model 2a).
Table 2.
Odds ratio for CAN in subjects with type 2 diabetes.
4. Discussion
In this study, we observed a positive relation between anemia and the prevalence of CAN in subjects with type 2 diabetes. In addition, our results suggest that the putative increased CAN risk associated with anemia might be mediated by a correlated decrease in serum bilirubin levels.
Anemia is commonly found in subjects with type 2 diabetes.[16] Previous studies have demonstrated a continuous decrease in Hb concentrations over time in subjects with type 2 diabetes in the absence of diabetic nephropathy, as well as in advanced diabetic kidney disease.[1,16,17] In addition, anemia itself might contribute to diabetes-associated organ damage as well as adverse cardiovascular outcomes such as coronary heart disease and heart failure.[2,3] Qiao et al[3] showed that anemia is positively related to diabetic retinopathy. Previous several studies have also reported that anemia is associated with diabetic somatic neuropathy in subjects with type 2 diabetes.[2,18,19] Therefore, in the present study, a positive association between anemia and CAN supports the hypothesis that anemia might be related to neuronal injury.[2,18,19]
Anemia results in tissue hypoxia.[2] Endoneural hypoxia has been suggested to play an important role in nerve injury in diabetes.[2,4] It is therefore interesting to find that the putative increased CAN risk associated with anemia might be linked to a correlated decrease in serum bilirubin levels. Our findings are supported by recent clinical and experimental evidence that bilirubin might have a protective role in oxidative stress-mediated diseases.[6,20,21] Serum bilirubin levels have been reported to be closely linked to the metabolic milieu in type 2 diabetes.[22–24] In addition, several studies have shown inverse associations between diabetic microangiopathy and serum bilirubin concentrations.[25–27] Serum total bilirubin concentrations are inversely correlated with the severity of diabetic retinopathy[28] and urine albumin excretion in subjects with type 2 diabetes.[25] Experimental researches have shown that bilirubin at physiologic levels might contribute to neuroprotection against oxidative injury.[5,20,29,30] Recently, serum total bilirubin concentration has been suggested to be associated with the severity of CAN.[30]
Although the mechanism has not been clearly defined yet, there are several possible explanations by which bilirubin might be involved in relations between anemia and CAN. First, bilirubin is formed by sequential catalytic degradation of heme present in Hb, which is mediated by heme oxygenase and biliverdin reductase.[5] In health subjects, approximately 80% of daily bilirubin production is suggested to be derived from Hb.[8] Thus, a decline in Hb level might be related with a decrease in serum bilirubin levels within the physiologic range. Second, diabetes mellitus is linked to increased oxidative stress, which is an important etiologic factor of autonomic neuropathy.[31] Bilirubin has a strong antioxidant capacity.[5,20] All types of bilirubin that encompass albumin-bound bilirubin, free bilirubin, unconjugated form, and conjugated form exhibit effective antioxidant activities.[32,33] In addition, bilirubin might be involved in the inhibition of protein kinase C and formation of advanced glycation end products,[34] and it is related to the immune response and the process of the inflammation.[35] These are key pathogenic pathways implicated in the pathogenesis of CAN, and the inhibitory effects of bilirubin on these pathways might explain the connection between anemia and CAN.
This study has some limitations. First, because the design of this study was cross-sectional, the causality of the relationships could not be established. Second, the etiology of anemia was not addressed in the present study. However, as anemia itself is characterized by the decrease in Hb levels,[36] it is not likely that this has affected our findings. In addition, although the well known risk factors were included in multivariable analysis to evaluate an association between anemia and CAN, not all possible factors that affect cardiovascular autonomic function might be controlled. Finally, patients with occult malignancies might not be completely excluded, although precautions were taken. In spite of these limitations, this study provides an important information with regard to the relationships among anemia, bilirubin, and CAN in patients with type 2 diabetes.
In conclusion, our results show that in patients with type 2 diabetes, anemia is positively associated with CAN, which may be mediated by a correlated decrease in serum total bilirubin levels within the physiologic range. Large prospective investigations are warranted to establish the causal associations among anemia, bilirubin, and cardiovascular autonomic dysfunction.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, CAN = cardiovascular autonomic neuropathy, CI = confidence interval, GFR = glomerular filtration rate, Hb = hemoglobin, HbA1c = glycated hemoglobin, HR = heart rate, OR = odds ratio, UACR = urinary albumin: creatinine ratio, ULN = upper limit of normal.
Authors’ contributions: JOC researched and analyzed data and wrote the manuscript. S-YP, DHC, and DJC researched the data, reviewed/edited the manuscript. MYC designed the study and reviewed/edited the manuscript. MYC is the guarantor of the manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Thomas MC, MacIsaac RJ, Tsalamandris C, et al. Unrecognized anemia in patients with diabetes: a cross-sectional survey. Diabetes Care 2003;26:1164–9. [DOI] [PubMed] [Google Scholar]
- [2].Thomas MC. Anemia in diabetes: marker or mediator of microvascular disease? Nat Clin Pract Nephrol 2007;3:20–30. [DOI] [PubMed] [Google Scholar]
- [3].Qiao Q, Keinanen-Kiukaanniemi S, Laara E. The relationship between hemoglobin levels and diabetic retinopathy. J Clin Epidemiol 1997;50:153–8. [DOI] [PubMed] [Google Scholar]
- [4].Greene DA, Sima AA, Stevens MJ, et al. Complications: neuropathy, pathogenetic considerations. Diabetes Care 1992;15:1902–25. [DOI] [PubMed] [Google Scholar]
- [5].Kapitulnik J. Bilirubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol Pharmacol 2004;66:773–9. [DOI] [PubMed] [Google Scholar]
- [6].Vitek L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol 2012;3:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care 2010;33:434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Berk PD, Howe RB, Bloomer JR, et al. Studies of bilirubin kinetics in normal adults. J Clin Invest 1969;48:2176–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(suppl 1):S5–20. [DOI] [PubMed] [Google Scholar]
- [10].World Health Organization Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva:World Health Organization; 2011. [Google Scholar]
- [11].Levey AS, Stevens LA, Schmid CH, et al. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kuehl M, Stevens MJ. Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol 2012;8:405–16. [DOI] [PubMed] [Google Scholar]
- [13].Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33:2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ewing DJ, Martyn CN, Young RJ, et al. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985;8:491–8. [DOI] [PubMed] [Google Scholar]
- [15].Vinik AI, Maser RE, Mitchell BD, et al. Diabetic autonomic neuropathy. Diabetes Care 2003;26:1553–79. [DOI] [PubMed] [Google Scholar]
- [16].Craig KJ, Williams JD, Riley SG, et al. Anemia and diabetes in the absence of nephropathy. Diabetes Care 2005;28:1118–23. [DOI] [PubMed] [Google Scholar]
- [17].Al-Eidi S, Tayel S, Al-Slail F, et al. Knowledge, attitude and practice of patients with type 2 diabetes mellitus towards complementary and alternative medicine. J Integr Med 2016;14:187–96. [DOI] [PubMed] [Google Scholar]
- [18].Ito H, Takeuchi Y, Ishida H, et al. Mild anemia is frequent and associated with micro- and macroangiopathies in patients with type 2 diabetes mellitus. J Diabetes Investig 2010;1:273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].He BB, Xu M, Wei L, et al. Relationship between anemia and chronic complications in Chinese patients with type 2 diabetes mellitus. Arch Iran Med 2015;18:277–83. [PubMed] [Google Scholar]
- [20].Stocker R, Yamamoto Y, McDonagh AF, et al. Bilirubin is an antioxidant of possible physiological importance. Science 1987;235:1043–6. [DOI] [PubMed] [Google Scholar]
- [21].Baranano DE, Rao M, Ferris CD, et al. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A 2002;99:16093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cheriyath P, Gorrepati VS, Peters I, et al. High total bilirubin as a protective factor for diabetes mellitus: an analysis of NHANES data from 1999–2006. J Clin Med Res 2010;2:201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ohnaka K, Kono S, Inoguchi T, et al. Inverse associations of serum bilirubin with high sensitivity C-reactive protein, glycated hemoglobin, and prevalence of type 2 diabetes in middle-aged and elderly Japanese men and women. Diabetes Res Clin Pract 2010;88:103–10. [DOI] [PubMed] [Google Scholar]
- [24].Chung JO, Cho DH, Chung DJ, et al. Serum bilirubin concentrations are positively associated with serum C-peptide levels in patients with type 2 diabetes. Diabet Med 2014;31:1316–22. [DOI] [PubMed] [Google Scholar]
- [25].Fukui M, Tanaka M, Shiraishi E, et al. Relationship between serum bilirubin and albuminuria in patients with type 2 diabetes. Kidney Int 2008;74:1197–201. [DOI] [PubMed] [Google Scholar]
- [26].Yasuda M, Kiyohara Y, Wang JJ, et al. High serum bilirubin levels and diabetic retinopathy: the Hisayama Study. Ophthalmology 2011;118:1423–8. [DOI] [PubMed] [Google Scholar]
- [27].Inoguchi T, Sasaki S, Kobayashi K, et al. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA 2007;298:1398–400. [DOI] [PubMed] [Google Scholar]
- [28].Sekioka R, Tanaka M, Nishimura T, et al. Serum total bilirubin concentration is negatively associated with increasing severity of retinopathy in patients with type 2 diabetes mellitus. J Diabetes Complications 2015;29:218–21. [DOI] [PubMed] [Google Scholar]
- [29].Ostrow JD, Pascolo L, Tiribelli C. Reassessment of the unbound concentrations of unconjugated bilirubin in relation to neurotoxicity in vitro. Pediatr Res 2003;54:98–104. [DOI] [PubMed] [Google Scholar]
- [30].Chung JO, Cho DH, Chung DJ, et al. Physiological serum bilirubin concentrations are inversely associated with the prevalence of cardiovascular autonomic neuropathy in patients with Type 2 diabetes. Diabet Med 2014;31:185–91. [DOI] [PubMed] [Google Scholar]
- [31].Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev 2006;22:257–73. [DOI] [PubMed] [Google Scholar]
- [32].Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J Biol Chem 1994;269:16712–9. [PubMed] [Google Scholar]
- [33].Wu TW, Fung KP, Wu J, et al. Antioxidation of human low density lipoprotein by unconjugated and conjugated bilirubins. Biochem Pharmacol 1996;51:859–62. [DOI] [PubMed] [Google Scholar]
- [34].Kalousova M, Novotny L, Zima T, et al. Decreased levels of advanced glycation end-products in patients with Gilbert syndrome. Cell Mol Biol (Noisy-le-grand) 2005;51:387–92. [PubMed] [Google Scholar]
- [35].Basiglio CL, Arriaga SM, Pelusa F, et al. Complement activation and disease: protective effects of hyperbilirubinaemia. Clin Sci (Lond) 2009;118:99–113. [DOI] [PubMed] [Google Scholar]
- [36].Buttarello M. Laboratory diagnosis of anemia: are the old and new red cell parameters useful in classification and treatment, how? Int J Lab Hematol 2016;38:123–32. [DOI] [PubMed] [Google Scholar]


