Supplemental Digital Content is available in the text
Keywords: adherence, drug delivery system, glaucoma medicine, peer adoption, physician recommendation, predicted uptake, sustained-release
Abstract
Sustained-release drug delivery systems that replace the need for daily glaucoma medications will improve outcomes for those who are nonadherent and reduce the inconvenience of having to take medications on a recurring basis.
The objective is to estimate uptake (i.e., demand) for a new technology that delivers sustained-release glaucoma medication and to investigate how uptake varies by product attributes, physician recommendations, peer adoption (i.e., percentage of patients seen in a clinic using the new technology), and patient characteristics.
In a web-enabled discrete-choice experiment survey, glaucoma patients in the United States were asked to choose between continuing eye drop use or purchasing the new delivery system. In a cross-sectional web-enabled survey, ophthalmologists were asked their likelihood of recommending the new technology based on product and patient characteristics.
Study participants were 500 glaucoma patients who were on topical administration of daily eye drops and 155 ophthalmologists who practice in the US.
Main outcomes were predicted uptake for patients and likelihood of recommending a new drug delivery system for ophthalmologists. Logistic models were used to analyze the choice data.
Uptake was estimated to be 18% at an annual cost of $1000 and to be 24% when the cost was $500. A physician's recommendation increased uptake by 6% to 12%, whereas an increase in peer adoption from 5% to 50% increased uptake by 3% to 7%. Patients aged ≥ 65 and those with lower income were more likely to remain on eye drops. Physicians were more likely to recommend a product if the interval between administrations is 6 months or longer and when long-term safety and efficacy data are available. They were less likely to recommend it to patients with lower income and no adherence problems.
Results suggest a significant interest in an injectable solution or other sustained-release alternatives to daily eye drops. However, in this survey, patient uptake was greatly influenced by out-of-pocket cost and the interval between treatment administrations. Few physicians were willing to recommend sustained-release technology if the treatment interval was less than 3 months.
1. Introduction
The most common cause of irreversible blindness in the world is glaucoma. It is estimated that 80 million people currently have glaucoma worldwide.[1] Glaucoma is a progressive optic neuropthay characterized by a signature optic nerve change that is associated with a corresponding visual field loss. Elevated intraocular pressure is the only known modifiable risk factor to date. First-line treatment for glaucoma is with topical administration of eye drops to lower intraocular pressure. Currently, if clinically effective, medical treatment is lifelong for most patients. To adequately control intraocular pressure and prevent disease progression, it is critical that the medication be administered according to the prescribed dosage, timing, and placement. Lack of adherence can lead to noncorrectable glaucomatous vision loss, and ultimately blindness. Despite the importance of being adherent to glaucoma medication, adherence is highly variable, with estimates ranging from 5% to 80%.[2]
Sustained-release drug delivery systems that replace the need for daily glaucoma medications are soon to be a reality. Several companies are experimenting with new technologies that will deliver daily medication through a timed release mechanism.[3] Innovations in the development of injectable biodegradable polymer depots help with increasing therapeutic benefits and providing controlled release for glaucoma treatment.[4,5] These technologies will improve outcomes for those who are nonadherent and reduce the hassles associated with having to take medications on a recurring basis. They also have the potential to decrease the variation in intraocular pressure during the day, especially for open-angle glaucoma patients.[6] As a result, they are likely to be an attractive alternative to eye drops for many patients. However, they are likely to be expensive and may not be covered by insurance. They also require a procedure that has to be performed by an ophthalmologist in the clinic on a recurring basis. As a result, there may be limited demand for this new technology.
The objective of this study was to estimate uptake (i.e., demand) for a prototypical product and how uptake is likely to vary as a function of product attributes (i.e., features), physician recommendations, peer adoption (i.e., percentage of patients seen in the clinic who adopted the new technology), and patient characteristics. The product attributes we considered were the interval between administrations and annual out-of-pocket costs. The previous literature shows that patients are swayed by recommendations from their physician [7,8] and by the influence of peers.[8–13] Patient characteristics included age, income, whether the patient is on monotherapy, and whether the patient reports difficulty remembering to administer eye drops. We expected that lower costs and longer intervals between injections will increase uptake of the new technology, and that patients will be more likely to adopt the new technology if their physician recommends it and/or if they think a large percentage of their peers are using it. We also hypothesized that patients past retirement age and those with lower income would be more likely to choose to remain on eye drops and patients who report difficulty with remembering to take eye drops and those on monotherapy would be less likely to remain on eye drops, as replacement for only 1 medication would offer lower value for these patients.
These hypotheses were tested using a web-enabled discrete choice experiment (DCE) survey administered to glaucoma patients in the United States. DCE is a survey method for assessing individual preferences through a structured set of tradeoffs and for quantifying the utility/satisfaction that people assign to a set of product attributes. The DCE method is based on the premise that products are characterized by a set of attributes (e.g., price, efficacy, safety) and that the attractiveness of a particular product is a function of the levels of these attributes.[14] DCE surveys have previously been used for estimating preferences for new medical technologies.[9,15]
Although the DCE survey can tell us the extent to which a physician's recommendation and other factors influence patient uptake, it cannot tell us whether physicians are likely to make such a recommendation. Therefore, we estimated factors that influenced the likelihood of a physicians’ recommendation as a function of product attributes and patient characteristics using a web-enabled cross-sectional survey targeting US ophthalmologists. The product attributes included interval between administrations and time since Food and Drug Administration (FDA) approval. Patient characteristics included patient income, patient adherence and whether the patient was on monotherapy or multiple therapies. We hypothesized that the longer the interval between administrations and the longer the product is in the market the more likely it is that physicians would recommend it. Contrarily, physicians would be less likely to recommend the technology to low-income patients, to those who have no adherence problems, and to patients on multiple medications. Based on the results of the 2 surveys we estimated uptake of a prototypical technology that could replace daily drops and simulated how uptake is likely to vary based on product attributes, physician recommendations, peer adoption, and patient characteristics.
2. Methods
2.1. Participants
A web-enabled DCE survey was fielded by a survey research firm to members of their US panel in July 2015. The eligibility criteria for the patient survey were self-reported diagnosis of glaucoma and being on topical administration of daily eye drops. Of the 15,000 panel members screened, 500 qualified to complete the patient questionnaire.
The physician survey was sent via the web in January and February 2016 to 989 ophthalmologists who were members of the American Glaucoma Society (AGS). The eligibility criteria for the physician survey were being a member of AGS and practicing in the United States. At the end, 155 physicians completed the survey.
Both patient and physician surveys were ethically reviewed and approved by the National University of Singapore's Institutional Review Board and a waiver of consent was given.i
2.2. Survey development
To facilitate patient's ability to respond to the DCE survey, we focused on an injectable solution that releases the drug over a period of months. Respondents were told that it would be administered by their ophthalmologist in clinic and their eye would be numbed before the injection. Respondents were also told that the risks associated with the injection were minimal but that they may feel temporary mild discomfort, irritation, or minor bleeding at the site of injection. These assumptions were made after discussions with the ophthalmologists.
After being presented with the above information, respondents were shown a series of DCE choice tasks where they were asked whether they would choose to continue on eye drops or adopt an injectable solution that was characterized by 4 attributes: interval between administrations, annual out-of-pocket cost, physician's recommendation, and perceived adoption rate defined as percentage of patients seen in the clinic who adopted the new technology. Table 1 lists the attributes and levels and Fig. A1 in Supplementary Digital Content 1 provides an example DCE choice task. The attributes and levels were identified after cognitive interviews with a convenience sample and discussions with ophthalmologists.
Table 1.
List of attributes and levels.
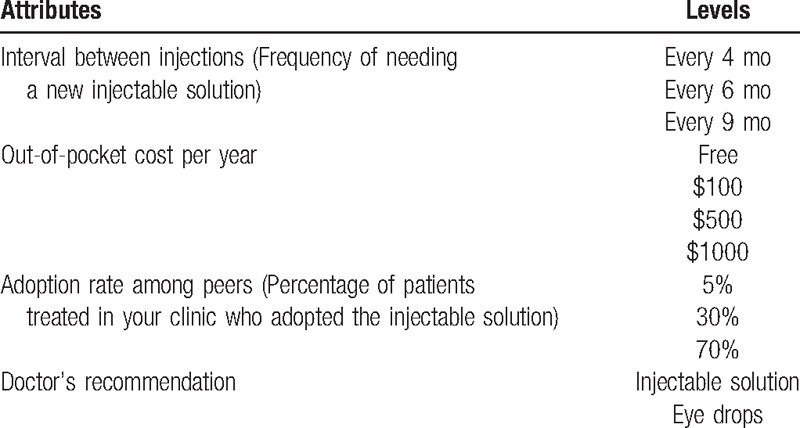
The DCE tasks were constructed via an experimental design that required identifying the specific level for each attribute and were created using SAS (SAS Institute, Cary, NC).[16] The tasks were then randomly divided into 3 blocks with 8 tasks per respondent to reduce the cognitive burden. The survey instrument also asked questions related to the number of glaucoma medicines used, difficulty remembering to take eye drops, and socio-demographic characteristics.
The physician survey asked whether they would recommend a sustained-release drug delivery system as a function of: interval between administrations, time since FDA approval, patient income, patient adherence, and whether the patient is on monotherapy or multiple therapies. Response options were on a 5-point Likert scale and ranged from “very likely” to “very unlikely.” To be conservative, in our demand estimation a recommendation was considered positive only when physicians reported that they were “very likely” to recommend it. (Patient and physician questionnaires are provided in Supplementary Digital Contents 2 and 3, respectively).
2.3. Analysis
The first step in the analysis of the patient data was to subdivide respondents into 3 groups: pro eye drops (those who chose eye drops in every choice task); pro injectable solution (those who chose injectable solution in every choice task); and traders. Traders made tradeoffs between the product attributes, physician recommendation and peer adoption, and sometimes chose eye drops and sometimes chose injectable solution depending on the levels of these factors. We assumed that respondents who were in the “pro eye drops” group would never purchase the injection, whereas the remaining 2 groups were considered potential consumers. We then used a logistic model to identify personal characteristics of those likely to be potential consumers.
As the traders may or may not purchase the injectable solution, their responses were analyzed using a mixed logit model [17] to understand the factors that influence uptake for this group and for quantifying uptake for specific combinations of the attributes. Overall uptake, taking into account uptake of Traders, those who were in the “pro eye drops” group (uptake was assumed to be 0%) and those who were in the “pro injectable solution” group (uptake was assumed to be 100%) was derived by taking a weighted average of uptake of the 3 groups. We used STATA version 12 for all statistical analyses. Methodological information on data analysis and uptake calculations can be found in Supplementary Digital Content 4.
3. Results
3.1. Respondent characteristics
Among the patients who completed the survey, about half were males, 24% were aged 65 or above and 60% were married; 18% were lower income, with household income less than US$25,000 a year and 62% had income between US$25,000 and US$100,000 a year, 47% reported being on monotherapy, and 14% reported having difficulty remembering to take their eye drops (Table A1 in Supplementary Digital Content 1).
From the physician survey, the mean number of years of experience treating glaucoma patients was 18 years and the mean number of patients treated per week was 100. Among the glaucoma patients treated by the physicians in the sample, the percentage of patients on monotherapy was 28% (Table A2 in Supplementary Digital Content 1).
3.2. Results from the patient survey
Based on responses to the DCE tasks, 9% of respondents always chose the injectable solution (“pro injectable solution” group) and 20% never did (“pro eye drops” group). The remaining 71% were considered “traders” who may or may not purchase the injectable solution depending on its characteristics. Respondents aged 65 and over and those with lower incomes were less likely to be potential customers; those who reported difficulty remembering taking eye drops were more likely to be potential customers of the injection (Table 2).
Table 2.
Logistic model to identify characteristics of respondents who are in the market for purchasing injectable solutions (vs not being interested in the product).
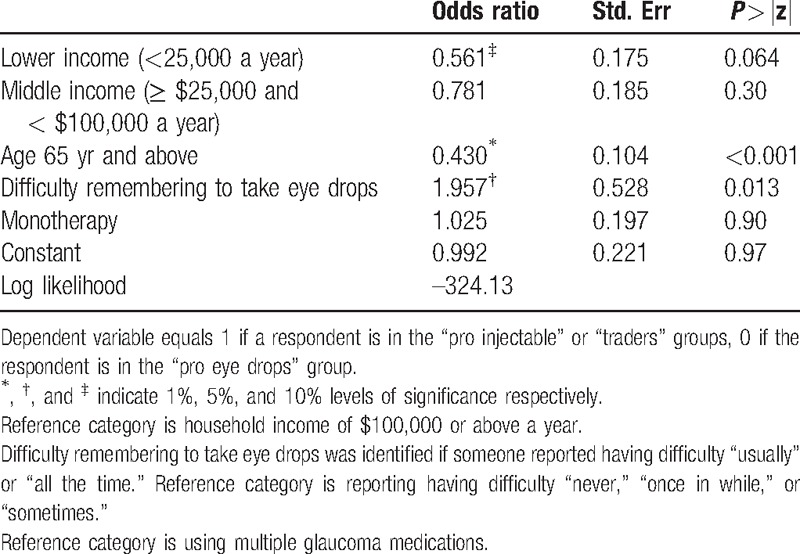
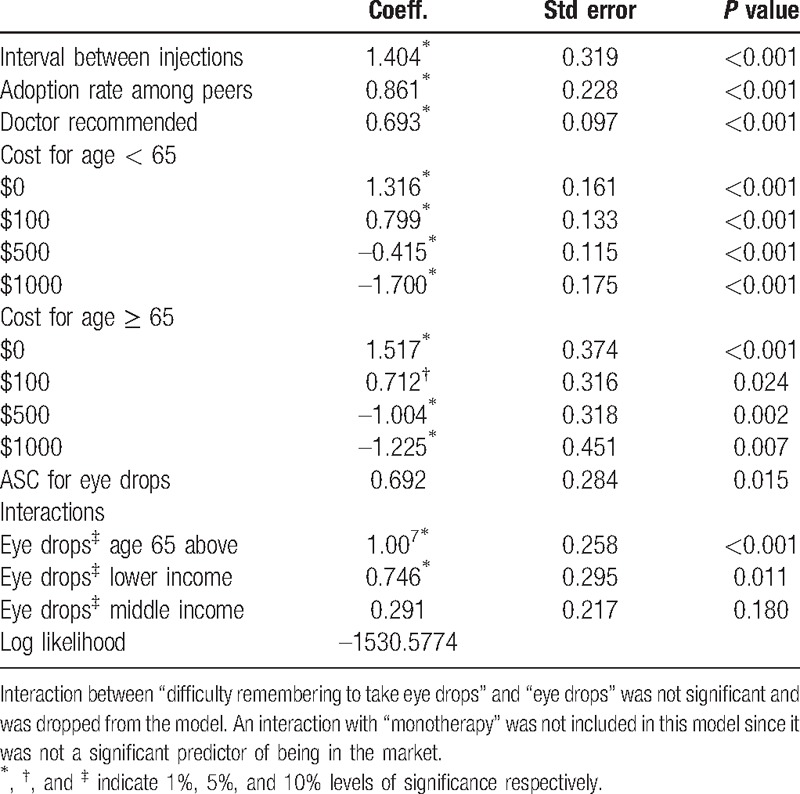
Table 3 presents the mixed logit estimates for traders. Consistent with our hypotheses, respondents preferred injectable solutions that have longer intervals between injections and lower costs. They were also more likely to choose injectable solutions if they are recommended by their physician or if they think that they are widely used by their peers. Respondents past retirement age were more sensitive to changes in the cost attribute compared with younger respondents. This age group and those with lower incomes were more likely to choose eye drops.
Table 3.
Mixed logit model estimates for traders (N = 354).
3.3. Results from the physician survey
Figure 1 provides the percentage of physicians recommending a new drug delivery system over eye drops. The interval between procedures was found to be highly influential in the likelihood of recommending the new system. Whereas only 11% of physicians would recommend a drug delivery system that required a minor surgical procedure every 3 months, 29% would recommend a system that required an administration no more than every 6 months and the majority (56%) would recommend one that required an administration no less than once annually. Recommendations were very sensitive to time since FDA approval. Although only 11% would recommend a delivery system soon after receiving FDA approval, 52% would recommend it after long-term safety and efficacy data became available. Physicians were also more likely to recommend a new drug delivery system for wealthier patients and patients with adherence problems. The likelihood of recommending the system was not influenced by whether the patient is on monotherapy or multiple therapies.
Figure 1.
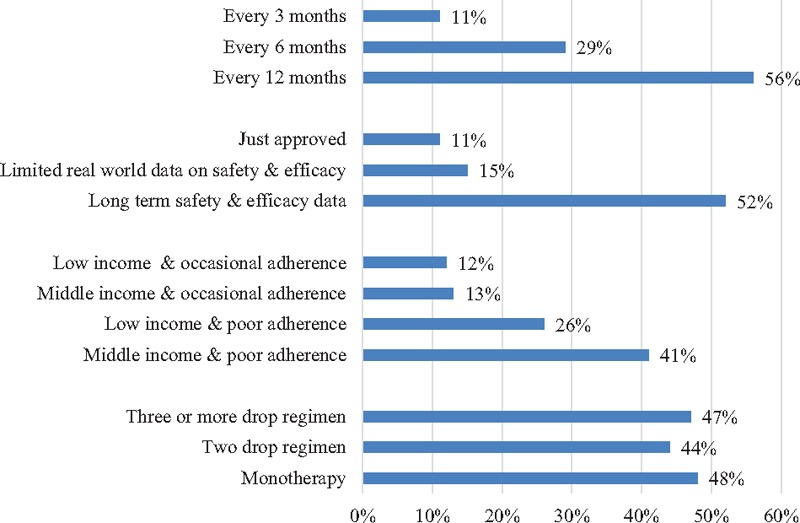
Percentage of physicians “very likely” recommending a new delivery system over eye drops.
3.4. Predicted uptake
Figure 2 predicts demand for a prototypical injectable solution that needs to be replaced every 6 months and that is in its early days of being in the market. Thus, we assumed a low peer adoption of 10% and a 0.29 probability of a physician recommendation, based on the results of the physician survey. Uptake was very sensitive to out-of-pocket cost. When free, uptake was estimated to be 60%; however, at an out-of-pocket cost of $1000 per year, this figure dropped to 18%. If administration is only required once annually, uptake could be as high as 71% (Fig. A2 in Supplementary Digital Content 1). A recommendation from the physician increased uptake by 6 to 12 percentage points (Fig. A3 in Supplementary Digital Content 1); whereas if the perceived adoption rate at the clinic increases from 5% to 50%, uptake increased by 3 to 7 percentage points (Fig. A4 in Supplementary Digital Content 1) depending on the out-of-pocket cost of the injectable solution. Those aged 65 and over were far less likely to adopt the injectable solution (Fig. A5 in Supplementary Digital Content 1). For this population subset, uptake was less than half of what it was for the younger age group when cost was low, but the difference narrowed at higher costs, largely because younger patients also opt out. Regardless of age, lower income patients had lower demand, with differences in uptake ranging from 6 to 11 percentage points (Fig. A6 in Supplementary Digital Content 1).
Figure 2.
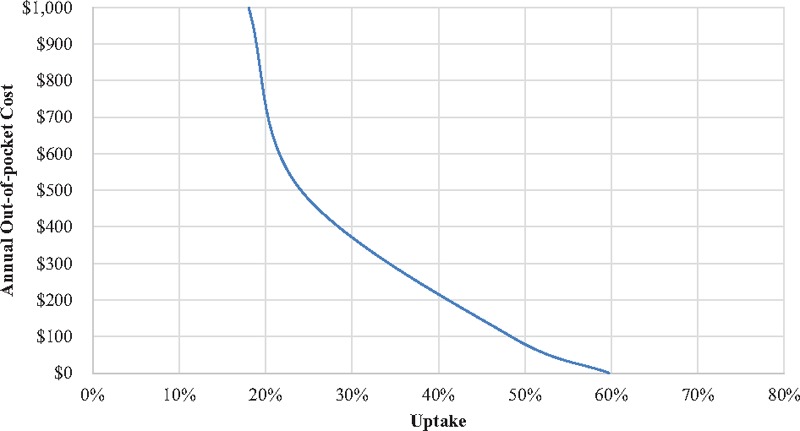
Stated uptake for a prototypical injectable solution.
4. Discussion
We estimated factors that influenced demand for a new drug delivery system that provides sustained release of glaucoma medication. We investigated this by using data from surveys conducted with glaucoma patients and ophthalmologists. The results from the patient data showed that 9% of patients were likely to be early adopters of the new drug delivery system even at fairly high prices, whereas 20% were unlikely to opt for the new system at any price. The remaining 71% may or may not choose the new drug delivery system depending on the product attributes, physician recommendation, and peer adoption. Early adopters would be younger and wealthier patients.
Even in a survey setting, uptake was sensitive to physician recommendations and peer adoption. This impact is likely to be higher in real life if patients receive a recommendation from their physician in person and/or when they witness other patients adopting the technology.[18] Results from the physician survey showed that physicians were likely to recommend the device if the intervals between administrations are at least 6 months. They were also more likely to recommend the technology to patients with higher income and to those with adherence problems.
Results suggest that for a prototypical injectable solution that is new to the market, lasts for 6 months between injections, and costs the patient $500 per year, uptake was likely to be roughly 24%. However, uptake was also very sensitive to out-of-pocket cost. We expected that the success of any discretionary technology that can replace daily eye medications would be highly dependent on the ability to obtain third-party reimbursement, including Medicare reimbursement. Lack of Medicare reimbursement would likely reduce demand for those above ages 65 given the finding that their demand was very price sensitive.[19,20] This would be especially true when the technology is first launched and the market price is relatively high. Over time, and assuming market data shows a new technology to be safe and efficacious, the physician survey data revealed that physicians would be far more likely to recommend it to their patients. This would increase peer adoption and push demand up even further. For example, simulations suggest that if the out-of-pocket cost decreases to $100 a year, as many as 48% of patients would adopt the new technology.
Our study had several limitations. First, the findings were based on survey responses. This was necessary given that the product does not currently exist on the market. It is possible that respondents, both patients and physicians, will behave differently to actual experience versus hypothetical situations. This may be more evident regarding patient responses to physician recommendations, since personal interactions with physicians influence patient behavior. To minimize this potential bias, in the physician survey, only responses to “very likely” were fed back into the patient model as positive recommendations. Second, we used a convenience sample of patients and physicians who are not representative of the patient or physician populations in the United States. Therefore, it is possible that our estimates suffer from population-related selection bias. Third, the predicted uptake for injectable solutions was estimated based on the attributes and levels used in the design and any changes in these attributes and study assumptions will affect the uptake. Concerning side-effects, the injectable solution was assumed to have only minimal risk. In addition, patients might have also considered any side-effects they experience with eye drops which makes them more likely to switch to injections. However, if injections are found to be associated with serious complications such as endophtalmitis, uveitis, or transient rise in intraocular pressure, uptake of the new technology would likely to be lower. Fourth, the study considered only 1 type of health technology as an alternative to eye drops. If more than 1 alternative to daily drops becomes available, the market would be segmented among all competitors and the uptake for this or any particular technology would be lower.
One significant strength of our study was the ability to model the likelihood of a physician recommendation. The highly significant coefficient associated with physician recommendation in the mixed logit results revealed that this variable is an important predictor of patient demand. As such, it is important to incorporate the likelihood of physician recommendations in estimates of patient uptake. Future studies on technologies that are also highly influenced by a physician's recommendation could incorporate a similar approach by combining results of both patient and physician surveys.
In summary, despite being an elective procedure, results suggest that there is significant interest in an injectable solution or other sustained-release alternatives to daily eye drops. However, patient demand will be greatly influenced by the extent of third-party reimbursement that minimizes out-of-pocket costs, the interval between administrations, and whether or not the procedure is recommended by their physician.
Acknowledgment
The authors thank Ms Isha Chaudhry for her excellent help with the survey development and analysis of the data.
Supplementary Material
A waiver of consent was given by the Institutional Review Board as both surveys were one-time anonymous surveys that posed no more than minimal risk to subjects.
Abbreviations: AGS = American Glaucoma Society, DCE = discrete choice experiment, FDA = Food and Drug Administration.
Financial support for data collection for this study was provided by a contract between Peregrine Ophthalmic Pte Ltd and Dr EAF as a private consultant. The funding agreement ensured the independence in designing the study, interpreting the data, writing the manuscript, and publishing the results.
Dr TTW is a cofounder of Peregrine Ophthalmic Pte Ltd.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Olthoff CM, Schouten JS, van de Borne BW, et al. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension: an evidence-based review. Ophthalmology 2005;112:953–61. [DOI] [PubMed] [Google Scholar]
- [3].Lavik E, Kuehn M, Kwon Y. Novel drug delivery systems for glaucoma. Eye 2011;25:578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chou S-F, Luo L-J, Lai J-Y. Gallic acid grafting effect on delivery performance and antiglaucoma efficacy of antioxidant-functionalized intracameral pilocarpine carriers. Acta Biomater 2016;38:116–28. [DOI] [PubMed] [Google Scholar]
- [5].Lai J-Y, Hsieh A-C. A gelatin-g-poly (N-isopropylacrylamide) biodegradable in situ gelling delivery system for the intracameral administration of pilocarpine. Biomaterials 2012;33:2372–87. [DOI] [PubMed] [Google Scholar]
- [6].Saccà SC, Rolando M, Marletta A, et al. Fluctuations of intraocular pressure during the day in open-angle glaucoma, normal-tension glaucoma and normal subjects. Ophthalmologica 1998;212:115–9. [DOI] [PubMed] [Google Scholar]
- [7].Gurmankin AD, Baron J, Hershey JC, et al. The role of physicians’ recommendations in medical treatment decisions. Med Decis Making 2002;22:262–71. [DOI] [PubMed] [Google Scholar]
- [8].Dong D, Ozdemir S, Bee YM, et al. Measuring high-risk patients’ preferences for pharmacogenetic testing to reduce severe adverse drug reaction: a discrete choice experiment. Value Health 2016;19:767–75. [DOI] [PubMed] [Google Scholar]
- [9].Hall J, Fiebig DG, King MT, et al. What influences participation in genetic carrier testing? Results from a discrete choice experiment. J Health Econ 2006;25:520–37. [DOI] [PubMed] [Google Scholar]
- [10].Brody JR, Kern SE. Stagnation and herd mentality in the biomedical sciences. Cancer Biol Ther 2004;3:903–10. [DOI] [PubMed] [Google Scholar]
- [11].Fred HL. Elephant medicine revisited. Tex Heart Inst J 2008;35:385. [PMC free article] [PubMed] [Google Scholar]
- [12].Spence D. Herd mentality. BMJ 2011;343:d5820. [DOI] [PubMed] [Google Scholar]
- [13].Hall J, Kenny P, Hossain I, et al. Providing informal care in terminal illness an analysis of preferences for support using a discrete choice experiment. Med Decis Making 2014;34:731–45. [DOI] [PubMed] [Google Scholar]
- [14].Johnson FR, Özdemir S, Mansfield C, et al. Crohn's disease patients’ risk-benefit preferences: serious adverse event risks versus treatment efficacy. Gastroenterology 2007;133:769–79. [DOI] [PubMed] [Google Scholar]
- [15].Brown DS, Finkelstein EA, Brown DR, et al. Estimating older adults’ preferences for walking programs via conjoint analysis. Am J Prev Med 2009;36:201–7. [DOI] [PubMed] [Google Scholar]
- [16].Johnson FR, Ozdemir S, Phillips KA. Effects of simplifying choice tasks on estimates of taste heterogeneity in stated-choice surveys. Soc Sci Med 2010;70:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].McFadden D. The choice theory approach to market research. Marketing Sci 1986;5:275–97. [Google Scholar]
- [18].Quill TE, Brody H. Physician recommendations and patient autonomy: finding a balance between physician power and patient choice. Ann Intern Med 1996;125:763–9. [DOI] [PubMed] [Google Scholar]
- [19].Trivedi AN, Rakowski W, Ayanian JZ. Effect of cost sharing on screening mammography in Medicare health plans. N Engl J Med 2008;358:375–83. [DOI] [PubMed] [Google Scholar]
- [20].Lillard LA, Rogowski J, Kington R. Insurance coverage for prescription drugs: effects on use and expenditures in the Medicare population. Med Care 1999;37:926–36. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


