Supplemental Digital Content is available in the text
Keywords: colorectal cancer, prognostic factors, serum albumin, survival
Abstract
The aim of the present study was to define the prognostic role of baseline serum albumin (BSA) in colorectal cancer (CRC) across tumor–node–metastasis (TNM) stages and other well defined prognostic factors. Many prognostic models in medicine employ BSA to define or refine treatments in very specific settings; in CRC, BSA has been found to be a prognostic factor as well. A retrospective cohort study of consecutive patients with CRC demonstrated by biopsy, who attended a cancer center during a 7-year period. Multivariate analysis was utilized to define prognostic factors associated with overall survival (OS) employing the Cox model. In this retrospective cohort study, 1465 patients were included; 46.6% were females and 53.4% males (mean age, 59.1 years). Mean BSA was inversely correlated with TNM stages. By multivariate analysis, it was an independent explanatory variable. TNM stages, “R” classification, age, lymphocyte count, neutrophil/platelet ratio, neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, postoperative morbidity, and BSA were independently associated with OS. Morbidities, surgery type, chemotherapy, and radiotherapy were considered confounders after adjusting by TNM stages. BSA is a significant and independent prognostic factor in patients with CRC, and its effect is maintained across TNM strata and other well known clinical prognostic factors. It can be easily used in prognostic models and should be employed to stratify prognosis in therapeutic randomized clinical trials.
1. Introduction
Worldwide, colorectal cancer (CRC) is the third cause of cancer-related deaths,[1] and this situation is similar in North America.[1,2] There is wide geographical variation in CRC incidence and mortality, with very similar regional patterns in women and men.[1] There are deep regional differences in screening programs and treatment practices,[3] but radical surgery is widely recognized as best curative option for patients with localized CRC.[4] Approximately, 45% cases of CRC will die as a result of the neoplasm, even when novel treatments have improved survival.[5]
Many reports describe regional disparities in the prognosis of patients with CRC, which cannot be completely explained by the tumor–node–metastasis (TNM) classification or by current known prognostic factors. Therefore, a better understanding of these factors and their interactions, including those related with patients, healthcare providers, treatments, or institutions, is required to expand our understanding of the problem as a prerequisite for improving the quality of care in CRC.[6] Moreover, determination of hematologic, immunological, and nutritional measurements are described with increasing frequency as associated with prognosis in cancer.[7,8] Serum albumin (SA) is a valuable biomarker in many diseases[9] and has been reported as a significant prognostic factor in healthy populations and in countless acute, chronic, and neoplastic diseases.[10,11]
Many prognostic models employ baseline SA (BSA) to define or refine treatments in very specific settings; in CRC, BSA has been described as a prognostic factor associated with survival[12–14] and also as a predictor of surgical morbidity and mortality.[14–16]
Measurement of BSA is widely available, inexpensive, precise, and reliable, and it is used commonly to define the general status of patients with any medical condition. Consequently, in this study, the association of BSA and prognosis is investigated by multivariate analysis, adjusting for TNM stages and for several well proven prognostic factors, in a cohort of patients with CRC treated at a cancer center.
2. Materials and methods
2.1. Patients
Consecutive patients with CRC who attended to the “Instituto Nacional de Cancerología” (INCan) at Mexico City, from January 2008 to December 2014, were included in a retrospective cohort. Inclusion criteria comprised complete colonoscopy and biopsy to confirm the diagnosis of CRC; female or male patients over 18 years of age were included, and chest X-rays, liver ultrasonography, computed tomography, positron emission tomography scans, and magnetic resonance imaging were required in the staging protocol as appropriate. Data were extracted from the patients’ electronic clinical records and included clinical history, physical examination, blood cytology and biochemistry (including BSA at diagnosis), tumor markers, surgical procedures, endoscopic mucosal resections, adjuvant chemotherapy, radiation or chemoradiation, and diverse palliative procedures. The INCan Institutional Review Board and the Bioethical Committee approved this study.
2.2. Prognostic factors
Location of the neoplasm was defined according to colonoscopy findings. Two independent pathologists reviewed the surgical pathology material, and disagreement was conciliated by consensus. SA was measured with the method of Doumas and Rodkey,[17] with a LX20 Automated Clinical Chemistry Analyzer (Beckman Coulter, Brea, CA). The Nutritional Prognostic Index (NPI) was calculated as follows: (BSA in g/dL × 10) + (0.005 × total lymphocyte count in cells/μL), as previously reported.[18] The 7th edition of the TNM staging system was used,[19] and patients treated before 2010 were restaged employing the new classification. Surgery was coded as radical right or left hemicolectomy, radical sigmoidectomy, low-anterior rectal resection, or abdominoperineal resection. Rectal cancers were treated according to the total mesorectal excision approach. Adjuvant chemotherapy was utilized as standard procedure in patients with positive lymph nodes or T4b disease in colon carcinoma, and locally advanced rectal cancer was treated with standard preoperative chemoradiation. Patients with synchronous or metachronic metastases, or with progressive or metastatic disease, were treated with monotherapy, doublets, or triplets according to the Medical Oncology specialist.
2.3. Statistical analysis
After descriptive analysis, bivariate analysis of prognostic factors was performed employing the Student t, analysis of variance, or squared chi test, as required for continuous or categorical variables. The Kaplan–Meier method was used to construct survival curves, and the log-rank method was employed for testing the differences. Multivariate analysis was performed with the proportional hazards (Cox) model. Interaction terms and proportionality assumptions were tested in the final models.[20] Any probability of 0.05 or less was considered significant; 2-tailed statistics were considered, and SPSS statistical software for Mac ver. 20 (IBM Corp., Armonk, NY) was utilized for computations.
3. Results
3.1. Patients
During the period of time of this study, 1465 patients who complied with the inclusion criteria were included: 683 females (46.6%) and 782 males (53.4%); mean age was 59.1 years (standard deviation [SD], 14.9; range, 19–97). This represents 209 cases per year; 323, 78, 89, 287, and 688 cases were located in right, transverse, left, sigmoid colon, and rectum, respectively. Ninety-six, 499, 203, 126, and 541 corresponded to stages I, II, IIIa/IIIb, IIIc, and IV, respectively. Frequency distribution of age groups is depicted in Fig. 1 of the Supplementary Material (SM).
Mean BSA level was 3.42 g/dL (SD, 0.59; range, 1.0–5.0) and was significantly associated with TNM stages: higher BSA levels were present in patients with earlier TNM stages (P < .0001) (Fig. 2, SM). Mean lymphocyte count was 2035 cells/μL (SD, 872.9; range, 200–13,700) and was also significantly associated with TNM stages (P = .022) (Fig. 3, SM). In addition, age, blood hemoglobin, neutrophil count, platelet count, neutrophil/lymphocyte ratio (NLR), neutrophil/platelet ratio (NPR), platelet/lymphocyte ratio (PLR), albumin/globulin ratio (AGR), and the NPI were defined. Complete surgical resections (R0) were performed in 821 cases (56%), and 52 patients (3.5%) had neoplastic disease in surgical margins (R1); in 358 patients (24.4%), palliative resections with macroscopic residual disease were performed, and 234 cases (15.9%) did not undergo surgical resection. The median lymph nodes harvested were 15 (range 90; minimum 0, maximum 90).
3.2. Bivariate analysis
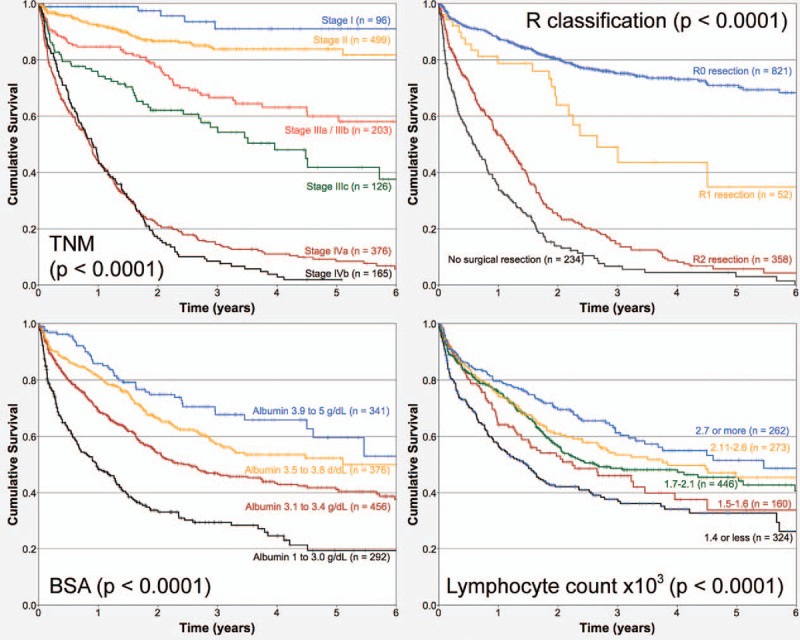
Mean follow-up of the cohort was 1.34 years, and 7.5% of patients were followed up at least for 5 years. Death during this period of time was registered in 630 patients (43%), and 835 (57%) cases were censored. TNM classification and BSA exhibited strong associations with overall survival (OS) (both P < .0001). Table 1 describes the Hazard ratios of the bivariate association of several clinical and surgical pathology factors and OS for the cohort of patients analyzed. Figure 1 depicts the associations of TNM stages, “R” classification, BSA or lymphocyte count, and OS, respectively. Figure 2 establishes the associations of NPI, NLR, NPR, and PLR, respectively.
Table 1.
Bivariate association of prognostic factors and OS (n = 1465).
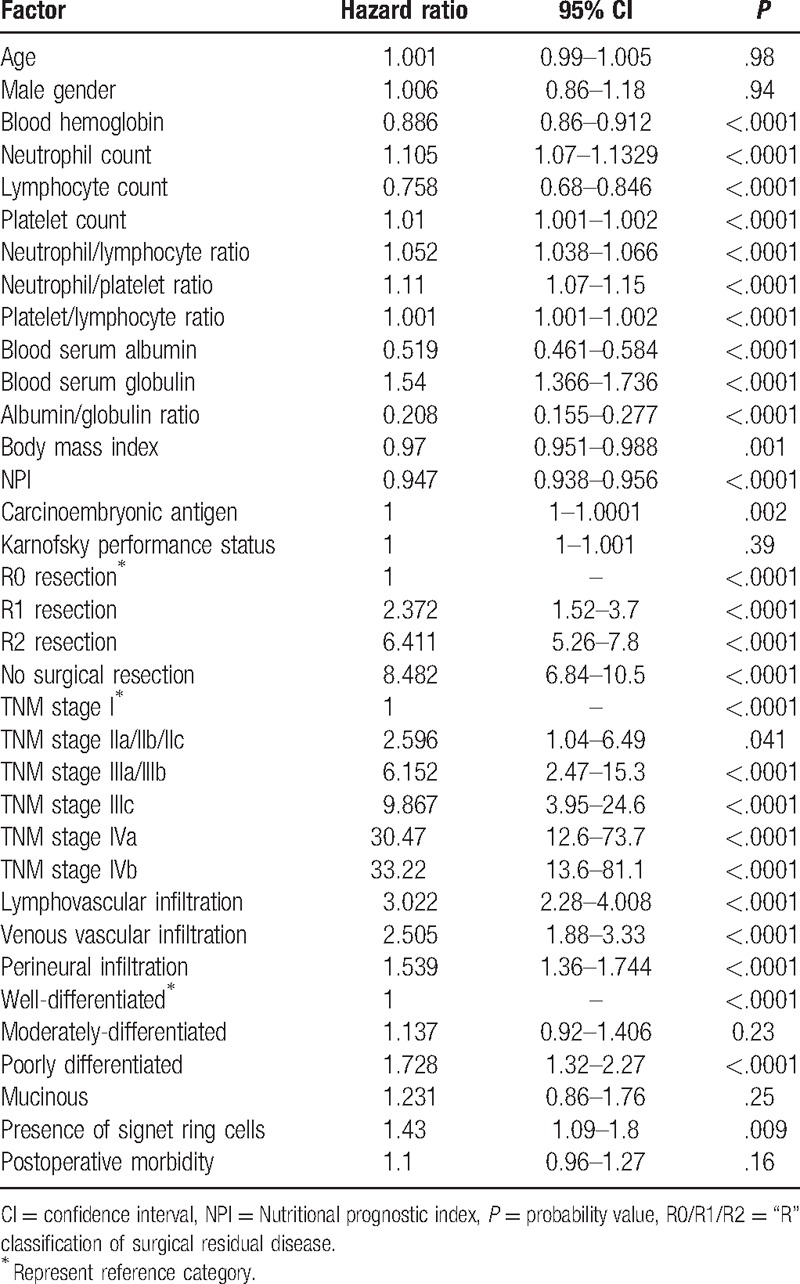
Figure 1.
Kaplan–Meier overall survival curves depending on (A) tumor–node–metastasis stages; (B) “R” classification; (C) baseline serum albumin; (D) lymphocyte count (n = 1465).
Figure 2.
Kaplan–Meier overall survival curves depending on (A) Nutritional Prognostic Index; (B) neutrophil/lymphocyte ratio; (C) neutrophil/platelet ratio, and (D) platelet/lymphocyte ratio (n = 1465).
In addition, blood hemoglobin, neutrophil count, platelet count, serum globulin, AGR, body mass index, carcinoembryonic antigen (CEA), lymphovascular infiltration, venous vascular infiltration, perineural infiltration, and differentiation grade were strongly associated with OS by bivariate analysis. Age was not associated with OS when analyzed as a continuous variable; however, it was categorized in 6 age groups and presented a multimodal association with OS. Figure 3 draws the Kaplan–Meier curves of the associations of BSA and OS, considering TNM stages as strata. The association was not clear for TNM stage I, but was strong in TNM stages II, III, and IV.
Figure 3.
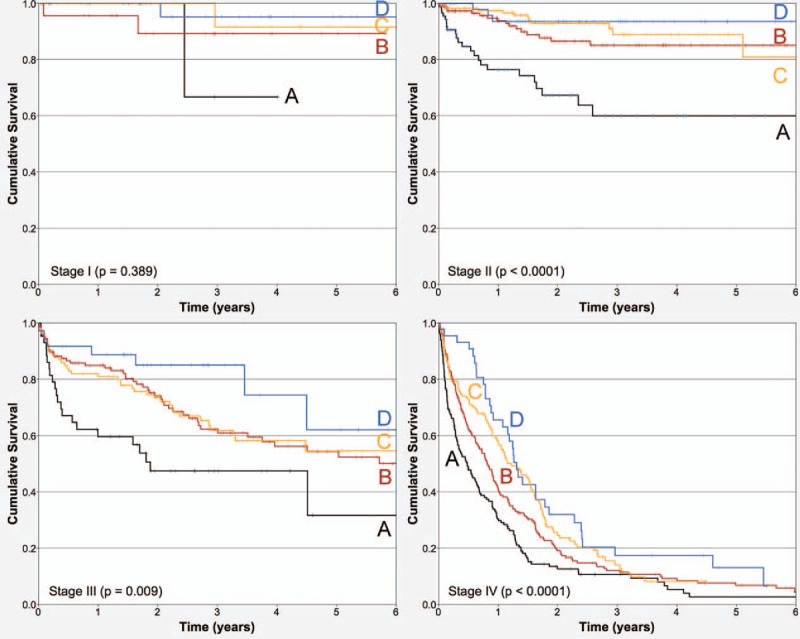
Kaplan–Meier overall survival curves depending on baseline serum albumin blood levels in the cohort by tumor–node–metastasis stages as strata (stratified analysis, P < .0001; n = 1465). (A) 1.0 to 3.0 g/dL; (B) 3.1 to 3.4 g/dL; (C) 3.5 to 3.8 g/dL, and (D) 3.9 to 5 g/dL.
3.3. Multivariate analysis
Factors associated with OS with a probability value of 0.1 or less were analyzed by the Cox model. BSA, lymphocyte count, NLR, NPR, PLR, gender, age groups, “R” classification of residual disease after surgery, surgical morbidity, and TNM stages were independent prognostic factors associated with OS. Table 2 reveals the parameters obtained by multivariate analysis for this final model. Analyses of interactions were negative, and proportionality assumptions were fulfilled. Albumin–lymphocyte interaction was highly significant, as well as NPI; however, both lost significance when the original terms were included in the model; hence, BSA and lymphocyte count were included as continuous variables. NPR was analyzed as a continuous variable, but NLR was analyzed as categorical because the impact on the model is substantially higher, as well as PLR.
Table 2.
Final model obtained by multivariate analysis of factors associated to OS in the cohort (n = 1465).

4. Discussion
The TNM staging system is the most valuable tool we have at present to define the prognosis of and to guide the treatment decisions for patients with CRC.[19] Nonetheless, it possesses important limitations for predicting prognosis in specific subgroups.[21] There is substantial variability in oncologic outcomes that are not completely explained by TNM staging or other prognostic factors: some patients with rectal cancer are less likely to undergo permanent colostomy if they are treated at high case-load centers; older age is associated with less frequency of use of adjuvant chemotherapy in CRC; black patients receive less-aggressive therapy and are more likely to die of CRC than white patients; low socioeconomic status is also associated with reduced OS in CRC; variations in treatment may also be linked with inadequate physician knowledge of treatment guidelines, differences in regional resources’ availability, with patient or physician preferences of treatment alternatives,[6] or by molecular heterogeneity in CRC.
This is a retrospective cohort study from a cancer center in the Mexico City with a large number of cases of CRC, and BSA is presented as a significant and independent prognostic factor within the range of patients with CRC from stage I to IVb. The main strengths of this report comprise its prolective hypothesis and data analyses, the comprehensive adjustment of relevant prognostic factors employed routinely in clinical practice including the TNM staging system, and the use of the BSA measurement as a continuous variable demonstrating a “biological gradient effect.” Moreover, other relevant prognostic factors were included in the multivariate analysis, such as the neutrophil, lymphocyte, and platelet counts; NLR; NPR; PLR; and NPI. In contrary, the main pitfalls of our study are the retrospective nature of data and that C-reactive protein (CRP) assessment was not available for the majority of our patients and was not considered in the present analysis.
BSA has been studied as a prognostic factor in CRC[11]; some reports utilize BSA as a single prognostic factor, while in others it is employed as part of the Glasgow Prognostic Score (GPS) or NPI; nevertheless, its impact has not been assessed by stratified or multivariate analysis, probably due to small sample sizes or insufficient follow-up times.
In our study, BSA is an important prognostic factor in stages II to IV; the association is not clear in stage I, which probably reflects its major importance in undernourished populations who present with advanced neoplastic disease like that of ours.
Recent reports describe novel prognostic factors that could improve the prognostic accuracy of TNM classification and pay specific attention to systemic inflammatory response markers in the prognosis of CRC.[7,8]
Many studies have established that BSA and numerous inflammatory markers, such as CRP, lymphocyte count, NLR, NPR, and PLR, are associated with different outcomes in patients with CRC. High NLR is associated with poor OS in many solid tumors. In a recent meta-analysis of 100 studies comprising 40,559 patients with diverse neoplastic diseases, it was found that NLR was associated with OS, an effect independent of disease subgroups, tumor sites, and stages.[22]
Another report evaluated the modified GPS in patients with CRC who underwent potentially curative surgical resection, and TNM stages were included in the multivariate analysis. TNM stages and modified GPS were independent explanatory variables associated with OS.[12] The association of NLR and OS was studied in CRC who underwent potentially curative resections. Baseline NLR >5 was associated with poor OS; however, NLR was not an independent explanatory variable when Dukes stage was adjusted.[23] A study of potentially curative resections for CRC investigated platelet count and NLR and found these to be associated with OS, along with histopathology, lymph node metastasis, serum levels of CEA, CRP, BSA, and GPS.[24] In a study of curative resections in CRC, NLR and PLR were associated with OS. PLR was an independent prognostic factor of OS based on multivariate analysis.[25] In a study of curative resections for CRC, NLR and PLR were associated with OS but, by multivariate analysis, only NLR retained independence as a prognostic factor. In addition, NLR was associated with age, mucinous morphology, T classification, and TNM stage.[26]
One of the best known and probably most commonly used prognostic models in CRC is the Memorial Sloan Kettering Cancer Center prediction tool. This model employs T and N classification, number of lymph nodes retrieved in surgery, number of positive lymph nodes, differentiation degree, patient gender, and patient age[27]; however, with the exception of T and N classification, plus gender, none of the remaining factors were relevant in our study. On increasing the sample size, the use of this model with readily available prognostic markers can outperform the TNM staging system.[28]
In a retrospective study comparing different models based on inflammatory biomarkers, the authors explore the prognostic role of NLR, PLR, lymphocyte/monocyte ratio (LMR) and AGR, but categorized these into 2 groups. They found that NLR, LMR, and AGR were significantly associated with OS and disease-free survival by multivariate analysis and proposed the use of this model to predict OS in CRC.[29]
In another report, the GPS has also been modified as the CRP/albumin ratio and proposed as a prognostic factor in CRC. However, TNM stage and other clinical variables were not adjusted by multivariate analysis.[30]
An exciting area of research is the cachexia–anorexia syndrome (CACS) that is associated with the activation of inflammatory response and physiologic alteration of mitochondria, which leads to anorexia, muscle wasting and atrophy, and fat loss. Inflammatory response to tumor growth appears to trigger many metabolic changes involving different cell types and organs that make CACS a multiorgan syndrome.[31] Although several blood biomarkers that could aid in measuring CACS have been suggested (acute-phase proteins, tumor-derived compounds, cytokines, and skeletal muscle degradation markers), common agreement has not yet been reached.[31] In this regard, BSA and nutritional and inflammation markers are excellent options for improving the TNM classification in its prognostic capability. These are widely available, inexpensive, precise, reliable, and commonly used as part of the clinical studies undertaken before the proposal of any treatment in practically all patients with CRC worldwide. Taken together, these are considered markers of host inflammation and nutritional state and may reflect a combination of defective albumin synthesis in the liver, cytokine activation, tumor-derived compounds, and in more aggressive disease.
In brief, increasing evidence indicates that inflammation plays a vital role in tumorigenesis, progression, and prognosis in CRC. Based on our data, the BSA level comprises a significant prognostic factor in CRC. This association presents a “biological gradient effect” and is congruent with many independent reports. BSA and the clinical markers reported herein should be evaluated in prospective studies to demonstrate its ubiquity before they can be integrated into the TNM staging system or into novel prognostic models. Accordingly, BSA and nutritional or inflammatory markers could be employed in clinical trials to better define baseline risk in patients with CRC. Visceral protein depletion, inflammatory response markers, nutritional therapy, and anabolic strategies should be evaluated together with CRC-specific therapies. Future research for BSA could be directed toward defining its role as a prognostic factor in specific subgroups such as stage II or liver metastases to aid the refinement of therapeutic decisions.
Acknowledgments
Many thanks to Professor Maggie Brunner, MA, for her English language editorial revision.
Supplementary Material
Footnotes
Abbreviations: AGR = albumin/globulin ratio, BSA = baseline serum albumin, CACS = cachexia–anorexia syndrome, CEA = carcinoembryonic antigen, CRC = colorectal cancer, CRP = C-reactive protein, GPS = Glasgow Prognostic Score, NLR = neutrophil/lymphocyte ratio, NPI = nutritional prognostic index, NPR = neutrophil/platelet ratio, OS = overall survival, PLR = platelet/lymphocyte ratio, SD = standard deviation, SM = supplementary material, TNM = tumor–node–metastasis.
This study was presented in part at the “Gastrointestinal Cancers Symposium 2016” (ASCO, SSO, ASTRO, and AGA) at San Francisco, CA. January 2016 (abstract number 740).
Funding/support: All resources for this study proceeded from INCan.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. 2013;Lyon, France:International Agency for Research on Cancer, Available at: http://globocan.iarc.fr. [Accessed on November 10, 2016]. [Google Scholar]
- [2].Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;in press. [DOI] [PubMed] [Google Scholar]
- [3].Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut 2015;64:1637–49. [DOI] [PubMed] [Google Scholar]
- [4].Liang J, Fazio V, Lavery I, et al. Primacy of surgery for colorectal cancer outcomes. Br J Surg 2015;102:847–52. [DOI] [PubMed] [Google Scholar]
- [5].Cremolini C, Schirripa M, Antoniotti C, et al. First-line chemotherapy for mCRC: a review and evidence-based algorithm. Nat Rev Clin Oncol 2015;12:607–19. [DOI] [PubMed] [Google Scholar]
- [6].Hodgson DC, Fuchs CS, Ayanian JZ. Impact of patient and provider characteristics on the treatment and outcomes of colorectal cancer. J Natl Cancer Inst 2001;93:501–15. [DOI] [PubMed] [Google Scholar]
- [7].McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534–40. [DOI] [PubMed] [Google Scholar]
- [8].Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218–30. [DOI] [PubMed] [Google Scholar]
- [9].Fanali G, di Masi A, Trezza V, et al. Human serum albumin: from bench to bedside. Mol Aspects Med 2012;33:209–90. [DOI] [PubMed] [Google Scholar]
- [10].Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol 1997;50:693–703. [DOI] [PubMed] [Google Scholar]
- [11].Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Park JH, Watt DG, Roxburgh CS, et al. Colorectal cancer, systemic inflammation, and outcome: staging the tumor and staging the host. Ann Surg 2016;263:326–36. [DOI] [PubMed] [Google Scholar]
- [13].Dixon MR, Haukoos JS, Udani SM, et al. Carcinoembryonic antigen and albumin predict survival in patients with advanced colon and rectal cancer. Arch Surg 2003;138:962–6. [DOI] [PubMed] [Google Scholar]
- [14].Tokunaga R, Sakamoto Y, Nakagawa S, et al. Prognostic nutritional index predicts severe complications, recurrence, and poor prognosis in patients with colorectal cancer undergoing primary tumor resection. Dis Colon Rectum 2015;58:1048–57. [DOI] [PubMed] [Google Scholar]
- [15].Chiang JM, Chang CJ, Jiang SF, et al. Pre-operative serum albumin level substantially predicts post-operative morbidity and mortality among patients with colorectal cancer who undergo elective colectomy. Eur J Cancer Care (Engl) 2017;26:1–8. [DOI] [PubMed] [Google Scholar]
- [16].Montomoli J, Erichsen R, Antonsen S, et al. Impact of preoperative serum albumin on 30-day mortality following surgery for colorectal cancer: a population-based cohort study. BMJ Open Gastroenterol 2015;2:e000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta 1971;31:87–96. [DOI] [PubMed] [Google Scholar]
- [18].Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi 1984;85:1001–5. [PubMed] [Google Scholar]
- [19].Edge SB, Byrd DR, Compton CC, et al. AJCC Cancer Staging Manual. 7th ed.New York:Springer; 2010. [Google Scholar]
- [20].Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York:John Wiley and Sons, Inc; 1999. [Google Scholar]
- [21].Nitsche U, Maak M, Schuster T, et al. Prediction of prognosis is not improved by the seventh and latest edition of the TNM classification for colorectal cancer in a single-center collective. Ann Surg 2011;254:793–800. [DOI] [PubMed] [Google Scholar]
- [22].Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- [23].Walsh SR, Cook EJ, Goulder F, et al. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol 2005;91:181–4. [DOI] [PubMed] [Google Scholar]
- [24].Ishizuka M, Nagata H, Takagi K, et al. Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer 2013;109:401–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kwon HC, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil-lymphocyte versus platelet-lymphocyte ratio in patients with operable colorectal cancer. Biomarkers 2012;17:216–22. [DOI] [PubMed] [Google Scholar]
- [26].Choi WJ, Cleghorn MC, Jiang H, et al. Preoperative neutrophil-to-lymphocyte ratio is a better prognostic serum biomarker than platelet-to-lymphocyte ratio in patients undergoing resection for nonmetastatic colorectal cancer. Ann Surg Oncol 2015;22(suppl 3):603–13. [DOI] [PubMed] [Google Scholar]
- [27].Weiser MR, Landmann RG, Kattan MW, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol 2008;26:380–5. [DOI] [PubMed] [Google Scholar]
- [28].Weiser MR, Gönen M, Chou JF, et al. Predicting survival after curative colectomy for cancer: individualizing colon cancer staging. J Clin Oncol 2011;29:4796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer 2016;139:220–31. [DOI] [PubMed] [Google Scholar]
- [30].Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal cancer. Anticancer Res 2016;36:995–1001. [PubMed] [Google Scholar]
- [31].Argilés JM, Busquets S, Stemmler B, et al. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


