1. Overview
Genomic sequencing studies indicate that the human gastrointestinal (GI) tract is a dynamic repository of about ~1014 micro-organisms that outnumber host cells by at least 100 to 1.1, 2, 3, 4, 5, 6 Collectively, these ~1014 microbes contain about 1017 genes; the number of microbial genes is at least 150-fold greater than the total number of human genes.3, 6, 7, 8, 9, 10 About 98% of the microbiota in the human GI tract consists of anaerobic bacteria, with archaebacteria, fungi, protozoa, prions, viruses of plant and animal origin, viroids and other micro-organisms, and other free nucleic acids, such as microRNAs, making up the remainder. Why evolution selected just 2 of the 54 recognized major divisions of bacteria Bacteroidetes and Firmicutes to populate the human GI tract is both enigmatic and open to speculation—these 2 major phyla represent the “bacterial core” of our microbiome. The ability of Bacteroidetes and Furmicutes to coexist symbiotically, to generate essential vitamins and cofactors, and to process dietary constituents such as fiber may have made them particularly suitable to support human immunity, physiology, biochemistry, and neurochemistry.3, 9, 10, 11, 12 Of these 2 phyla, the Bacteroidetes, the largest class of obligate anaerobic gram-negative (G-) bacteria of the human GI tract, release extraordinarily complex mixtures of amyloids, lipopolysaccharides (LPSs), enterotoxins, and neurotoxins. Microbiome-derived exudates appear to be noxious to multiple aspects of microbiome–host interactions, including (1) GI tract and blood–brain barrier structure and integrity; (2) systemic, central nervous system (CNS), and peripheral nervous system (PNS) homeostasis and equilibrium; and (3) progressive inflammatory degeneration within the human nervous system. Interestingly, unique LPS toxins of the microbiome-abundant Bacteroides fragilis (BF-LPS) and the B. fragilis toxin (BFT) fragilysin are among the most barrier-disruptive and proinflammatory neurotoxins known. This paper overviews some current research and emerging concepts published over the past 6 months on (1) the toxic array of substances generated by B. fragilis; (2) the potential contribution of microbiome-generated factors such as BF-LPS in driving proinflammatory signaling both systemically and within the nervous system; and (3) the recent recognition of the beneficial effects of dietary fiber on the growth and proliferation of B. fragilis and other prolific microbial species in the human GI tract microbiome, with specific reference to Alzheimer's disease (AD) neuropathology wherever possible.
2. BF-LPS amyloids, LPSs, and enterotoxins
A priori, we would like to note that in this paper we are using as the principal illustration a major anaerobic microbial species of the GI tract, B. fragilis, as a prime example of an abundant, G- bacteria of the microbiome; it should be kept in mind that at least 1000 additional species of GI tract bacteria have both a dynamic and opportunistic potential to similarly contribute to (1) the homeostatic maintenance of the GI tract–CNS axis in health and (2) the development of both systemic and inflammatory neurodegenerative disease (Fig. 1). First, B. fragilis produces 3 major classes of toxic secretory products: amyloid, LPS, and enterotoxins. They are briefly defined here.
-
1.
Amyloid. The term amyloid is generic for any insoluble, lipoprotein-enriched molecule exhibiting β-pleated sheet structures oriented perpendicular to its fibrillar axis; a remarkably wide variety of microbiome-resident species, particularly bacteria and fungi, generate significant quantities of functional amyloids and related microbial exudates.6, 7, 8, 9, 10 Interestingly, well over half of all known proteins contain “unstructured” regions of amino acids that are intrinsically amyloidogenic.7, 8, 9, 10, 11 For example, the amyloids that characterize AD consist largely of “perivascular” amyloid deposits enriched in the 40-amino acid Aβ40 peptide; “parenchymal” amyloid, enriched in the 42-amino acid Aβ42 peptide; and “nuclear” amyloids, which contain highly complex mixtures of lipoprotein fibrils and dense amyloid aggregates.9, 10, 11, 12 Interestingly, common tertiary protein structures or pathogenic-associated molecular patterns between microbial and host amyloids (1) may be involved in propagation or acceleration of amyloidogenesis through “molecular mimicry” and (2) may be important in the priming of the host innate immune system and/or microglial cell activation by microbiota, a factor that may enhance inflammatory and immunologic responses to AD amyloid.9, 10, 11, 12, 13, 14, 15, 16
-
2.
LPS. Distinguishing components of the outer leaflet of the outer membrane of G- bacteria shed into the extracellular space, LPSs have historically been thought to play some host-pathogen immune-evasion strategy useful to bacterial survival while eliciting strong immune and proinflammatory responses within the host.8, 10, 17, 18, 19, 20 Although LPSs contain large and hypervariable polysaccharide/oligosaccharide regions, a relatively conserved lipid region (known as the “lipid A” core) is the endotoxic and biologically active moiety that is responsible for the induction of systemic inflammation and ensuing septic shock.9, 10, 11, 12, 14, 15, 16, 17, 18 Interestingly, it has been recently shown by several independent groups that (1) LPS strongly promotes amyloid aggregation,9, 10, 20 and (2) BF-LPS is one of the most potent inducers of nuclear factor kappa B (NF-κB) activation in primary human neuronal-glial cocultures known.17, 18, 19, 20
-
3.
Enterotoxins: fragilysin. This is also known as BFT, a Zn2+-requiring metalloprotease highly cytopathic to intestinal epithelial cells with the following characteristics: (1) possesses broad proteolytic specificity of hydrolytic cleavage adjacent to leucines;17, 18, 19 (2) hydrolyzes gelatin, fibrinogen, and extracellular matrix proteins such as the extracellular domain of type 1 transmembrane proteins such as E-cadherin;15, 19 (3) rapidly degrades intracellular proteins such as actin and myosin;15, 16, 20 and (4) disrupts the tight-junction zona occludens-1 protein of the intestinal epithelial cell.16, 17, 18, 19, 20, 21 Taken together, these recent findings indicate that fragilysin's pathogenic actions (1) interrupt the integrity of intercellular adhesion;15, 18, 19 (2) increase mucosal permeability and the permeability of the intestinal epithelium to GI tract contents;14, 15, 16, 17 and (3) induce epithelial cell–cell detachment, laying the morphologic basis for a compromised and “leaky” GI tract barrier.17, 18, 19, 20, 21, 22, 23
Fig. 1.
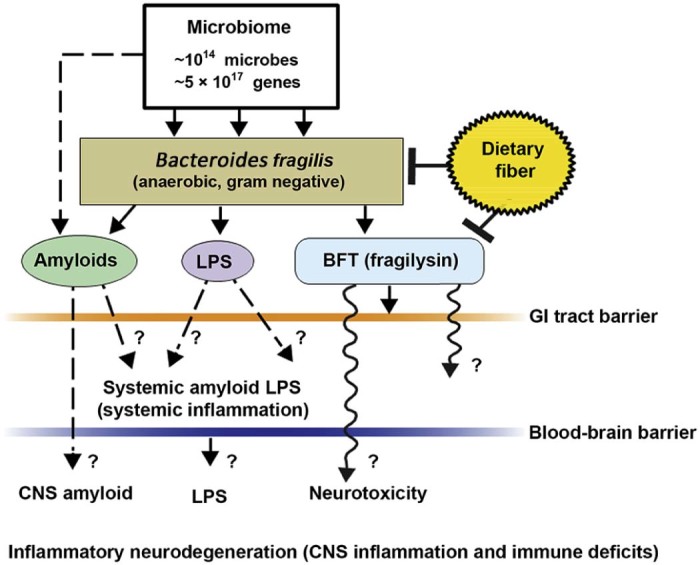
Schematic representation of the potential contribution of gastrointestinal (GI) tract microbiome-derived amyloids, lipopolysaccharides (LPS), and endotoxins to systemic inflammation and/or to central nervous system (CNS) neurotoxicity and immune deficits. One of the major microbial species in the human GI tract is Bacteroides fragilis. In concert with other microbiome components, this anaerobic gram-negative bacillus generates complex mixtures of amyloid, LPS, and/or potent neurotoxins such as B. fragilis toxin (BFT; fragilysin), one of the most potent proinflammatory molecules known.20, 21, 22, 23 Fragilysin is recognized to both (1) increase the paracellular permeability of the intestinal epithelium largely via the dissolution of tight junctions in epithelial cells16, 17, 18, 19 and (2) at low, physiologically realistic (nanomolar) concentrations, induce robust inflammatory signaling (such as activation of nuclear factor kappa B (NF-κB)-DNA binding) in human brain cells in primary culture20, 21, 22, 23, 24 (unpublished observations). The presence of B. fragilis and/or B. fragilis-derived amyloids, LPSs, or endotoxins such as BFT in the bloodstream during systemic inflammation (bacteremia) is more common than any for any other anaerobe of the microbiome.15, 16, 17, 18, 19, 20, 21, 22, 23 In combination with other facultative/obligate anaerobic microbes, their secretory exudates are extremely powerful proinflammatory and innate immune system activators in the CNS once they pass GI tract and blood–brain barriers. These actions would further induce vascular permeability, trigger host immunogenicity, and induce the generation of reactive oxygen species and NF-κB signaling. For example, (1) these neuropathogenic signals further promote amyloid aggregation and inflammatory degeneration characteristic of age-related neurologic diseases such as AD and other neurologic disorders that exhibit defective Aβ42 peptide clearance mechanisms and progressive amyloidogenesis,9, 24, 25 and (2) B. fragilis-derived toxins are also responsible for the majority of localized abscesses within the cranium.24, 25 As a major component of the human microbiome, GI tract microbial sources of amyloid, LPS, and/or other microbial-derived endotoxins have a remarkable potential to contribute to both systemic amyloid and CNS amyloid burden in their respective CNS compartments. This contribution of noxious, proinflammatory molecules from the GI tract microbiome may be increasingly important during the course of aging, when both the GI tract and blood–brain barriers become significantly more permeable.14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Interestingly, high intake of dietary fiber is a strong inhibitor of B. fragilis abundance and proliferation in the human GI tract and as such is a potent inhibitor of the neurotoxic B. fragilis-derived amyloids, LPSs, and enterotoxins. Hence dietary fiber-mediated suppression of B. fragilis abundance may be beneficial for both human GI tract and CNS health.14, 21, 22, 23, 24, 25, 26
Clinically, the presence of BF-LPS in the blood serum signifies (1) a breach of epithelial cell–GI tract barriers; (2) an early, major, and contributing factor to the initiation and propagation of systemic inflammatory disease; and (3) the first major biophysical barrier crossed for complex mixtures of proinflammatory toxins of the GI tract contents to gain access to the systemic circulation, and onward to the blood–brain barrier of the CNS. It would be surprising if BF-LPS were not similarly effective in weakening tight junctions of the blood–brain barrier, and recent data support this concept.17, 19, 20 Furthermore, in primary human neuronal-glial cocultures BF-LPS is (1) an unusually potent inducer of the proinflammatory transcription factor NF-κB (p50/p65 complex) and hence proinflammatory gene expression programs and (2) recognized by toll-like receptors 2 and 4 (TLR2, TLR4) and/or cluster of differentiation 14 (CD14) microglial cell receptors, as are the amyloid peptides that characterize AD neuropathology.9, 10, 20
Of related interest is that B. fragilis and its toxins, such as BF-LPS and fragilysin, also appear to play critical roles in the postmortem microbiome, when at the point of death the human microbiome rapidly transforms into the “thanato-microbiome” (thanatos-, Greek, death) that plays a primary role in the rapid decomposition of host tissues.9, 21
3. Proliferation of BF-LPS in the absence of dietary fiber
The trillions of micro-organisms that constitute the human GI tract microbiome are reliant on sufficient sources of complex dietary fibers (sometimes called “roughage”) to promote and maintain their dynamism and diversity, which support efficient microbiome–host functions. Indeed, microbiome-accessible carbohydrates found in dietary fiber provide a critical contribution to shaping the microbial ecosystem of the GI tract; their individual distributions are notably altered in high-fat, high-cholesterol “Western diets” (high in fat and processed carbohydrates and low in fiber) compared with more traditional “Paleolithic diets” (moderate in fat and processed carbohydrates and high in fiber).16, 17, 18 Several recent studies indicate that in high-fat, high-cholesterol diets deprived of sufficient dietary fiber, there is an upset in the balance of normal bacterial stoichiometry conducive to human health and a proliferation in B. fragilis and similar opportunistic anaerobic bacteria.14, 15, 16, 17, 18, 19, 20 It is perhaps not too surprising that diets low in the fiber provided by complex carbohydrates that favor B. fragilis proliferation are also associated with increased amyloid, LPS, and endotoxins such as fragilysin, which are systemically detrimental to health, supporting leaky barrier functions, which are ultimately proinflammatory toward the PNS and CNS in a variety of ways.
4. Concluding remarks
The microbiome represents a dynamic ecosystem in which structure and function are influenced by multiple interactive factors including age, maternal influences, antibiotic or drug presence, environment, exercise, metabolism, and stress. Microbial amyloids, LPSs, and endotoxins of abundant bacterial species such as B. fragilis may be the most obvious examples of microbiome-resident micro-organisms that can affect GI tract and blood–brain barrier function, stimulate proinflammatory signaling systemically, and promote inflammatory neurodegeneration of the PNS and CNS. It has been known for some time that the functional optimization of the microbiome–GI tract–CNS axis, via studies on GI tract “microbial imbalance” or “dysbiosis” in germ-free animals, the administration of probiotics, and bacterial infections with enteric pathogens have strong effects that can ultimately modulate cognitive behavior, learning, memory, and healthy brain aging.22, 23 Recent studies underscore the concept that just as exercise requires the replenishment of efficient and sufficient energy stores, diets that support optimal CNS and cognitive health require both a healthy dietary intake and sufficient dietary fiber to ensure the “best possible performance” of our microbiome,14, 15, 16, 17, 18, 19, 20 leading to the optimization of microbial speciation and stoichiometry, functional symbiosis, and communication along the microbiome–GI tract–CNS axis.
Competing interests
The author declares no competing financial interests.
Acknowledgments
This work was presented in part at the Society for Neuroscience Annual Meeting, October 17–21, 2015, Chicago, IL, USA; at the Association for Research in Vision and Ophthalmology Annual Conference, May 1–5, 2016, Seattle, WA, USA; and at the Scientific Committee of Aging and Anti-aging, Gerontological Society of China, May 6–8, 2016, Shanghai, China. Sincere thanks are extended to the late Dr. James M. Hill and Drs. Cristoph Eicken, Chris Hebel, Yuhai Zhao, and Vivian Jaber for LPS extracts, BFT, and other microbiome-derived proinflammatory factors, DNA array, and RNA sequencing data and initial data interpretation, and to Darlene Guillot and Aileen Ivy Pogue, the latter for expert technical assistance in the preparation of this manuscript. Research on microRNA and messenger RNA in the Lukiw laboratory involving the innate immune response, neuroinflammation, amyloidogenesis, and the microbiome in AD, prion disease, age-related macular degeneration, and other neurologic or retinal diseases was supported through an unrestricted grant to the LSU Eye Center from Research to Prevent Blindness; the Louisiana Biotechnology Research Network, and National Institutes of Health grants NEI EY006311, NIA AG18031, and NIA AG038834.
Footnotes
Peer review under responsibility of Shanghai University of Sport.
References
- 1.Hamady M., Knight R. Microbial community profiling for human microbiome projects: tools, techniques, and challenges. Genome Res. 2009;19:1141–1152. doi: 10.1101/gr.085464.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turnbaugh P.J., Ley R.E., Hamady M., Fraser-Liggett C.M., Knight R., Gordon J.I. The human microbiome project. Nature. 2007;449:804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu B., Wang X., Li L. Human gut microbiome: the second genome of human body. Protein Cell. 2010;1:718–725. doi: 10.1007/s13238-010-0093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jandhyala S.M., Talukdar R., Subramanyam C., Vuyyuru H., Sasikala M., Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21:8787–8803. doi: 10.3748/wjg.v21.i29.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sankar S.A., Lagier J.C., Pontarotti P., Raoult D., Fournier P.E. The human gut microbiome, a taxonomic conundrum. Syst Appl Microbiol. 2015;38:276–286. doi: 10.1016/j.syapm.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Hugon P., Dufour J.C., Colson P., Fournier P.E., Sallah K., Raoult D. A comprehensive repertoire of prokaryotic species identified in human beings. Lancet Infect Dis. 2015;15:1211–1219. doi: 10.1016/S1473-3099(15)00293-5. [DOI] [PubMed] [Google Scholar]
- 7.Buxbaum J.N., Linke R.P. A molecular history of the amyloidoses. J Mol Biol. 2012;421:142–159. doi: 10.1016/j.jmb.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 8.Blanco L.P., Evans M.L., Smith D.R., Badtke M.P., Chapman M.R. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 2012;20:66–73. doi: 10.1016/j.tim.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao Y., Dua P., Lukiw W.J. Microbial sources of amyloid and relevance to amyloidogenesis and Alzheimer's disease (AD) J Alzheimers Dis Parkinsonism. 2015;5:177. doi: 10.4172/2161-0460.1000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill J.M., Lukiw W.J. Microbial-generated amyloids and Alzheimer's disease (AD) Front Aging Neurosci. 2015;7:9. doi: 10.3389/fnagi.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson T.R., Mazmanian S.K. Control of brain development, function, and behavior by the microbiome cell host microbe. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.von Mikecz A. Pathology and function of nuclear amyloid. Protein homeostasis matters. Nucleus. 2014;5:311–317. doi: 10.4161/nucl.29404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maldonado R.F., Sá-Correia I., Valvano M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol Rev. 2016;40:480–493. doi: 10.1093/femsre/fuw007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barczynska R., Slizewska K., Litwin M., Szalecki M., Kapusniak J. Effects of dietary fiber preparations made from maize starch on the growth and activity of selected bacteria from the Firmicutes, Bacteroidetes, and Actinobacteria phyla in fecal samples from obese children. Acta Biochim Pol. 2016;63:261–266. doi: 10.18388/abp.2015_1068. [DOI] [PubMed] [Google Scholar]
- 15.Choi V.M., Herrou J., Hecht A.L., Teoh W.P., Turner J.R., Crosson S. Activation of Bacteroides fragilis toxin by a novel bacterial protease contributes to anaerobic sepsis in mice. Nat Med. 2016;22:563–567. doi: 10.1038/nm.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnenburg E.D., Smits S.A., Tikhonov M., Higginbottom S.K., Wingreen N.S., Sonnenburg J.L. Diet-induced extinction in gut microbiota compound over generations. Nature. 2016;529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sears C.L., Geis A.L., Housseau F. Bacteroides fragilis subverts mucosal biology: from symbiont to colon carcinogenesis. J Clin Invest. 2014;124:4166–4172. doi: 10.1172/JCI72334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vines R.R., Perdue S.S., Moncrief J.S., Sentz D.R., Barroso L.A., Wright R.L. Fragilysin, the enterotoxin from Bacteroides fragilis, enhances the serum antibody response to antigen co-administered by the intranasal route. Vaccine. 2000;19:655–660. doi: 10.1016/s0264-410x(00)00254-1. [DOI] [PubMed] [Google Scholar]
- 19.Varatharaj A., Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2016 doi: 10.1016/j.bbi.2016.03.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 20.Lukiw W.J. Bacteroidetes fragilis lipopolysaccharide (BF-LPS) and inflammatory signaling in Alzheimer's disease (AD) Front Microbiol. 2016;7:1544. doi: 10.3389/fmicb.2016.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javan G.T., Finley S.J., Abidin Z., Mulle J.G. The Thanatomicrobiome: a missing piece of the microbial puzzle of death. Front Microbiol. 2016;7:225. doi: 10.3389/fmicb.2016.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan L.S., Davies S.S. Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions. Genome Med. 2016;8:46. doi: 10.1186/s13073-016-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troletti C.D., de Goede P., Kamermans A., de Vries H.E. Molecular alterations of the blood-brain barrier under inflammatory conditions: the role of endothelial to mesenchymal transition. Biochim Biophys Acta. 2016;1862:452–460. doi: 10.1016/j.bbadis.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Greenlee J.E. Brain abscess. 2016. http://www.merckmanuals.com/professional/neurologic-disorders/brain-infections/brain-abscess Available at: accessed 15.08.2016.
- 25.Clement C., Hill J.M., Dua P., Culicchia F., Lukiw W.J. Analysis of RNA from Alzheimer's disease post-mortem brain tissues. Mol Neurobiol. 2016;53:1322–1328. doi: 10.1007/s12035-015-9105-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y., Jaber V., Lukiw W.J. Over-expressed pathogenic miRNAs in Alzheimer's disease and prion disease drive deficits in TREM2-mediated Aβ42 peptide clearance. Front Aging Neurosci. 2016;8:140. doi: 10.3389/fnagi.2016.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]


