Abstract
Despite the advances in the microsurgical technique and anatomical understanding of the anterior and middle skull base, anterior clinoidal meningiomas are still challenging lesions to resect completely and safely due to their intimate relationship with vital neurovascular structures. We report predictive factors for tumor recurrence and postoperative complications based on surgical outcome of patients with anterior clinoidal meningiomas treated at our institution.
Fifty-nine consecutive patients with anterior clinoidal meningioma who were surgically treated between March, 1993, and July, 2015, were reviewed retrospectively. For microsurgical tumor removal, orbitocranial or orbitozygomatic (78.0%), extended pterional (15.3%) and subfrontal approach (6.8%) were performed.
The median follow-up duration was 54.1 months. Gross total resection (GTR, Simpson's grade I or II) was achieved in 38 patients (64.4%). The overall recurrence rate (new lesion in GTR cases and re-growth in non-GTR cases) was 18.6%. GTR (Hazard ratio [HR] 0.014, 95% confidence interval [CI] 0.001–0.256; P = .004), absence of internal feeder (HR 0.058, 95% CI 0.004–0.759; P = .030) and benign pathology (WHO grade I, HR 0.056, 95% CI 0.005–0.674; P = .023) were independent prognostic factors for recurrence-free. Fourteen patients (23.7%) developed permanent complications. The most common complication was cranial nerve injury (n = 6; 10.2%), followed by postoperative hemorrhage/infarction, hydrocephalus and infection. Larger size (≥ 40 mm) was significant as an independent predictive factor for permanent complication (HR 0.139, 95% CI 0.030–0.653; P = .012). Old age (≥60 years, P = .056) and peritumoral edema (thickness ≥ 5 mm, P = .303) did not reach statistical significance in multivariate analysis.
In surgical resection of anterior clinoidal meningiomas, various clinicoradiological factors were related with resection degree, complication, and progression rate. Although our results showed acceptable resection degree and morbidity, mortality, and recurrence rate, compared to the results of past, anterior clinoidal meningioma remain as neurosurgical challenges because of their close contact to critical vascular and neural structures.
Keywords: anterior clinoid process, complication, intracranial meningioma, recurrence, surgical outcome
1. Introduction
Anterior clinoidal meningiomas can be considered medial sphenoidal or spheno-cavernous meningiomas because of their proximity to these anatomical points around the anterior clinoid process (ACP).[1] Since large clinoidal meningiomas (≥3 cm) can incorporate an wide range of the anterior cranial base, it is difficult to define if the tumor truly originates from the ACP or adjacent structures including the sphenoid ridge, tuberculum sellae, and planum sphenoidale.[2] Therefore, we define anterior clinoidal meningiomas as globular tumors with an epicenter on ACP, hyperostotic ACP, and upward growing feature. Despite remarkable advancements in anterior skull base surgery and anatomical understanding, anterior clinoidal meningiomas remain challenging for skull base surgeons due to their intimate relationship with vital neurovascular structures such as the optic apparatus, internal carotid artery (ICA) including its major branches and other cranial nerves of the cavernous sinus.[3] In the previously published cases, recurrence or re-growth rates of anterior clinoidal meningioma ranged from 0% to 21% with various follow-up durations, and the mortality rates remained high, up to 15.4% with range of morbidity incidence from 8.8 to 60%.[1–12]
In the present study, we analyzed surgical outcomes of patients with anterior clinoidal meningiomas treated at our institution to clarify the predictors for tumor recurrence and postoperative complications.
2. Methods
2.1. Patients
The study was conducted in compliance with the Declaration of Helsinki (sixth revision, 2008), and fulfilled all of the requirements for patient anonymity. This study was approved by the Institutional Review Board of the Chonnam National University Hwasun Hospital (CNUHH-2016–31). Between March, 1993, and July, 2015, 981 cases of intracranial meningioma were surgically resected at our institution. We clearly re-defined anterior clinoidal meningioma based on the radiological characteristics including tumoral epicenter of ACP, presence of hyperostosis of ACP or upward growing direction of tumor. By definition, experienced neuroradiologists reviewed intracranial meningiomas of the middle cranial fossa on computed tomography (CT) scans or magnetic resonance imaging (MRI), and 59 cases (6.0%) were enrolled in this study as anterior clinoidal meningiomas. The 59 cases could be categorized into 3 different year group such as 1990s, 2000s, and 2010s if it was intended to minimize the influence of the rapidly developing modern microsurgical techniques. However, basic surgical techniques for anterior clinoidal meningioma have been changed minimally over the past decades and the selected cases were predominantly from the recent decases; 9 cases were operated in 1990s, 32 cases in 2000s, and 18 cases in 2010s, respectively. Recurrent cases or previously radiated cases were excluded.
2.2. Operative technique
There are many controversial issues regarding the most appropriate surgical approach of anterior clinoidal meningiomas. Selection of the optimal approach for each case is essential for better surgical outcome. The basic surgical component is frontotemporal craniotomy (pterional) with anterior clinoidectomy. In addition, orbital roof and/or zygomatic arch is typically also resected in 1/2-piece manner. As described previously,[13] extradural clinoidectomy using a microdrill was performed for unroofing of the optic canal and to open the optic nerve sheath, following exposure of the superior orbital fissure and transacting of the meningo-orbital band. After sufficient resection of ACP and devascularization, the sylvian fissure was split widely, the brain was gradually relaxed with cerebrospinal fluid (CSF) drainage, and the tumor was resected. Whenever possible, gross total resection (GTR) with removal or coagulation of involved dura mater was tried. However, residual tumor which had invaded the cavernous sinus or adhered avidly to the optic nerve, occulomotor nerve, and ICA and its branches were inevitable. In some cases, intradural clinoidectomy was performed to prevent injury to the already compressed optic nerve, in large sized cases.
2.3. Analysis variables
The clinical and radiological data of the enrolled patients were retrospectively reviewed. Clinical data included age, sex, presenting symptoms, pathological grade, resection degree, postoperative complications and follow-up duration. Radiological data included the size of tumor, degree of peritumoral edema, the presence of calcification and internal feeding artery and signal intensity (SI) on T2-wighted MRI. The relationship between ICA including its major branches and tumor was also analyzed based on MRI and conventional 4-vessel angiography (DSA). The presence of internal feeders was also evaluated using DSA.
The relationship between tumor and ICA or its branches was divided into 4 groups using a previously described method[14]; Group A: just displaced ICA, Group B: encased but not narrowed ICA, Group C: encased and narrowed ICA, Group D: extended contralaterally or into basilar arterial system (Fig. 1). Tumor size was evaluated by measuring the maximal diameter on 3-dimensional T1-weighted MRI with enhancement. The cut-off size value was 4 cm (<4 cm vs ≥4 cm). Peritumoral edema, evaluated on axial T2-weighted MRI, classified the patients into 2 groups based on edema thickness (none or mild [<5 mm in edema thickness] vs moderate to severe [≥5 mm in edema thickness]). The extent of tumor resection was classified according to Simpson's classification. Simpson's grade I or II resection without remnant tumor on follow-up MRI was defined as GTR. The World Health Organization (WHO) classification system was applied for pathological grading for all tumors. A newly developed neurological deficit or aggravated pre-existing deficit after surgery was regarded as a surgery-related complication, which was transient or permanent. Transient complications typically resolved within 3 to 6 months post-operatively. If the problem persisted longer than 6 months or another operation was needed, it was regarded as a permanent complication. All surviving patients were followed up with a median duration of 54.1 months (range, 1–246 months). To evaluate tumor recurrence, postoperative follow-up MRI was obtained from all patients 6 months after surgery and every year of the patient's life. On follow-up MRI, a newly-enhanced mass in a completely resected case or a re-growing symptomatic mass in an incompletely resected case was regarded as recurrence.
Figure 1.
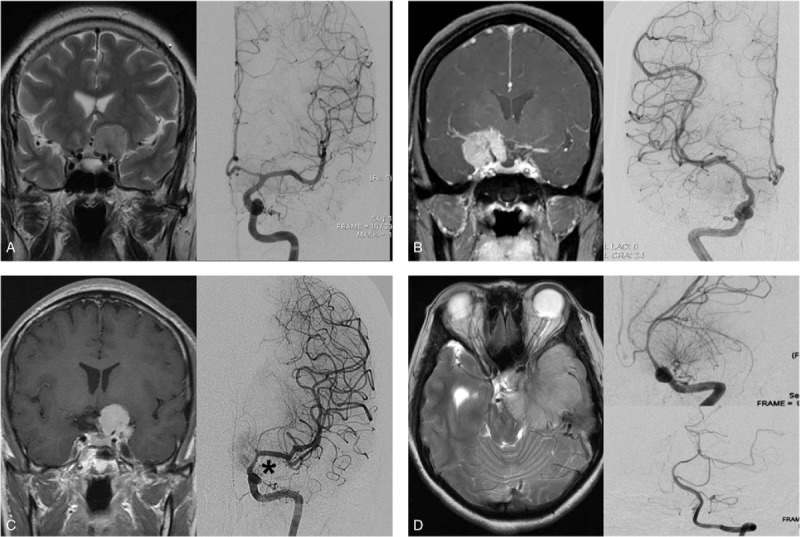
Representative radiographic images according to Goel's classification. Type A; Coronal T2-weighted magnetic resonance imaging (MRI) (left) and angiography (right) show just a medially displaced internal cerebral artery (ICA). Type B; Coronal T1-contrast enhanced MRI (left) and angiography (right) shows encased but not narrowed ICA by the tumor. Type C; Coronal T1-contrast enhanced MRI (left) and angiography (right) reveals encased and narrowed ICA by the tumor (asterisk). Type D; axial T2-weighted MRI (left) and angiography (right-upper; ICA, right-lower; vertebral artery) shows displaced ICA and basilar artery by the tumor. ICA = internal carotid artery, MRI = magnetic resonance imaging.
2.4. Statistical analyses
Recurrence-free survival (RFS) was calculated as the time from surgery to the date of recurrence. The probability of RFS was analyzed according to the Kaplan–Meier method, and the resulting values were compared using log-rank tests. Overall recurrence was also calculated as the time from surgery to the date of progression or last follow-up MRI. For the multivariate analysis, independent prognostic factors were determined using the Cox proportional hazards model. The comparison of a permanent complication occurrence and a categorical variable was done by the Chi-square test or the Fisher-exact probability test. Furthermore, the binary logistic regression test was applied for multivariate analysis. All statistical analyses were performed using SPSS version 20.0 for Windows (SPSS, Chicago, IL); P < .05 was considered statistically significant.
3. Results
3.1. Patient characteristics
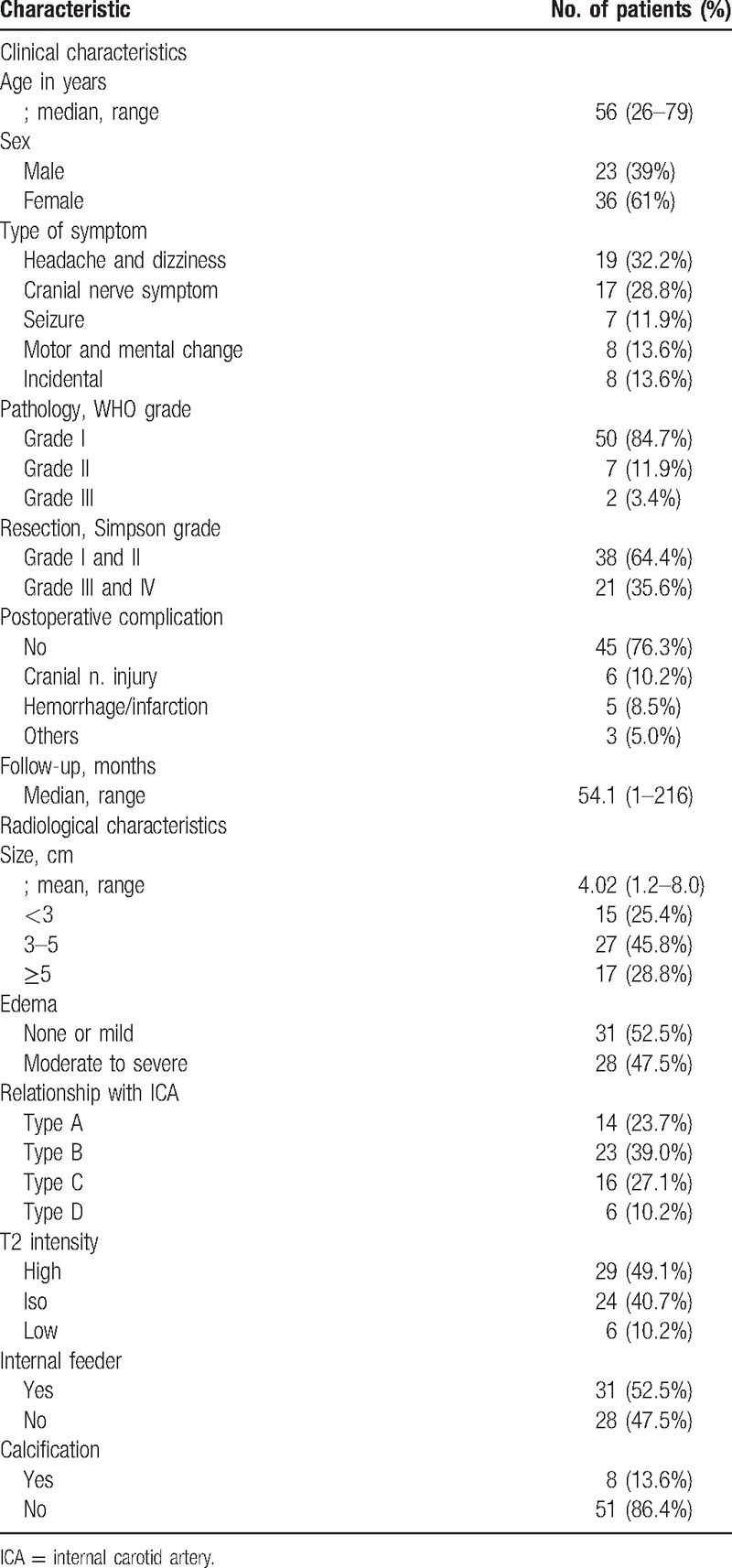
Clinicoradiological characteristics of the 59 patients with anterior clinoidal meningioma are summarized in Table 1. The patients consisted of 23 males (39%) and 36 females (61%), with a median age of 56 years (range, 26–79 years). Twenty-seven (46%) of the 59 tumors were detected by minimal symptoms (headache or dizziness) or incidentally. Tumors ranged in size from 1.2 to 8 cm (median, 4 cm). Intratumoral calcification was documented in 8 patients (13.6%). On T2-weighted MRI, only 10% of the cases showed low SI, presumed as hard tumoral consistency. Moderate to severe peritumoral edema formation was evident in 28 (47.5%) of the 59 cases. Based on the preoperative DSA and intraoperative confirmation, ICA was encased (Type B and C) in 39 patients (66.1%). Fourteen patients (23.7%) revealed only displacement of ICA by tumor. Internal feeders were found in more than half of the cases (32/59, 54.2%). The majority of the patients (50/59, 84.7%) were diagnosed as a WHO grade I.
Table 1.
Clinicoradiological characteristics of 59 patients with anterior clinoidal meningioma.
3.2. Treatment
Orbitocranial or orbitozygomatic approach was used in the majority of the cases (46/59, 78.0%), and extended pterional (9/59, 15.3%) or subfrontal (4/59, 6.8%) approaches were used mainly for small tumors. GTR (Simpson's grade I or II) was possible in 38 cases (64.4%), grade III in 10 cases (16.9%) and grade IV in 11 cases (18.6%). Main causes of interrupting GTR in Simpson's grade III tumors were the involvement of cavernous sinus (8/10, 80%) and optic canal (2/10, 20%). In Simpson's grade IV tumors, encasement of ICA and/or cranial nerve was the main cause of subtotal resection (10/11, 90.9%).
3.3. Recurrence
The recurrence rate was 18.6% (11/59). RFS for anterior clinoidal meningiomas was 86.5%, 84.7% and 81.4% at 3, 5, and 10 years, respectively. Median time for recurrence was 102 months after surgery (Fig. 2). The results of analyses of the variables that could be correlated with recurrence are shown in Table 2 and Fig. 3. In the GTR group (Simpson's grade I or II), the recurrence rate was 5.3% (2/38) without reaching median time. Recurrence occurred in 9 cases (42.9%) with a median period of 73.5 months in the non-GTR group (Simpson's grade III or IV). This difference was statistically significant (P = .002). The absence of internal feeders was related with longer RFS than presence of internal feeders (P = .012). Multivariate analysis also confirmed that GTR (Hazard ratio [HR] 0.014, 95% confidence interval [CI] 0.001–0.256; P = .004) and no internal feeders (HR 0.058, 95% CI 0.004–0.759; P = .030) were significant as independent prognostic factor for recurrence-free.
Figure 2.
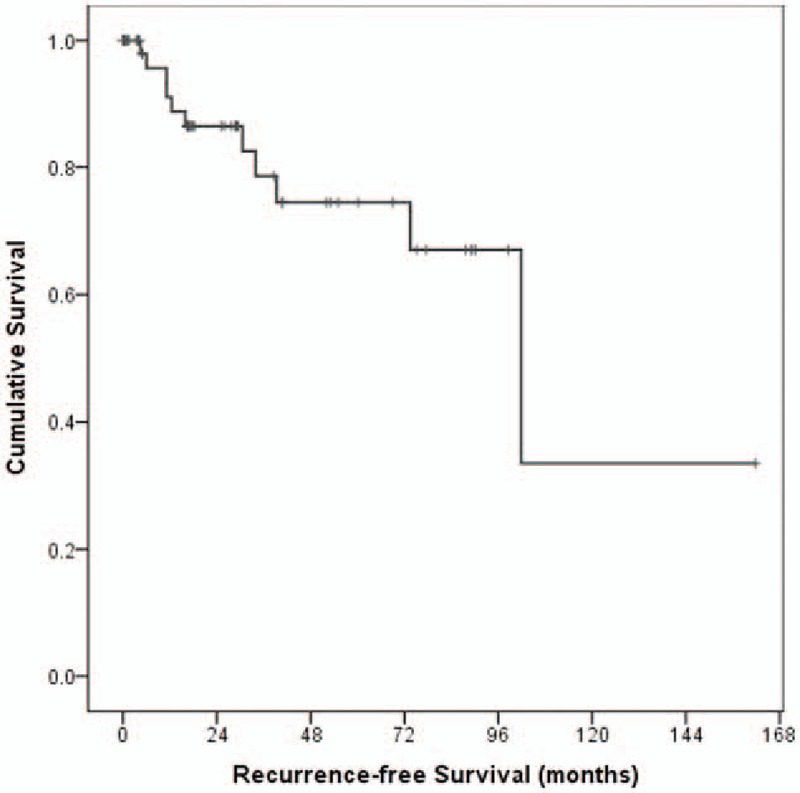
Kaplan–Meier curves showing recurrence-free survival (RFS) and cumulative survival of 59 study patients (overall comparison was estimated using a log-rank test). RFS = recurrence-free survival.
Table 2.
Univariate and multivariate analyses for tumor recurrence in patients with anterior clinoidal meningioma.
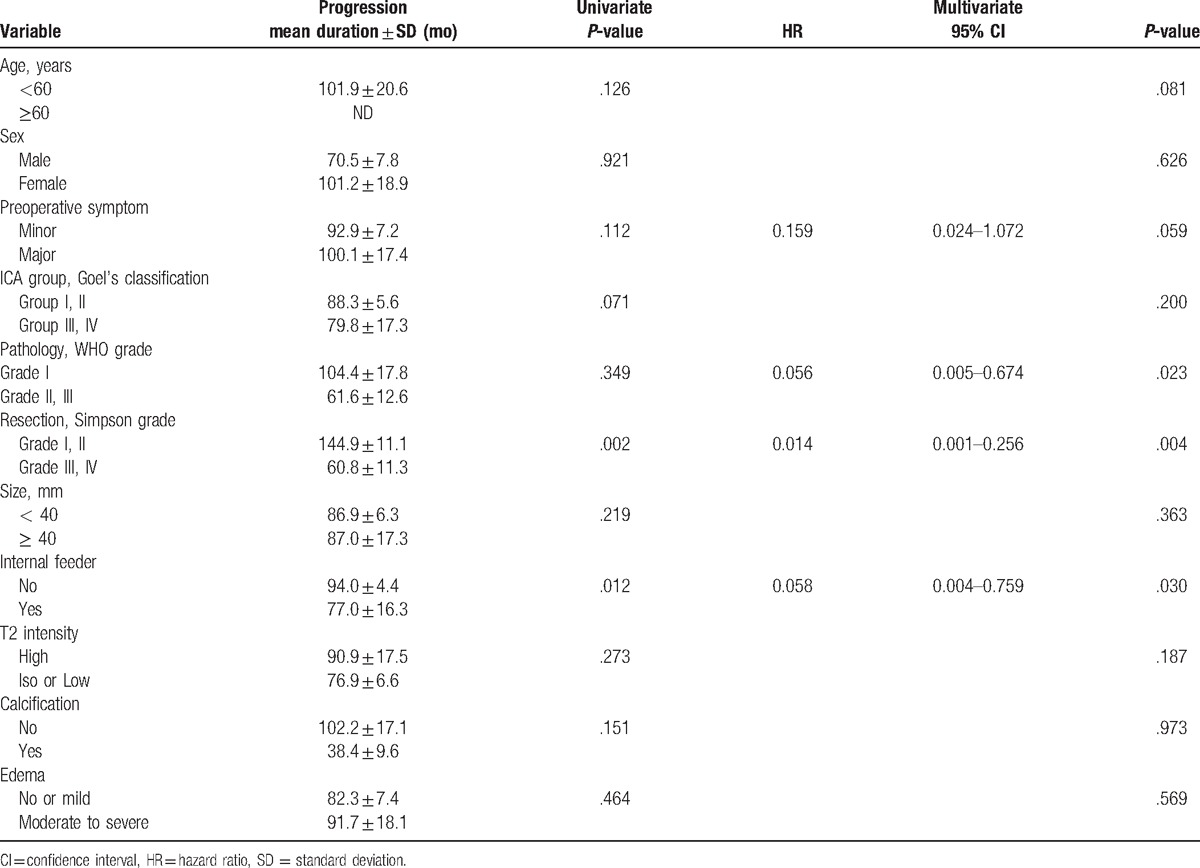
Figure 3.
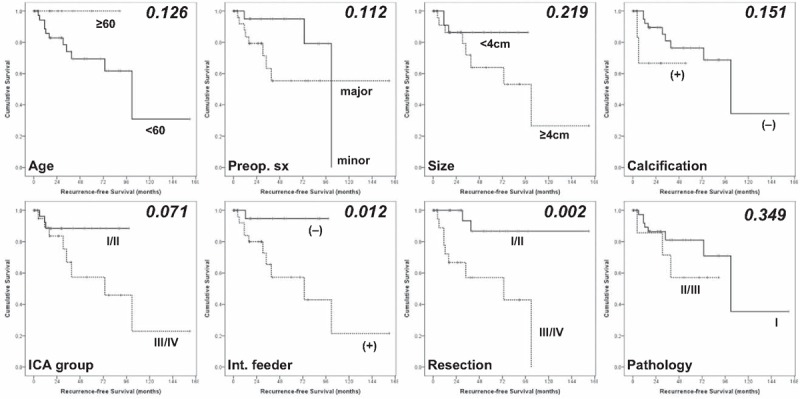
Kaplan–Meier curves showing recurrence-free survival (RFS) of 59 study patients according to different predictors (overall comparison was estimated using a log-rank test). The number on right upper in each curve represents the P-value. RFS = recurrence-free survival.
Preoperative symptoms (P = .112) and relationship between ICA and tumor (P = .071) were marginally significant in univariate statistical analyses. Preoperative symptoms also reached marginal statistical significance in multivariate analysis (HR 0.159, 95% CI 0.024–1.072; P = .059). The relationship between ICA and tumor did not reach the statistical significance in multivariate analysis (P = .200). Although pathological WHO grade was not related with RFS in univariate analysis (P = .349), benign pathology was significant as an independent prognostic factor for recurrence-free (HR 0.056, 95% CI 0.005–0.674; P = .023). Age, sex, tumor size and consistency, intratumoral calcification, and peritumoral edema were not statistically significant.
3.4. Complications
Permanent complication occurred in 14 patients (23.7%). The most common complication was cranial nerve injury (n = 6, 10.2%), followed by operation-related hemorrhage or infarction (n = 5, 8.5%), hydrocephalus (n = 2, 3.4%), and infection (n = 1, 1.7%). The results of analyses of the variables that could be correlated with permanent complications are shown in Table 3.
Table 3.
Univariate and multivariate analyses for postoperative complications in patients with anterior clinoidal meningioma.
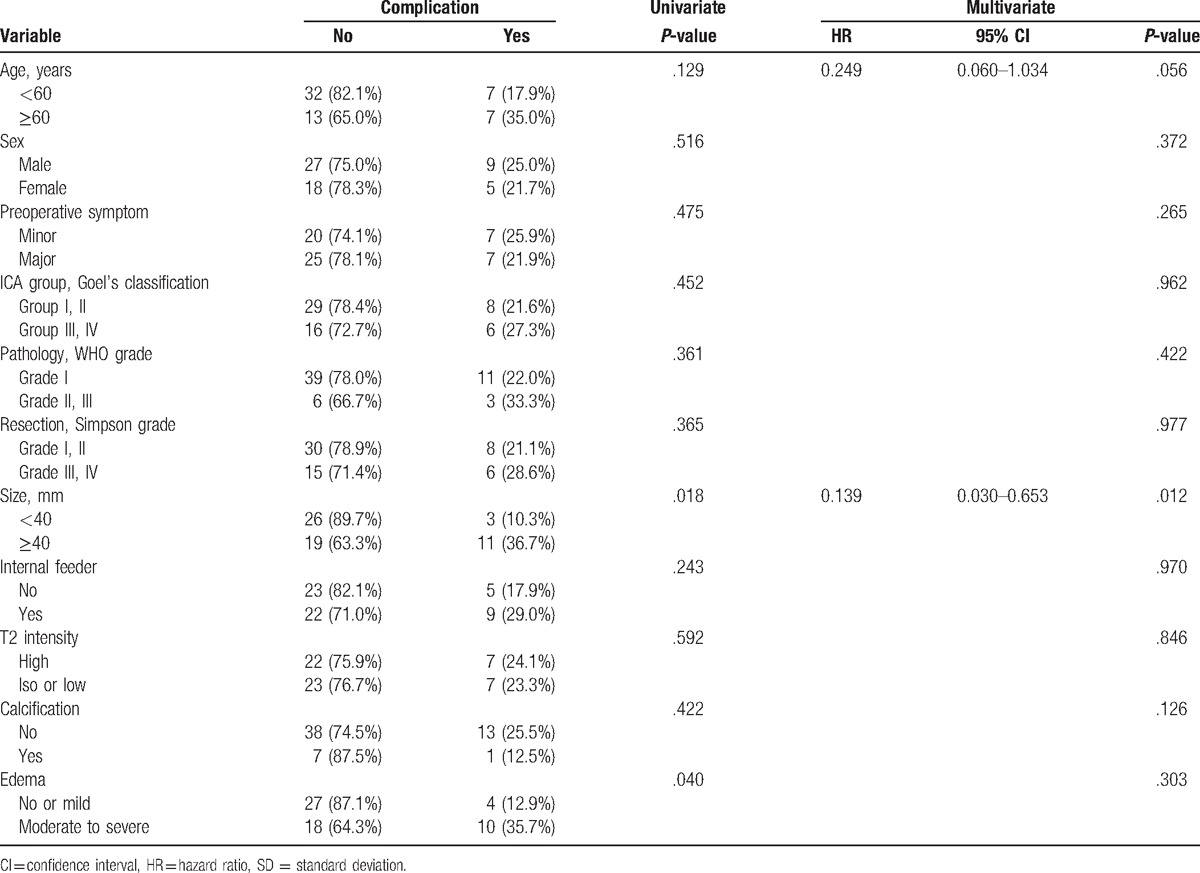
On univariate analysis, tumor size and peritumoral edema showed statistical significance. Permanent complications were more prevalent in patient with tumor ≥4 cm than those with tumors <4 cm (36.7% vs 10.3%, P = .018). Patients with moderate to severe peritumoral edema had a higher complication rate compared to the patients with no or mild perilesional edema (35.7% vs 12.9%, P = .040). Multivariate analysis confirmed that larger tumor size (HR 0.139, 95% CI 0.030–0.653; P = .012) was significant as an independent prognostic factor for development of complications. Peritumoral edema did not reach statistical significance in multivariate analysis (P = .303).
Old age (≥60 years) as a prognostic factor was not significant in univariate statistical analyses (P = .129) and approached significance in multivariate analysis (HR 0.249, 95% CI 0.060–1.034; P = .056). Sex, preoperative symptoms, relationship between ICA and tumor, presence of internal feeders, WHO grade, resection degree, tumor consistency, and intratumoral calcification were not statistically significant.
4. Discussion
Advances in microsurgical anatomy have greatly expanded our understanding of meningiomas of the anterior and middle skull base.[5] Among these meningiomas, some large-sized meningiomas, originating from the medial sphenoid ridge, optic canal or cavernous sinus can be classified as clinoidal meningiomas when they extend to the region of the ACP.[1] To define the anterior clinoidal meningiomas, some characteristic radiologic findings including mass formation with epicenter on ACP, hyperostotic changes on ACP, and upward growing feature should be considered.
Although the ideal surgical method for anterior clinoidal meningioma is controversial, more assertive techniques (i.e., anterior clinoidectomy with/without optic canal unroofing or optic sheath opening) have been proposed to improve the resection degree and visual deterioration.[2,6–8] Nevertheless, anterior clinoidal meningiomas are still thought-provoking lesions in complete and safe resection because they are in close contact with the ICA and its branches, optic nerve, and sometimes cavernous sinus.[1] The recurrence rates of anterior clinoidal meningiomas are difficult to compare with those of meningiomas from other sites, but it seems one of the highest among intracranial meningiomas. Even in early surgical series, Mirimanoff et al. reported recurrence or progression rates of 34% at 5 years and 54% at 10 years after surgery for anterior clinoidal meningiomas (vs 3% at 5 years and 25% at 10 years for convexity meningiomas).[14] Based on recently published data, recurrence or regrowth rates range from 0% to 21% with various follow-up durations (Table 4).[1–12]
Table 4.
Previously published case-series in the literature of anterior clinoidal meningioma.
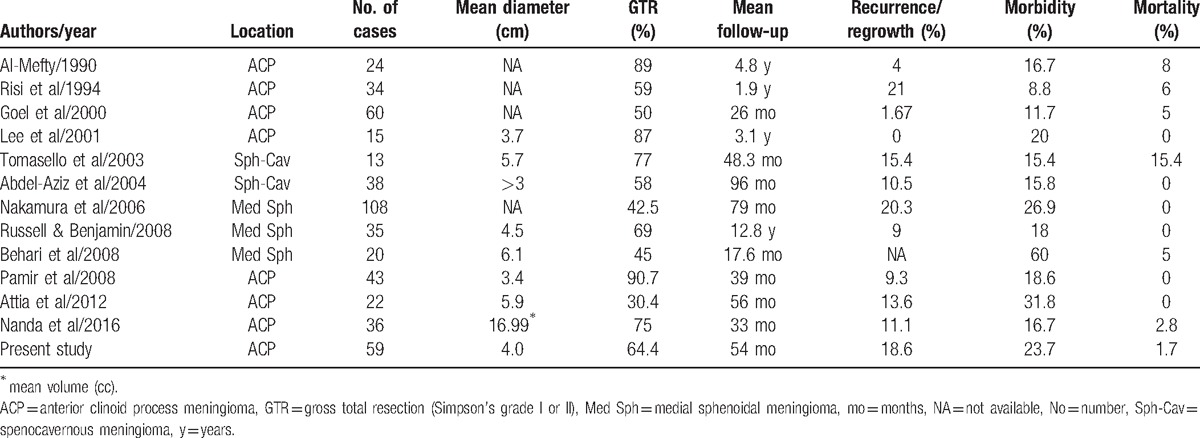
There have been various tries to seek related factors for tumor recurrence and better surgical outcome. Aggressive surgical removal of anterior clinoidal meningioma can be supported by the inference that the resection degree is inversely related to the recurrence rate.[15] The GTR rate for anterior clinoidal meningiomas ranged from 23% to 50% in an earlier series.[16] In more recent surgical series, however, it was reported to range between 30.4 and 90.7% (Table 4). Al-Mefty[8] already mentioned the importance of total removal of tumor to avoid post-operative tumor recurrence. GTR may pose a great risk of mortality and morbidity.[17] Our study also revealed a positive correlation between extent of tumor resection and recurrence (HR 0.014, 95% CI 0.001–0.256; P = .004). The collective data seems to lay to rest the controversy surrounding the pursuit of total removal.
Nakamura et al[1] reported a higher recurrence rate of 27.5% in clinoidal meningiomas with cavernous sinus involvement in contrast to a recurrence rate of 7.7% in tumors without involvement. Nanda et al[12] considered the tumoral involvement of ICA and cavernous sinus as related factors for deciding the extent of tumor resection. Our study also revealed the involvement of cavernous sinus (80% in Simpson's grade III) and encasement of ICA and/or cranial nerve (90.9% in Simpson's grade IV) as the main cause of subtotal resection. Presently, the relationship between tumor and ICA was a marginally significant factor for recurrence only in univariate analysis (P = .071). However, the presence of internal feeder was an independent predictive factor for recurrence in our study (HR 0.058, 95% CI 0.004–0.759; P = .030). In accordance with a previous study that linked the presence of mitoses, brain invasion and focal necrosis to a higher tumor recurrence rate,[18] the WHO grade was also an independent predictive factor for recurrence in our study (HR 0.056, 95% CI 0.005–0.674; P = .023).
The mortality rate of anterior clinoidal meningioma remained high, up to 15.4% with incidence of permanent profound neurological deficit from 8.8 to 60% (Table 4). Hao et al[19] suggested that the level of extension to the cavernous sinus is significantly related with post-operative complications including ptosis. Several other studies also found that in cases of tumor invasion to the cavernous sinus, tumor removal from the sinus is significantly associated with a risk of a new or worsening oculomotor nerve deficit.[1,7,20–22] Bassiouni et al[21] reported that the relationship between the tumor and the major ICA branches or the optic structures and tumor extension into cavernous sinus were prognostic factors for complications and surgical outcome. However, in our study, the relationship between tumor and ICA did not have statistical significance (P = .962).
Tumor consistency as well as vascular encasement and tumor size have been related with a high complication rate. Goel et al[4] observed more severe visual deficits were associated with firmer tumors. However, presently, tumor consistency represented by T-2 weighted SI did not show statistical significance (P = .846). In accordance with previous studies showing that the complication rate was higher in large tumors (≥3 cm),[4,13] tumor size ≥4 cm was significantly related with permanent complications (HR 0.139, 95% CI 0.030–0.653; P = .012) in our study. Tomasello et al[10] reported a positive correlation between grade of peritumoral edema and cortical penetration or complication rate. Presently, moderate to severe peritumoral edema was related with a higher complication rate (P = .040) in univariate analysis. Peritumoral edema did not reach statistical significance in multivariate analysis. Additionally, old age (≥60 years) was related with complications with marginal significance in multivariate analysis (P = .056).
This study should be interpreted with caution because of single-institution analysis, which could lead to selection bias, relatively small number of patients and treatment bias. The retrospective study design and limited follow-up makes it difficult to validate the results. So, the value in clinical studies of benign tumors is unclear.
5. Conclusions
Various patients and tumor factors are related with the degree of resection, disease progression and postoperative complication rate. To achieve maximized resection with minimized morbidity or mortality, these factors should be considered to try to decide on an appropriate surgical approach and plan for each case. Nevertheless, anterior clinoidal meningiomas still represent a challenge for neurosurgeons because of their close location to critical neurovascular structures, such as ICA and its major branches, optic and occulomotor nerves.
Acknowledgments
The authors thank Drs. Jeong, Min-Young, and Chung, Tae-Woong for review of neuroradiology of intracranial meningiomas in our hospital.
Footnotes
Abbreviations: ACP = anterior clinoid process, CT = computed tomography, GTR = gross total resection, ICA = internal carotid artery, MRI = magnetic resonance imaging.
Authorship: JHK, WYJ, KSM analyzed the data and drafted manuscript. KHL and TYJ revised manuscript critically for important intellectually content. KHL and KSM performed the statistical analysis. WDK, TYJ, and WYJ helped acquisition and interpretation of data. WDK, TYJ, and IYK participated in reviewing literatures and helped in conception and design of the study. KSM and SJ conceived the study, participated in the design of it and coordination. All authors read and approved the final manuscript.
Funding: This work was supported by Chonnam National University Hospital Biomedical Research Institute (HCRI15014-21), partly by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Minist (2016R1C1B2007494).
The authors have no conflicts of interest to disclose.
References
- [1].Nakamura M, Roser F, Jacobs C, et al. Medial sphenoid wing meningiomas: clinical outcome and recurrence rate. Neurosurgery 2006;58:626–39. [DOI] [PubMed] [Google Scholar]
- [2].Attia M, Umansky F, Paldor I, et al. Giant anterior clinoidal meningiomas: surgical technique and outcomes. J Neurosurg 2012;117:654–65. [DOI] [PubMed] [Google Scholar]
- [3].Behari S, Giri PJ, Shukla D, et al. Surgical strategies for giant medial sphenoid wing meningiomas: a new scoring system for predicting extent of resection. Acta Neurochir 2008;150:865–77. [DOI] [PubMed] [Google Scholar]
- [4].Goel A, Gupta S, Desai K. New grading system to predict resectability of anterior clinoid meningiomas. Neurol Med Chir 2000;40:610–6. [DOI] [PubMed] [Google Scholar]
- [5].Pamir MN, Belirgen M, Ozduman K, et al. Anterior clinoidal meningiomas: analysis of 43 consecutive surgically treated cases. Acta Neurochir 2008;150:625–35. [DOI] [PubMed] [Google Scholar]
- [6].Lee JH, Jeun SS, Evans J, et al. Surgical management of clinoidal meningiomas. Neurosurgery 2001;48:1012–9. [DOI] [PubMed] [Google Scholar]
- [7].Abdel-Aziz KM, Froelich SC, Dagnew E, et al. Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes. Neurosurgery 2004;54:1375–83. [DOI] [PubMed] [Google Scholar]
- [8].Al-Mefty O. Clinoidal meningiomas. J Neurosurg 1990;73:840–9. [DOI] [PubMed] [Google Scholar]
- [9].Risi P, Uske A, de Tribolet N. Meningiomas involving the anterior clinoid process. Br J Neurosurg 1994;8:295–305. [DOI] [PubMed] [Google Scholar]
- [10].Tomasello F, de Divitiis O, Angileri FF, et al. Large sphenocavernous meningiomas: is there still a role for the intradural approach via the pterional-transsylvian route? Acta Neurochir 2003;145:273–82. [DOI] [PubMed] [Google Scholar]
- [11].Russell SM, Benjamin V. Medial sphenoid ridge meningiomas: classification, microsurgical anatomy, operative nuances, and long-term surgical outcome in 35 consecutive patients. Neurosurgery 2008;62(3 suppl 1):38–50. discussion 50. [DOI] [PubMed] [Google Scholar]
- [12].Nanda A, Konar SK, Maiti TK, et al. Stratification of predictive factors to assess resectability and surgical outcome in clinoidal meningioma. Clin Neurol Neurosurg 2016;142:31–7. [DOI] [PubMed] [Google Scholar]
- [13].Lee JH, Sade B, Park BJ. A surgical technique for the removal of clinoidal meningiomas. Neurosurgery 2006;59(1 suppl 1): ONS108-14. [DOI] [PubMed] [Google Scholar]
- [14].Mirimanoff RO, Dosoretz DE, Linggood RM, et al. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg 1985;62:18–24. [DOI] [PubMed] [Google Scholar]
- [15].DeMonte F, Smith HK, al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg 1994;81:245–51. [DOI] [PubMed] [Google Scholar]
- [16].Pompili A, Derome PJ, Visot A, et al. Hyperostosing meningiomas of the sphenoid ridge—clinical features, surgical therapy, and long-term observations: review of 49 cases. Surg Neurol 1982;17:411–6. [DOI] [PubMed] [Google Scholar]
- [17].Olivecrona H. Olivecrona H, Tonnis W. The surgical treatment of intracranial tumors. Handbuch der Neurochirurgie. Berlin:Springer-Verlag; 1967. 1–301. [Google Scholar]
- [18].Boker DK, Meurer H, Gullotta F. Recurring intracranial meningiomas. Evaluation of some factors predisposing for tumor recurrence. J Neurosurg Sci 1985;29:11–7. [PubMed] [Google Scholar]
- [19].Hao S, Tian R, Wu Z, et al. Clinical characteristics and prognosis factors analysis for post-operative ptosis of sphenocavernous meningiomas: a single institution study. Clin Neurol Neurosurg 2015;131:35–41. [DOI] [PubMed] [Google Scholar]
- [20].Tobias S, Kim CH, Kosmorsky G, et al. Management of surgical clinoidal meningiomas. Neurosurg Focus 2003;14:e5. [DOI] [PubMed] [Google Scholar]
- [21].Bassiouni H, Asgari S, Sandalcioglu IE, et al. Anterior clinoidal meningiomas: functional outcome after microsurgical resection in a consecutive series of 106 patients. Clinical article. J Neurosurg 2009;111:1078–90. [DOI] [PubMed] [Google Scholar]
- [22].Puzzilli F, Ruggeri A, Mastronardi L, et al. Anterior clinoidal meningiomas: report of a series of 33 patients operated on through the pterional approach. Neuro Oncol 1999;1:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]


