Abstract
Rationale:
Primary bone lymphoma (PBL) is a rare malignant entity. There is a better survival of PBL than any other malignant bone tumors and extranodal lymphomas.
Patient concerns:
We report a rare case of PBL involving radius and tibia. The patient was a 14-year-old girl with left forearm pain and swelling after trauma. Six months later after the last chemotherapy and radiotherapy, pain and swelling of left knee was presented.
Diagnoses:
Radiological imaging revealed a lytic destruction, periosteal reaction, and pathological fracture of radius and tibia with soft tissue mass. Surgical biopsy was performed, and the result of histopathological diagnosis was diffused large B-cell lymphoma (stage IV, group A).
Intervention:
Chemotherapy combined with radiotherapy was applied before curation.
Lessons:
Due to its uncommon presentation, PBL should be taken into consideration if differential diagnosis from other bone tumors is necessary in clinic.
Keywords: computed tomography, magnetic resonance imaging, primary bone lymphoma, radius, tibia
1. Introduction
Primary bone lymphoma (PBL) is a rare malignant entity, accounting for 2% of all bone tumors and approximately 5% of all extranodal lymphomas.[1,2] The majority of PBL are non-Hodgkin lymphomas. It was first described by Oberling in 1928, and further reported by Parker and Jackson in their series on primary reticulum cell sarcoma of bone in 1939.[3] It is mostly located in femur or pelvis (50%), long bones of upper limbs (20%), and it can also occur in other locations such as ribs, mandible, or scapula (30%).[4] The most common symptoms of PBL are pain and swelling of affected bone. PBL is extremely uncommon in children, with few cases reported in literature.[5] We report a rare case of PBL of radius and tibia in a female child with literature review.
2. Case report
The patient provided informed consent for the publication of his clinical and radiological data. The study was approved by the Institutional Ethics Committee of First Affiliated Hospital of Dalian Medical University (Dalian, China).
A 14-year-old girl presented with pain and swelling of left forearm for 20 days after trauma. The patient denied fever, weakness, night sweats, and weight loss. At the same time, appetite and sleeping were well. No other system abnormalities were observed. Physical examination showed swelling and tenderness of the distal of left forearm without superficial lymphadenopathy. The palpable mass was ill-defined, hard, and about 7 cm in diameter, with restricted external rotation of left forearm. The skin around the mass did not show red and rupture.
Computed tomography (CT) showed a large mass with diffused destruction in the left distal radius with surrounding muscles involved (Fig. 1). The mass showed hypo-density compared with muscles. On the contrary, periosteal reaction and pathological fracture can be found. Magnetic resonance imaging (MRI) demonstrated bone marrow involved and surrounding soft tissues swollen. The lesion showed hyperintensity on T2-weighted imaging and iso-intensity on T1-weighted imaging, with significantly homogeneous enhancement after contrast administration (Fig. 2).
Figure 1.
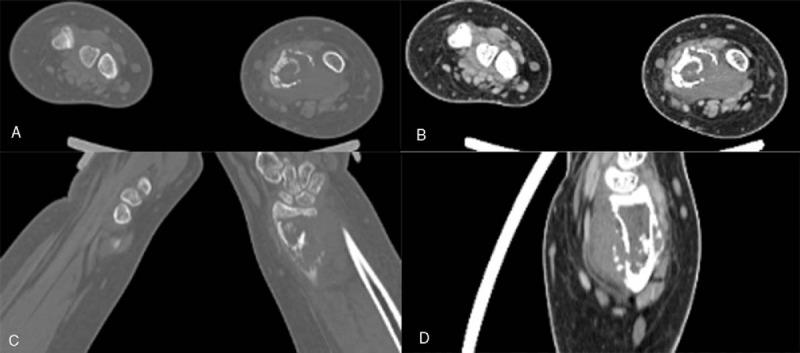
Computed tomography (CT) multiplanar reconstruction images (A, C) showed an osteolytic lesion in the distal of left radius, with periosteal reaction, pathologic fracture with surrounding soft tissue mass (B, D).
Figure 2.
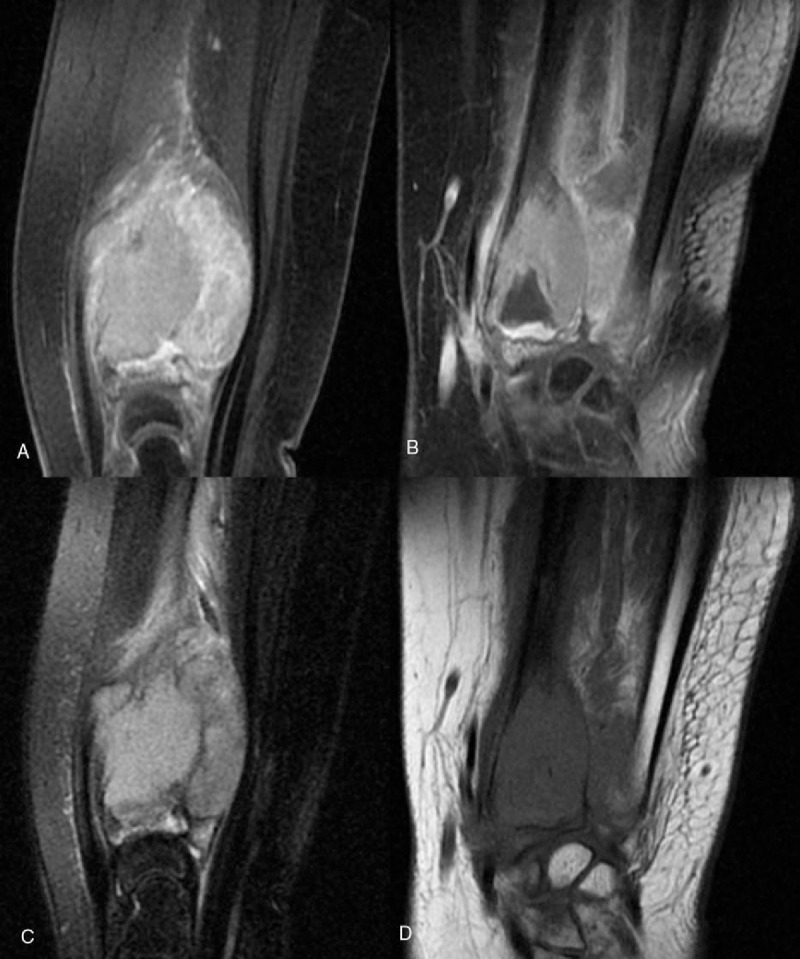
Primary bone lymphoma, with circumferential soft-tissue mass was shown as homogeneously hyperintensity on T2-weighted image (A), homogeneously isointensity on T1-weighted image (B) with obvious enhancement (C, D).
The laboratory examination revealed lymphocyte ratio of 43% (reference range: 20%–40%), platelet of 427 × 109/L (reference range: 101–320 × 109/L), serum glutamic-pyruvic transaminase 75 IU/L (reference range: 6–27 IU/L), serum cholinesterase 465 U/L (reference range: 203–460 U/L), serum phosphorus 1.67 mmol/L (reference range: 0.87–1.45 mmol/L), and uric acid 452 μmol/L (reference range: 155–357 μmol/L), without abnormalities of other laboratory index. Although glucose quantity was normal at the first time in hospital, it increased after 2 months (glucose 7.09 mmol/L, reference range: 3.83–6.11 mmol/L), which was possible related to the application of hormone. The patient denied history of diabetes. Ultrasound showed fatty liver, without other organs abnormality.
The surgical biopsy of the distal part of left radius was performed. Macroscopically, the mass was slight gray, solid, and tough. Light microscopic examination of the mass showed large cell infiltration between the striated muscle and connective tissues with obvious hyperplasia, apoptosis, and necrosis. The cell cytoplasm was dyed pink, and the cell nucleus was large, round, or ovoid with small nucleoli visible. The immuno-histochemical analysis was as follows (Fig. 3): positive for LCA(+), CD20(+), Mum-1(+), Bcl-6(+), CD99(+); and negative for CD3(−), CD43(−), NSE (−), MPO(−), S-100(−); and greater than 85% for Ki-67 proliferation index. As a result, a diffuse large B-cell lymphoma (stage IV, group A) was diagnosed.
Figure 3.
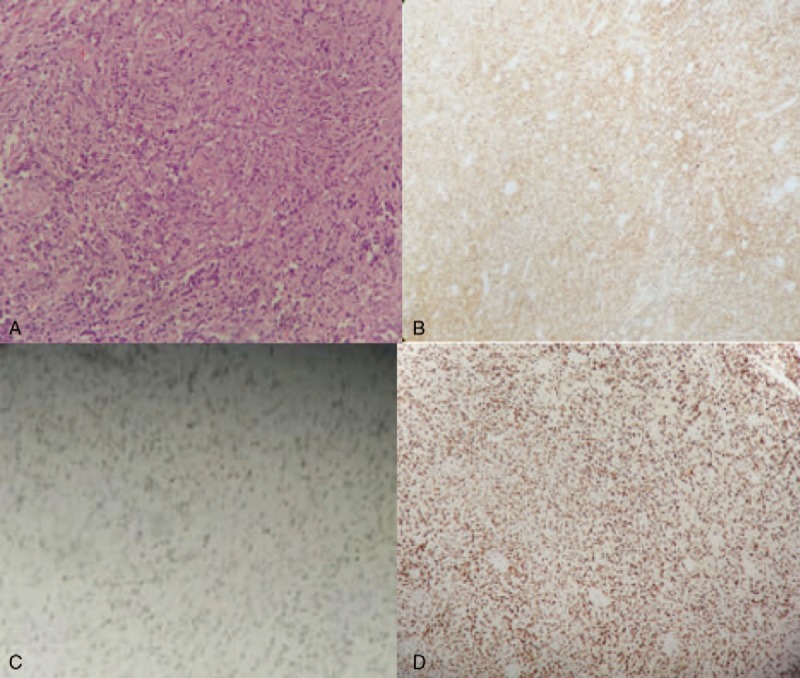
Histopathologic examination (200×) showed lymphoma cells diffuse infiltrating in bone tissue (A) with CD20 positivity (100×) (B), Bcl-6 positivity (100×) (C), and strong Ki-67 positivity (100×) (D).
The patient was treated with 8 courses of CHOP (cyclophosphamide 600 mg d1–2; pirarubicin 40 mg d1–2; vincristine 2 mg d1; prednisone 100 mg d1–5), followed by obvious swelling improvement of left forearm. Subsequently, the patient was treated with 1 course of local radiotherapy. After that, bone marrow biopsy of left radius showed no lymphomatous infiltration. Then, the chemotherapy and radiotherapy were not applied to the patient any more.
Six months later, the patient was readmitted into hospital, presented with pain and swelling of left knee for 1 month, without other abnormal clinical symptoms except for a history of diabetes for 1 year. CT revealed a lytic destruction of left tibia with swelling of surrounding soft tissues (Fig. 4). Also, periosteal reaction and pathological fracture could be found. At the same time, chronic inflammatory synovitis and joint effusion was found in the left knee-joint cavity.
Figure 4.
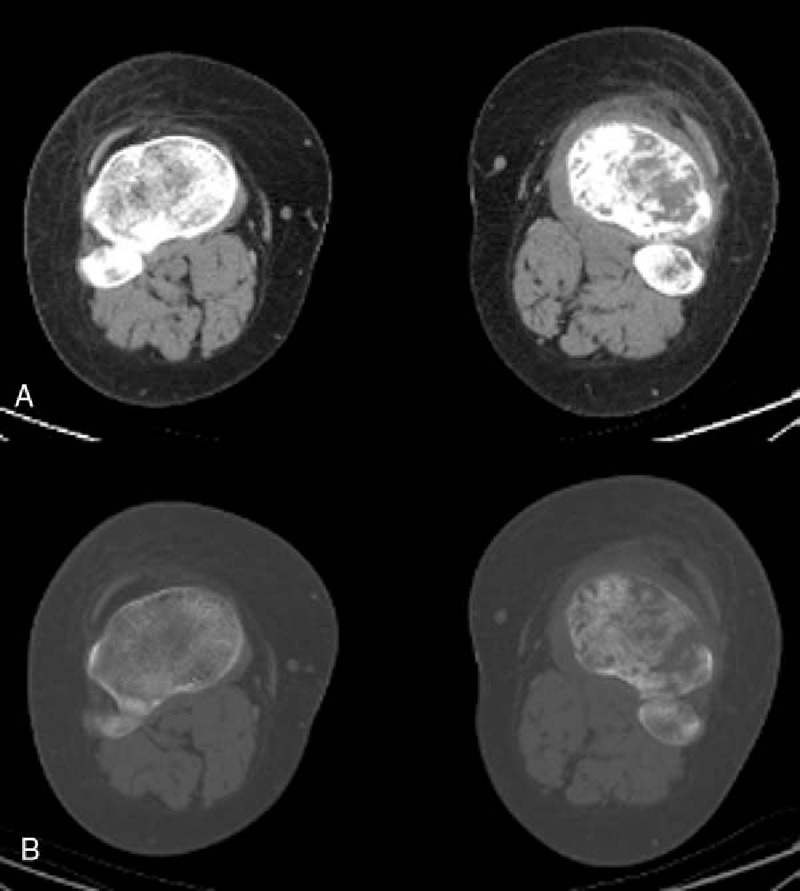
Soft-tissue swelling and osteolytic destruction of left tibia with cortical fracture were shown in CT images (A, B). CT = computed tomography.
The laboratory examination revealed glucose quantitative of 8.37 mmol/L (reference range: 3.83–6.11 mmol/L), lactate dehydrogenase of 301 U/L (reference range: 109–245 U/L), creatinine 34 μmol/L (reference range: 35–71 μmol/L), low-density lipoprotein 327 mg/dL (reference range: <120 mg/dL), and platelet 386 × 109/L (reference range: 125–350 × 109/L).
In clinic, the patient was treated with 6 courses of EPOCH (etoposide 100 mg for duration 1–4; pirarubicin 20 mg for duration 1–4; vincristine 1 mg for duration 1–4; prednison 100 mg for duration 1–5; cyclophosphamide 1200 mg for duration 5), 1-week duration per course; after the treatment, symptoms of pain and swelling of left knee lessened. After that, the laboratory examination revealed that lymphocytes, monocytes, eosinophils, and mean corpuscular count decreased, along with increased neutrophils. However, the patient died nearly 1 month later after last time of inpatient. The whole clinical process of this patient was shown in Table 1.
Table 1.
Detailed information of treatment and follow-up.

3. Discussion
The definition of PBL was still controversial. However, currently, there is a prevalent agreement that it can be considered PBL when primary involvement site of bone with no evidence of extrabone lesions or any other extrabone lesions 6 months after bone lesion is diagnosed by both pathological morphology and immunohistochemistry.[4] Non-Hodgkin lymphoma accounts for up to 94% of the PBL[6]; almost all cases are diffused large B-cell lymphoma among them.[3] The mechanism of non-Hodgkin lymphoma is not definite in clinic. It is reported[7] that non-Hodgkin lymphoma is mainly related to some uncertain factors such as: immune deficiency, genetic susceptibility, infection, and autoimmune abnormality. The possible factors which need to be further studied are as follows: blood transfusions, drugs, diet, and environment. For example, HIV infection increases the risk of developing lymphomas, leading to severe impairment of the immune system, especially the CD4 lymphocytes.[8] There is a male prevalence for PBL. It can occur in any age, most common in the sixth and seventh decade of life. The clinical presentation varies from pain, surrounding soft-tissue swelling to palpable mass with or without systemic symptoms.
The radiological features of a PBL are not specific. The common appearance of plain radiographs of PBL includes lytic destruction, periosteal reactions, cortical destruction, pathological fracture, and soft tissue masses. On MRI, the lesions often show hypointensity on T1-weighted imaging and hyperintensity on T2-weighted imaging.[9] The contrast-enhanced images will demonstrate areas of enhancement within lesion.[10]
Differential diagnosis should be considered, such as Ewing sarcoma, osteosarcoma, chronic osteomyelitis,[11] and lymphoblastic lymphoma/leukemia. Ewing sarcoma often occurs in the diaphysis of long bones in people younger than 20 years and in flat bones in people older than 20 years. Ewing sarcoma typically shows a permeative or “moth-eaten” cortical destruction, and lamellated or perpendicular periosteal reaction, a soft tissue mass on plain film and CT image.[12] Osteosarcoma has 2 age peaks, firstly mostly seen in the patients during the second decade of life, and secondly often observed in patients older than 50 years. According to Lee et al,[13] the difference of the plain radiological findings of osteosarcoma in 2 groups (adolescent patients and older patients) is statistically significant. Osteosarcoma in older patients usually shows osteolytic lesions with insignificant or no periosteal reactions, a small extent of extraskeletal soft tissue mass. However, the classical radiological findings of osteosarcoma in adolescent patients are sclerotic bone metaphyseal, with cortical disruption, significant periosteal reaction, and a large circumferential extraskeletal soft tissue mass. Then, a Codman triangle can be seen where the elevated periosteum and bone come together. Chronic osteomyelitis can occur in any age. It is a benign lesion with sharp margins, sclerotic border, and periosteal reaction. Cortical involvement and soft tissue mass can also be seen in chronic osteomyelitis; however, this is absent.[14] Immunohistochemistry is the main and important means for lymphoma differentiating from T/B lymphoblastic lymphoma/leukemia. The TdT is the most specific and sensitive marker of lymphoblastic lymphoma/leukemia, with positive diagnosis rate of 95%, which can be expressed by T and B lymphoblasts. Then, CD34, CD99, and CD43 are also the sensitive markers for diagnosis of lymphoblastic lymphoma/leukemia. As imaging features of PBL are atypical, histopathological diagnosis is necessary.
For treatment of PBL, chemotherapy combined with radiotherapy is commonly applied for decreasing the risk of local recurrence, which is better than chemotherapy or radiotherapy alone. However, it is considered that surgery is indicated for biopsy, prophylactic fixation of impending fractures, treatment of fractures before or after radiotherapy and systemic therapy, and theoretically in patients with disease unresponsive to conventional therapy.[2]
The prognosis of PBL is excellent with overall survival at 5 years, ranging from 58% to 88%, which depends mainly on staging and histopathological classification.[15] Even so far, an overall survival of 95% at 8 years has been reported.[2] It has also been reported that age is a prognosis factor; patients younger than 60 years have a better prognosis than others.[16]
Chemotherapy combined with radiotherapy is a favorable treatment for PBL. Some studies suggested that it is the age of patient, not the location of lymphoma, that influences the prognosis of the pediatric PBL.[17] In our case, the outcome of the 14-year-old girl may be related to immature of immune system development, which is necessary for further study.
4. Conclusions
This study can help clinicians to get acquaintance with PBL. PBL in pediatric age is very rare and it should be taken into consideration as a differential diagnosis for osteolytic lesions of bone. A correct diagnose of PBL requires biopsy because the clinical manifestations and imaging findings are usually nonspecific.
Footnotes
Abbreviations: CHOP = cyclophosphamide 600 mg d1–2; pirarubicin 40 mg d1–2; vincristine 2 mg d1; prednisone 100 mg d1–5, CT = computed tomography, EPOCH = etoposide 100 mg for duration 1–4; pirarubicin 20 mg for duration 1–4; vincristine 1 mg for duration 1–4; prednison 100 mg for duration 1–5; cyclophosphamide 1200 mg for duration 5, MRI = magnetic resonance imaging, PBL = primary bone lymphoma.
Y.H. and Y.Q., as co-first authors, contributed equally to this study.
The authors report no conflicts of interest.
References
- [1].Chang H, Tang TC. Surgical site spread of skeletal diffuse large B-cell lymphoma. J Clin Oncol 2013;31:e141–3. [DOI] [PubMed] [Google Scholar]
- [2].Scoccianti G, Rigacci L, Puccini B, et al. Primary lymphoma of bone: outcome and role of surgery. Int Orthop 2013;37:2437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Singh R, Al Wattar BH, Mohanty K. A rare case of primary bone lymphoma mimicking a pelvic abscess. Ann R Coll Surg Engl 2011;93:e141–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Undabeitia J, Noboa R, Boix M, et al. Primary bone non-Hodgkin lymphoma of the cervical spine: case report and review. Turk Neurosurg 2014;24:438–42. [DOI] [PubMed] [Google Scholar]
- [5].Fox MG, Marti JK, Bachmann KR, et al. Epiphyseal presentation of non-Hodgkin's lymphoma of bone in two pediatric patients-one with primary lymphoma of bone. Skeletal Radiol 2015;44:587–95. [DOI] [PubMed] [Google Scholar]
- [6].Mulligan ME, McRae GA, Murphey MD. Imaging features of primary lymphoma of bone. Am J Roentgenol 1999;173:1691–7. [DOI] [PubMed] [Google Scholar]
- [7].Grulich AE, Vajdic CM, Cozen W. Altered immunity as a risk factor for non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2007;16:405–8. [DOI] [PubMed] [Google Scholar]
- [8].Katusiime C, Kambugu A. A rare entity of primary extranodal diffuse large B cell lymphoma of the lower limb calf in an HIV-infected young adult on highly active antiretroviral therapy. BMJ Case Rep 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Krishnan A, Shirkhoda A, Tehranzadeh J, et al. Primary bone lymphoma: radiographic-MR imaging correlation. Radiographics 2003;23:1371–87. [DOI] [PubMed] [Google Scholar]
- [10].Rahmat K, Wastie M, Abdullah B. Primary bone lymphoma: report of a case with multifocal skeletal involvement. Biomed Imaging Interv J 2008;3:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Giardino AA, Shinagare AB, Shinagare SA, et al. Primary bone lymphoma involving bilateral tibia. Am J Hematol 2012;87:924–5. [DOI] [PubMed] [Google Scholar]
- [12].Henninger B, Glodny B, Rudisch A, et al. Ewing sarcoma versus osteomyelitis: differential diagnosis with magnetic resonance imaging. Skeletal Radiol 2013;42:1097–104. [DOI] [PubMed] [Google Scholar]
- [13].Lee SY, Cho WH, Song WS, et al. Different radiological findings with the same pathologic diagnosis due to different age in primary osteosarcoma. Acta Radiol 2006;47:841–4. [DOI] [PubMed] [Google Scholar]
- [14].Miller TT. Bone tumors and tumor-like conditions: analysis with conventional radiography. radiology 2008;246:662–74. [DOI] [PubMed] [Google Scholar]
- [15].Heyning FH, Kroon HM, Hogendoorn PC, et al. MR imaging characteristics in primary lymphoma of bone with emphasis on non-aggressive appearance. Skeletal Radiol 2007;36:937–44. [DOI] [PubMed] [Google Scholar]
- [16].Liu M, Liu B, Han F, et al. Primary bone lymphoma of the left radius: a case report and related literature review. Eur J Med Res 2014;19:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chisholm KM, Ohgami RS, Tan B, et al. Primary lymphoma of bone in the pediatric and young adult population. Hum Pathol 2016;1–30. [DOI] [PubMed] [Google Scholar]


