Abstract
The objective of this study was to evaluate the impact of occupation and education level of Chinese female breast cancer patients on their cancer staging at diagnosis, clinical and pathological features, rate of implementation, and selection of treatment.
The medical charts of 4211 confirmed female breast cancer cases diagnosed between 1999 and 2008, from 7 breast cancer centers spread across the whole of China, were reviewed. Data including information on the patient's sociodemographic status, clinical and pathological characteristics, implementation of clinical examination and treatment modalities were analyzed. In parallel, the associations between different occupations and level of educational attainment were analyzed in relation to tumor stage through TNM staging, clinical and pathological characteristics, implementation of clinical examination, and treatment patterns. Multivariate logistic regression was used to identify whether the occupation and education level of patients are independent factors of TNM staging at diagnosis.
There were significant differences among different occupation groups and the education level of patients in regards to pathological characteristics and treatment choice. Both the occupation and education level of patients were independent factors of TNM staging at diagnosis. For patients within the lower-income occupation or lower educational attainment group, the tumor stage was later, the rates of implementation of relevant investigations were lower, as were the rates of radiotherapy, chemotherapy, and endocrine therapy.
This study suggests that strategies should work toward developing more accurate and effective breast cancer prevention and treatment strategies aimed specifically at patients with lower educational attainment levels and at specific occupation groups.
Keywords: breast cancer, clinical and pathological characteristics, education level, occupation, TNM staging, treatment patterns
1. Introduction
Breast cancer is the most common cancer and the leading cause of cancer death among females worldwide, accounting for 25% of all cancer cases and 15% of all cancer-related deaths among females.[1] In 2012, there were nearly 190,000 new diagnoses of breast cancer in China alone and >47,000 deaths representing a substantial economic and societal burden.[2,3] Early detection and early treatment of breast cancer have been shown to among the most effective methods to address the impact of this devastating disease.[3]
Substantial evidence indicates that the socioeconomic status (SES) of breast cancer patients has a significant impact on prognosis through its associated influence on the cancer stage at diagnosis. Previous findings suggest people with lower incomes have a later cancer stage at point of diagnosis and a worse overall prognosis.[4–6] Socioeconomic status is also significantly associated with education level and occupation, both of which can greatly influence patients’ perception of the tumor, thereby affecting the level of early detection, diagnosis, and treatment.[7,8] Our previous single-center study confirmed that occupation and education level of patients had a significant impact on TNM staging at diagnosis, clinical and pathological characteristics, rate of implementation of clinical examination, and the selection of treatment modalities.[9] We hypothesized that similar findings also existed across the whole of China.
This study looked to further confirm these associations in an epidemiological retrospective study of 10 years (1999–2008) with the participation of 7 regional representative hospitals in China, which related data can represent the status quo of diagnosis and treatment of breast cancer in recent China.[5,10–12] These data will enable us to adapt diagnosis and treatment modalities for patients with different social backgrounds to improve their overall prognosis. What's more, as China is a typical developing country, the results can also provide a reference for other developing countries to formulate corresponding policies of diagnosis and treatment.
2. Methods
2.1. Ethics statement
This study was approved by the institutional review board, Cancer Foundation of China. Patient consent was not required for this study because there were no anticipated risks for the participants of the study. All patient identifiers were removed per the board-approved procedures. Deidentified data were maintained in a secure database, to which only research team members had access.[13]
2.2. Selection of hospitals and patients
Hospital selection and case sampling methods have been previously described in detail.[13] In brief, China was stratified into 7 geographical areas (north, northeast, northwest, east, central, south, and southwest). We selected 1 tertiary public cancer hospital in each region with the following characteristics. First of all, participant hospitals had to be the leading regional public cancer hospitals and referral centers that provide pathology diagnosis, surgery, medical oncology, radiotherapy, and routine follow-up care for breast cancer patients. Second, patients of the selected hospitals must include the entire study region. Another criterion was that the hospitals had to be located in a major city.[13]
The participant hospitals provided us with the medical records of female breast cancer patients diagnosed during 1999 to 2008. One month was randomly selected within each year and all inpatient cases for that month were reviewed. January and February were excluded because Chinese traditional spring festival always fall in these 2 months and there are much fewer inpatients during this period.[13] All cases within the selected month were reviewed and patient's information was collected based on the designed case report form (CRF). If qualifying cases were <50 in a selected month, additional cases from the following months were reviewed until the total number reaches 50. Whereas, if the number of patients exceeded 50 in the selected month, all cases should be reviewed.[13] Patients’ information was collected based on the standard CRF designed by the Cancer Institute of the Chinese Academy of Medical Sciences. As such, a total of 4211 female breast cancer patients were included in the study.[5,10–12]
2.3. Pathology diagnostic criteria
The histological subtype categorization was based on the 1981 and 2003 WHO histological classification criteria.[11,14] Staging of breast cancer was categorized according to the American Joint Committee on Cancer (AJCC) TNM System of year 1997[15] and 2002.[16] The specific details were described in our previous study,[13] and we grouped the staging of breast cancer into 2 categories: “early” (stages 0, I, and II) or “advanced” (stages III and IV).[13]
2.4. Categorization of occupation and education level
According to our previous study, the occupations of patients were classified into 5 groups: manual worker, housewife, private sector worker, professional, and unspecified.[9] By convention, we grouped the education levels of patients into 6 categories: none (never having received any formal education), those having received formal education of primary, middle, high, or university, and above, and unspecified.[13,17]
2.5. Statistical analysis
Descriptive statistics were used to summarize the clinical and pathologic characteristics, implementation of clinical examination, and treatment options of the study population. Measurement data were expressed as mean ± standard deviation (SD), and the differences in the distribution of variables among groups were analyzed using χ2 tests. Additionally, multivariate logistic regression analysis was performed to further explore the associations between individual demographic characteristics and breast cancer stage at diagnosis (advanced stage vs. early stage). SPSS statistical software version 17.0 (SPSS Inc, Chicago, IL) was used to analyze the data. A 2-tailed P < 0.05 was considered statistically significant.
3. Results
3.1. General information of patients
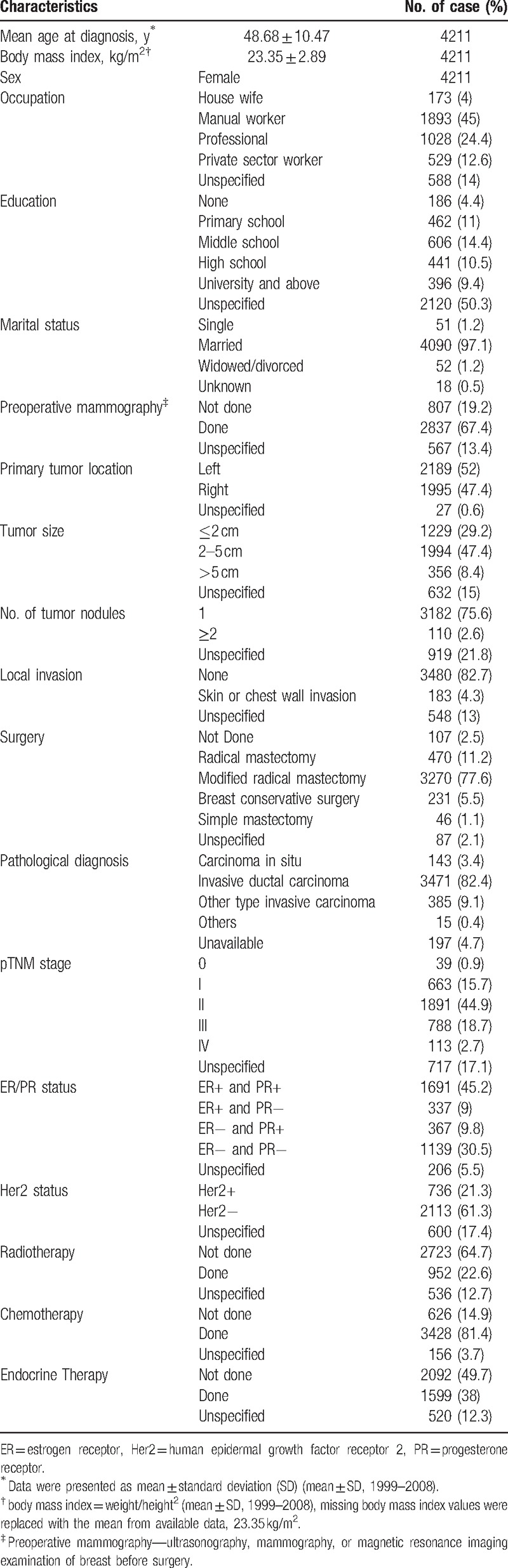
A total of 4211 cases were included in this multicenter study, in which the distribution of patient's occupation within the 5 categories was composed of 4% housewives (173/4211), 45% manual workers (1893/4211), 24.4% professionals (1028/4211), 12.6% private sector workers (529/4211), and 14% unspecified (588/4211). The proportion of patients’ levels of education were 4.4% uneducated (186/4211), 11% primary (462/4211), 14.4% middle school (606/4211), 10.5% high school (441/4211), 9.4% university and above (396/4211), and 50.3% unknown (2120/4211). Further details were shown in Table 1 including the average age at diagnosis, mean body mass index (BMI), clinical and pathologic characteristics, and treatment patterns of all patients.
Table 1.
Overview of characteristics of the 4211 breast cancer patients included in the study.
3.2. Occupation
3.2.1. Comparison of clinical and pathologic characteristics among different occupations
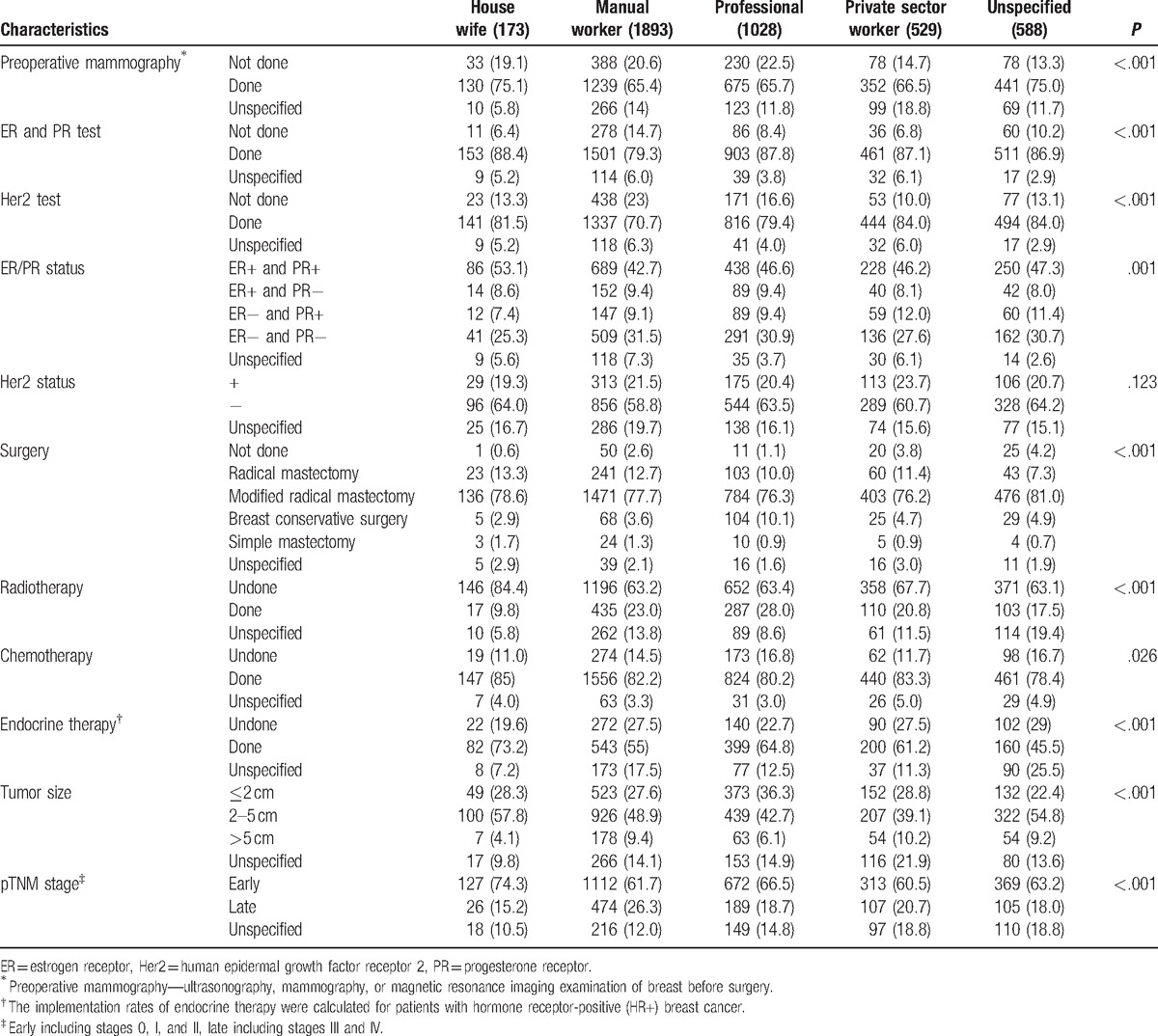
There were significant differences between different occupations in several clinical and pathological characteristics in this study. For tumor size, the proportion of professionals with tumors <2 cm was 36.3%, significantly higher than in other occupations (P < .001); for comparison, the proportion of manual workers was 27.6%. Significant differences were also seen with regards to TNM staging. Manual workers had fewer reports of early breast cancer (61.7%) and the highest proportion of advanced breast cancer (26.3%) (P < .001). Hormone receptor (HR) expression status (estrogen receptor [ER] and/or progesterone receptor [PR]) was significantly different (P = .001), with manual workers having the lowest HR-positive rate (61.2%) and the highest negative rate (31.5%). No significant differences were observed in expression of human epidermal growth factor receptor 2 (Her2) status among different occupations (P = .123) (Table 2).
Table 2.
Comparison of different characteristics amongst occupations.
3.2.2. Comparison of implementation of clinical examination among different occupations
The data in Table 2 showed there were significant differences between different occupations in the preoperative imaging implementation rate (underwent breast ultrasound, mammography or magnetic resonance imaging [MRI] before surgery), and the implementation rate of postoperative detection of ER/PR and Her2 (P < .001), which both showed similar trends that the implementation rate of manual workers was lower than that of professionals (65.4% vs. 65.7%; 79.3% vs. 87.8%; 70.7% vs. 79.4%).
3.2.3. Comparison of treatment patterns among different occupations
The above results indicated that breast cancer patients with different occupations had significant differences in their clinical and pathological characteristics and implementation rate of related evaluation, which may further affect patients and doctor's choice of treatment patterns. The results in Table 2 showed first that the choice of surgical approach of patients in different occupation groups was significantly different (P < .001), in which implementation of radical surgery (radical mastectomy + modified radical mastectomy) in the professionals group was lowest (86.3%), whereas implementation of breast-conserving surgery was highest (10.1%). In contrast, corresponding implementation rates in manual workers were 90.4% and 3.6%, respectively. Second, there were significant differences (P < .001) between patients with different occupations in considering whether to receive radiotherapy; the implementation rates were 28% for professionals, 23% for manual workers, 20.8% for private sector staff, and 9.8% for housewives. Third, patients within different occupation groups also showed significant differences (P = .026) in whether they received chemotherapy; the implementation rates were 85% for housewives, 83.3% for private sector staff, 82.2% for manual workers, and 80.2% for professionals. Fourth, finally there were significant differences in whether patients with hormone-receptor positive (HR+) breast cancer received endocrine therapy among the different occupation groups (P < .001) with implementation rates of 73.2% for housewives, 64.8% for professionals, 61.2% for private sector staff, and 55% for manual workers.
3.3. Education level
3.3.1. Comparison of clinical and pathologic characteristics of patients with differing education levels
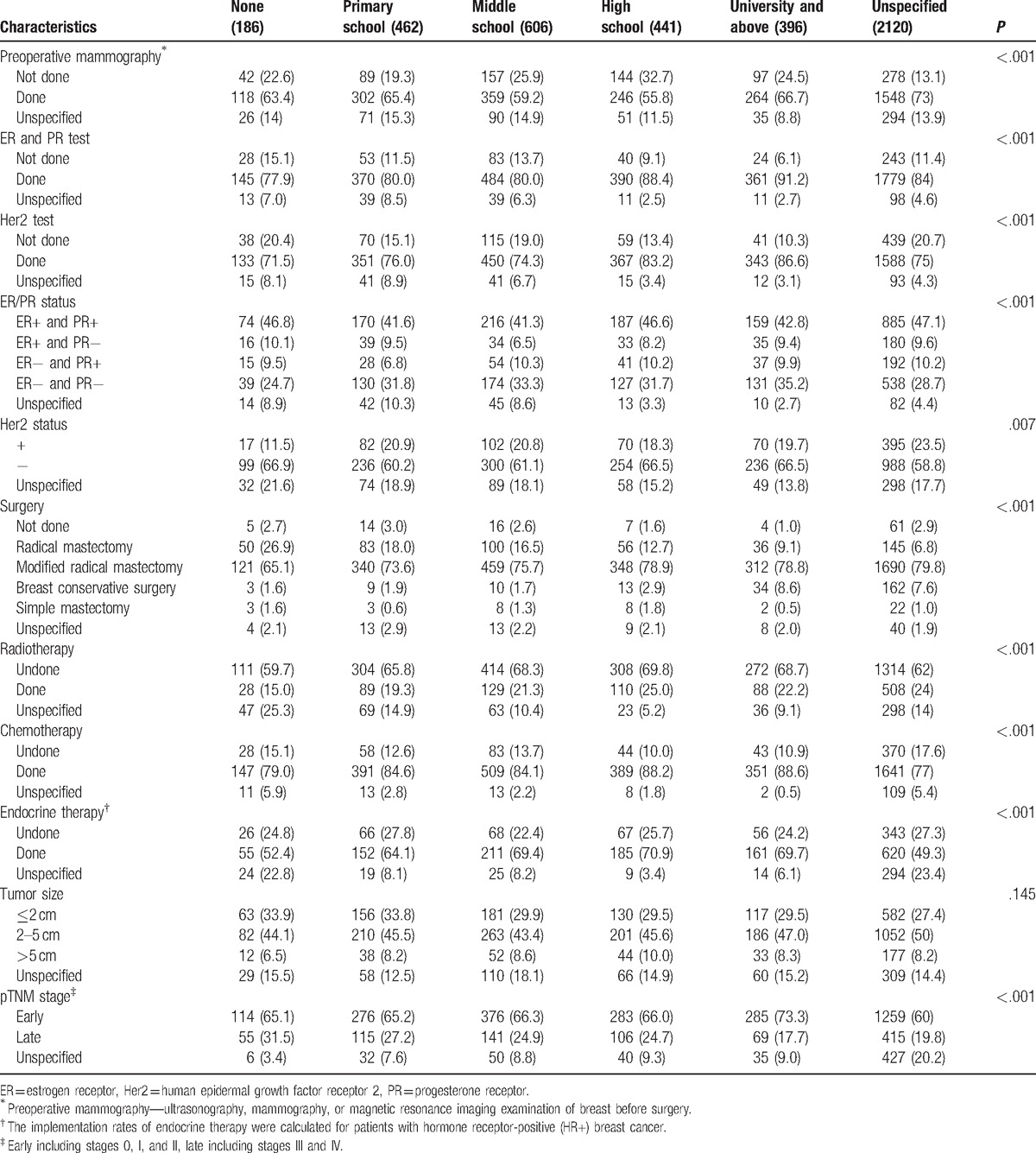
The data in Table 3 showed that different education backgrounds had a significant impact on TNM staging distribution (P < .001); the group with the highest rate of early breast cancer was those educated to university and above (73.3%) whilst the lowest was within the uneducated group (65.1%). Conversely the highest rate of advanced breast cancer at diagnosis was found within the uneducated group (31.5%) and the lowest was university and above (17.7%). There were no significant differences in other clinical and pathologic characteristics such as tumor size for each group. Different levels of education did however have a significant impact on HR (ER and/or PR) expression status (P < .001), in which the highest HR-positive rate was within the uneducated group (66.4%). The highest HR-negative rate was found in the university and above education group (35.2%) and the lowest was within the uneducated group (24.7%). The status of Her2 expression between different groups also showed significant differences (P = .007); the uneducated group had the lowest Her2 positive rate (11.5%) and the highest negative rate (66.9%).
Table 3.
Comparison of different characteristics amongst education levels.
3.3.2. Comparison of implementation of clinical examination across different education levels
The data in Table 3 showed there were significant differences among different education backgrounds in the implementation rate of preoperative imaging examination and postoperative detection of ER/PR and Her2 (P < .001). The implementation rate of preoperative imaging examination was highest in the group of education level of university and above (66.7%) and the lowest was within the high school group (55.8%). And the overall trend of implementation of detecting ER/PR and Her2 was that the higher the education level was, the higher the implementation rate of detection (Table 3).
3.3.3. Comparison of treatment patterns across different education levels
Different education levels may result in different awareness of breast cancer which can affect the patient's choice in treatment. The data in Table 3 indicated that the implementation rate of radical surgery (radical mastectomy + modified radical mastectomy) was lower in groups with higher educational status. Nevertheless, the higher the education level was, the higher the rate of implementation of breast-conserving surgery. Patients with different education backgrounds also had significant differences in implementation of radiotherapy, chemotherapy, and endocrine therapy (P < .001) (Table 3).
3.3.4. Multivariate analysis
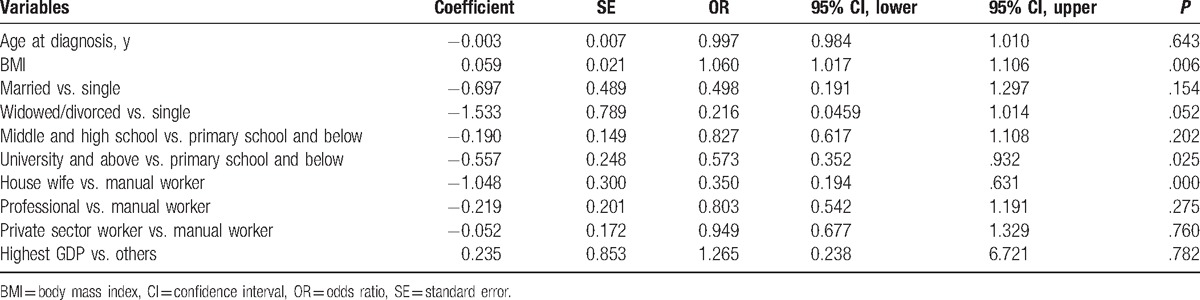
Univariate analysis described above showed that occupation and education levels of patients can significantly affect TNM staging of breast cancer at diagnosis. To determine whether the occupation and education status of patients were independent factors affecting tumor stage, we next conducted multivariate analysis. In response to the results of previous studies, we included a number of other factors, which may be related to TNM staging such as age at diagnosis, BMI, marital status (divided into 3 types: single, married, and widowed or divorced) at diagnosis, and regional Gross Domestic Product (GDP).[5,10] As GDP of Beijing is much higher than across other areas within the study, we classified this area as a separate category.[5]
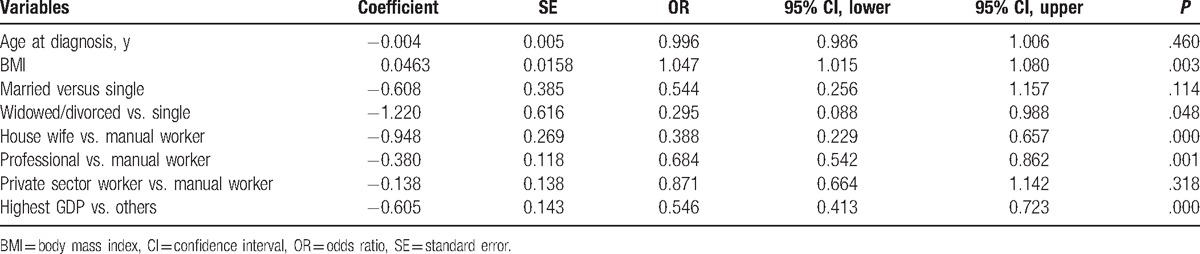
Owing to incomplete data regarding education status in Beijing, we did not include education level in the first multivariate analysis. The results of the 2343 cases included in the multivariate analysis (1787 early cases and 556 advanced cases) suggested that BMI, marital status, occupation, and GDP were all independent factors affecting the TNM staging at diagnosis, within which BMI was an independent risk factor. In the case of controlling other risk factors, each unit increase in BMI was associated with 4.7% increased risk of an advanced breast cancer. Compared to manual workers, the other occupations were protective factors. Compared with the unmarried group, divorced or widowed were protective factors. Finally, compared with the low GDP regions, high GDP was a protective factor (Table 4).
Table 4.
Multivariable logistic regression for association between individual demographic characteristics and breast cancer stage (stage 0, I, and II, stages III and IV) at diagnosis.
A smaller subset for which we were able to gain education status information was then analyzed through multivariate analysis. We classified the education level for this analysis into 3 types, including uneducated and primary education, middle and high school education, and universities and above. We included a total of 1431 cases this time (1074 early cases and 357 advanced cases). The results suggested that BMI, education, and occupation were independent factors affecting tumor staging, in which BMI was once again an independent risk factor. Compared with primary education, University and above education was an independent protective factor. Compared to manual workers, housewives were independent protective factors, whereas the professionals were not (Table 5). Owing to some debate over the classification of clinical stage of breast cancer, wherein certain researchers believe early stage should be defined as stage 0 and I only, with the remaining stages being classified as advanced stage,[5] we also adopted this variation of classification to analyze the data a second time. The results showed that occupation and education were still independent factors that influence tumor stage, which is consistent with the results of the analysis using the initial classification.[5]
Table 5.
Multivariable logistic regression for association between individual demographic characteristics and breast cancer stage (stage 0, I, and II, stages III and IV) at diagnosis (including education level).
4. Discussion
With the rapid growth of China's economy, the levels of economic development across different regions show significant imbalance. Previous studies indicate that socioeconomic status of the region can influence the diagnosis and treatment of breast cancer which can affect course and prognosis.[4,5,18] Our early single-center study in China's Shaanxi Province showed that the occupation and education of patients with breast cancer had a significant impact on TNM staging, clinical and pathological features, implementation of clinical examination, and the selection of treatment patterns.[9] To further confirm these associations, we conducted this novel multicenter study in which the results confirmed that our previously observed associations also existed across the whole of China.
A number of studies indicate that occurrence and development of breast cancer are closely related to the level of economic income. For example, higher income groups have a higher incidence of early breast cancer diagnosis and a better prognosis. Income level is closely related to occupation and education level.[9] In this study, we found that the diameters of tumor size of patients in the professional occupation group were smaller, and the proportion of early breast cancer at diagnosis was higher, which was similar to the results within the higher educated groups. We and several other groups have previously reported this phenomenon.[9,19,20]
There are several potential explanations for the observed results. Individuals in a professional occupation and those that are well educated tend to live in more developed cities and subsequently have a higher probability of receiving breast cancer screening.[21,22] Apart from organizational and funding obstacles, inherent reluctance from cultural barriers and cancer fatalism in Chinese women often hampers screening efforts, particularly in older women.[23,24] Failure to screen regularly leads to the loss of opportunities for early detection of breast cancer, and as a consequence, increases treatment costs which leads to greater economic burden at a personal and societal level. At present, the early discovery rate of breast cancer in a developing country such as China has large disparities with that of developed countries. Findings from a study in Beijing showed that only 5.2% of new cases were detected by routine mammographic screening, whereas 82.1% of women who were diagnosed had experienced obvious symptoms. Conversely, the proportion of breast cancers detected through screening is about 60% in the United States.[25,26] This significant delay in diagnosis results in prolonged treatment time of patients, resulting in a worse prognosis. Individuals in professional occupations and those who have received higher education have more possibilities to obtain appropriate medical knowledge; subsequently, they can recognize the symptoms of breast cancer earlier.[27,28] In addition, they also have sufficient income to access preoperative examination, so implementation rate of their preoperative examination may be higher, and as a consequence, they could find breast cancer earlier. Our study confirmed that the rate of implementation of the relevant presurgery examination was the lowest in manual workers. For education, the highest execution rate was in the higher group of education level of university and above.
Interestingly, in this study, we found that manual laborers had the lowest HR (ER and/or PR) positive rate and the highest HR-negative rate. When considering the impact of education levels on HR rates, the highest HR-positive rate was within the uneducated group. The results of the remaining 4 groups showed that patients with higher education levels exhibited higher HR-positive rate. This result was consistent with reports that breast cancer patients in a better socioeconomic status had a higher proportion of ER-positive.[29,30] The possible reason is that the occupation and education level are closely related to BMI of patients. Generally, manual laborers in China are more likely to have low BMI because of extensive physical workload. In comparison, patients with higher education levels may have higher BMI because of superior living conditions and lack of time for physical activities.[31] It is reported that higher BMI is associated with increased risk of HR-positive breast cancer in postmenopausal women.[32,33] In addition, we also found that uneducated individuals had the highest rate of Her2 negative expression and patients in the group of education level of university and above had the highest HR-negative rate. But at present, we have no reasonable explanations for the findings, and as such it requires further study.
Economic levels, awareness of breast cancer, and the clinical and pathological features of patients can affect the doctors’ and patients’ choice of treatment patterns. In this study, we found there were significant differences among the different groups when choosing treatment patterns. First, professionals tended to prefer breast-conserving surgery rather than radical surgery in the operation mode selection. The reason could be that professionals tend to have earlier tumor stage at diagnosis, they can accept new ideas and new technologies easier, and they have perceived higher requirements of quality of life after surgery, which means they are more receptive to sentinel lymph node biopsy and breast-conserving surgery.[34,35] Manual workers and housewives would delay their treatment because of lower income[27,36]; therefore the tumor stage is later at diagnosis, so they lose the opportunity for sentinel lymph node biopsy and breast-conserving surgery. In addition, they cannot afford the cost of breast-conserving surgery and postoperative radiotherapy.[35] Second, people of different occupations had significant differences in the selection of radiotherapy, chemotherapy and endocrine therapy. The implementation rate of radiotherapy and endocrine therapy in the professional group were high which is similar to our previous findings,[9] but their minimum implementation rate of chemotherapy is somewhat different with our previous works.[9] The possible reasons include 3 reasons. First, professionals have a higher proportion of breast-conserving surgery and radiation therapy is standard treatment after breast-conserving surgery.[37,38] Second, professionals have a higher HR-positive rate which results in a higher proportion receiving endocrine therapy. In addition, because endocrine treatment needs spanning for 5 to 10 years, patients’ affordability and awareness of the disease can also affect their compliance.[39] Third, professionals have a high proportion of early breast cancer and subsequently some of them do not require chemotherapy, such as the case with carcinoma in situ.[40]
In this multicenter study, we also found that there were significant differences among breast cancer patients with different education backgrounds regarding their choice of treatment patterns, in which the rate of breast-conserving surgery was highest in the highest education levels. The opposite trend was observed for radical surgery. In addition, we found that the highest implementation rate of breast-conserving surgery was only 8.6%, which is still significantly lower than within the United States and other developed countries. At present, lymph node biopsy and breast-conserving surgery have become the main treatments for breast cancer in developed countries[41,42] with a rate of >50% in the United States, >30% in Japan, and 70% to 80% in Singapore.[9,43] Such findings suggest that we should further intensify breast-conserving surgery so that more patients can benefit from advanced surgical treatment.
This study also exhibits some shortcomings. First, the hospital and month may unavoidably exhibit some degree of selection bias. Second, there are some serious missing data such as lost education information in Beijing, which will have some impact on the conclusions. To weaken the influence of missing data, we conducted 2 multivariate analyses without or with education status information. We found that occupation and education were independent factors affecting the TNM staging at diagnosis. And the results were to a certain extent convincing; what is most regrettable is that there are no follow-up clinical data which are very limited for study of the prognosis. We plan to collect the follow-up data in a future study. But after all, this is the first nationwide representative clinical research on breast cancer.
In summary, the prognosis of breast cancer patients is affected by the stage at diagnosis and standard of treatment patterns. This study showed that occupation and education have a significant impact on these 2 key prognostic factors, suggesting efforts should be made to address imbalances across different socioeconomic groups. This could be addressed through increases in regular breast cancer screening programs among individuals with lower-income occupations or lower education levels and improve the coverage and penetration of screening to improve early detection rate. Second, there is a need to strengthen publicity of breast cancer-related knowledge for lower-income occupational groups or those with lower education levels to enable them to better understand the importance of recognizing early clinical symptoms and treatment options. The results of this study also suggest that changes regarding health insurance policy may be necessary with the proportion of reimbursements needing to be higher for those with lower-income occupations or lower education levels so to ensure they do not encounter financial barriers that stop them from reporting their symptoms earlier and subsequently receiving more timely and comprehensively treatment. These recommendations are also significant for other developing countries.
5. Conclusions
We found that occupation and education level have a significant impact on the TNM staging, clinical and pathological features, implementation of examination, and the selection of treatment patterns and occupation and education level of patient are independent factors for TNM staging at diagnosis. These suggest that the government should focus on characteristics of lower-income occupation and lower educational attainment group to develop more accurate and effective prevention and treatment strategies for breast cancer.
Acknowledgments
The authors thank all the patients who participate in this study. We thank the local investigators from Beijing, Liaoning (Shenyang), Zhejiang (Hangzhou), Hunan (Changsha), Shaanxi (Xi’an), Guangdong (Guangzhou), and Sichuan (Chengdu) for data collection and assisting them in the successful completion of the project.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, BMI = body mass index, CI = confidence interval, CRF = case report form, ER = estrogen receptor, Her2 = human epidermal growth factor receptor 2, MRI = magnetic resonance imaging, OR = odds ratio, PR = progesterone receptor, SE = standard error.
The authors report no conflicts of interest.
References
- [1].Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. [DOI] [PubMed] [Google Scholar]
- [2].Peng Z, Wei J, Lu X, et al. Treatment and survival patterns of Chinese patients diagnosed with breast cancer between 2005 and 2009 in Southwest China: an observational, population-based cohort study. Medicine (Baltimore) 2016;95:e3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang F, Yu Z. Current status of breast cancer prevention in China. Chronic Diseases and Translational Medicine 2015;1:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Macleod U, Ross S, Gillis C, et al. Socio-economic deprivation and stage of disease at presentation in women with breast cancer. Ann Oncol 2000;11:105–7. [DOI] [PubMed] [Google Scholar]
- [5].Wang Q, Li J, Zheng S, et al. Breast cancer stage at diagnosis and area-based socioeconomic status: a multicenter 10-year retrospective clinical epidemiological study in China. BMC Cancer 2012;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wilf-Miron R, Peled R, Yaari E, et al. The association between socio-demographic characteristics and adherence to breast and colorectal cancer screening: analysis of large sub populations. BMC Cancer 2011;11:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chung HW, Noh SH, Lim JB. Analysis of demographic characteristics in 3242 young age gastric cancer patients in Korea. World J Gastroenterol 2010;16:256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Baade PD, Aitken JF, Ferguson M, et al. Diagnostic and treatment pathways for men with prostate cancer in Queensland: investigating spatial and demographic inequalities. BMC Cancer 2010;10:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang K, Li X, Zhou C, et al. Socio-economic factors influencing tumor presentation and treatment options in Chinese breast cancer patients. Asian Pac J Cancer Prev 2013;14:267–74. [DOI] [PubMed] [Google Scholar]
- [10].Lee H, Li JY, Fan JH, et al. Risk factors for breast cancer among Chinese women: a 10-year nationwide multicenter cross-sectional study. J Epidemiol 2014;24:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kong Y, Yang L, Tang H, et al. A nation-wide multicenter retrospective study of the epidemiological, pathological and clinical characteristics of breast cancer in situ in Chinese women in 1999-2008. PLoS One 2013;8:e81055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhou C, He J, Li J, et al. A nation-wide multicenter 10-year (1999-2008) retrospective clinical study of endocrine therapy for Chinese females with breast cancer. PLoS One 2014;9:e100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li J, Zhang BN, Fan JH, et al. A nation-wide multicenter 10-year (1999-2008) retrospective clinical epidemiological study of female breast cancer in China. BMC Cancer 2011;11:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bocker W. WHO classification of breast tumors and tumors of the female genital organs: pathology and genetics. Verh Dtsch Ges Pathol 2002;86:116–9. [PubMed] [Google Scholar]
- [15].Fleming ID. American Joint Committee on Cancer AJCC Cancer Staging Manual. 1997;Philadelphia:Lippincott-Raven, 5. [Google Scholar]
- [16].Greene FL. American Joint Committee on Cancer AJCC Cancer Staging Manual. 2002;New York:Springer Verlag, 6. [Google Scholar]
- [17].Norsa’adah B, Rampal KG, Rahmah MA, et al. Diagnosis delay of breast cancer and its associated factors in Malaysian women. BMC Cancer 2011;11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Saxena N, Hartman M, Bhoo-Pathy N, et al. Breast cancer in South East Asia: comparison of presentation and outcome between a middle income and a high income country. World J Surg 2012;36:2838–46. [DOI] [PubMed] [Google Scholar]
- [19].Huang Y, Zhou K, Li H, et al. Knowledge, attitudes, and behaviour regarding breast cancer screening among women from different socio-economic regions in southwest China: a cross-sectional study. Asian Pac J Cancer Prev 2011;12:203–9. [PubMed] [Google Scholar]
- [20].Damiani G, Federico B, Basso D, et al. Socioeconomic disparities in the uptake of breast and cervical cancer screening in Italy: a cross sectional study. BMC Public Health 2012;12:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Barry J, Breen N. The importance of place of residence in predicting late-stage diagnosis of breast or cervical cancer. Health Place 2005;11:15–29. [DOI] [PubMed] [Google Scholar]
- [22].Sekikawa A, Curb JD, Edmundowicz D, et al. Coronary artery calcification by computed tomography in epidemiologic research and cardiovascular disease prevention. J Epidemiol 2012;22:188–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wardle J, Steptoe A. Socioeconomic differences in attitudes and beliefs about healthy lifestyles. J Epidemiol Community Health 2003;57:440–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tang TS, Solomon LJ, McCracken LM. Cultural barriers to mammography, clinical breast exam, and breast self-exam among Chinese-American women 60 and older. Prev Med 2000;31:575–83. [DOI] [PubMed] [Google Scholar]
- [25].Yuan XM, Wang N, Ouyang T, et al. Current status of diagnosis and treatment of primary breast cancer in beijing, 2008. Chin J Cancer Res 2011;23:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Breen N, Yabroff KR, Meissner HI. What proportion of breast cancers are detected by mammography in the United States? Cancer Detect Prev 2007;31:220–4. [DOI] [PubMed] [Google Scholar]
- [27].Bish A, Ramirez A, Burgess C, et al. Understanding why women delay in seeking help for breast cancer symptoms. J Psychosom Res 2005;58:321–6. [DOI] [PubMed] [Google Scholar]
- [28].Grunfeld EA, Hunter MS, Ramirez AJ, et al. Perceptions of breast cancer across the lifespan. J Psychosom Res 2003;54:141–6. [DOI] [PubMed] [Google Scholar]
- [29].Twelves CJ, Thomson CS, Gould A, et al. Variation in the survival of women with breast cancer in Scotland. The Scottish Breast Cancer Focus Group and The Scottish Cancer Therapy Network. Br J Cancer 1998;78:566–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thomson CS, Hole DJ, Twelves CJ, et al. Prognostic factors in women with breast cancer: distribution by socioeconomic status and effect on differences in survival. J Epidemiol Community Health 2001;55:308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pampel FC, Denney JT, Krueger PM. Obesity, SES, and economic development: a test of the reversal hypothesis. Soc Sci Med 2012;74:1073–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Suzuki R, Rylander-Rudqvist T, Ye W, et al. Body weight and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status among Swedish women: a prospective cohort study. Int J Cancer 2006;119:1683–9. [DOI] [PubMed] [Google Scholar]
- [33].Phipps AI, Buist DS, Malone KE, et al. Breast density, body mass index, and risk of tumor marker-defined subtypes of breast cancer. Ann Epidemiol 2012;22:340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Keating NL, Weeks JC, Borbas C, et al. Treatment of early stage breast cancer: do surgeons and patients agree regarding whether treatment alternatives were discussed? Breast Cancer Res Treat 2003;79:225–31. [DOI] [PubMed] [Google Scholar]
- [35].Gilligan MA, Kneusel RT, Hoffmann RG, et al. Persistent differences in sociodemographic determinants of breast conserving treatment despite overall increased adoption. Med Care 2002;40:181–9. [DOI] [PubMed] [Google Scholar]
- [36].Grunfeld EA, Ramirez AJ, Hunter MS, et al. Women's knowledge and beliefs regarding breast cancer. Br J Cancer 2002;86:1373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].van Maaren MC, de Munck L, de Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol 2016;17:1158–70. [DOI] [PubMed] [Google Scholar]
- [38].Eaton BR, Jiang R, Torres MA, et al. Benefit of adjuvant radiotherapy after breast-conserving therapy among elderly women with T1-T2N0 estrogen receptor-negative breast cancer. Cancer 2016;122:3059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Johnston SR. Enhancing endocrine therapy for hormone receptor-positive advanced breast cancer: cotargeting signaling pathways. J Natl Cancer Inst 2015;107:djv212. [DOI] [PubMed] [Google Scholar]
- [40].Stuart KE, Houssami N, Taylor R, et al. Long-term outcomes of ductal carcinoma in situ of the breast: a systematic review, meta-analysis and meta-regression analysis. BMC Cancer 2015;15:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Povoski SP, Jimenez RE, Wang WP, et al. Standardized and reproducible methodology for the comprehensive and systematic assessment of surgical resection margins during breast-conserving surgery for invasive breast cancer. BMC Cancer 2009;9:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Goyal A, Mansel RE. Recent advances in sentinel lymph node biopsy for breast cancer. Curr Opin Oncol 2008;20:621–6. [DOI] [PubMed] [Google Scholar]
- [43].Nguyen BC, Alawadi ZM, Roife D, et al. Do socioeconomic factors and race determine the likelihood of breast-conserving surgery? Clin Breast Cancer 2016;16:e93–7. [DOI] [PubMed] [Google Scholar]


