Abstract
Objectives
We tested the hypothesis that oral progesterone administration attenuates drug-induced QT interval lengthening.
Background
Evidence from preclinical and human investigations suggests that higher serum progesterone concentrations may be protective against drug-induced QT interval lengthening.
Methods
In this prospective, double-blind, crossover study, 19 healthy female volunteers (21-40 years) were randomized to receive progesterone 400 mg or matching placebo orally once daily for 7 days timed to the menses phase of the menstrual cycle (between-phase washout period = 49 days). On day 7, ibutilide 0.003 mg/kg was infused over 10 minutes, after which QT intervals were recorded and blood samples collected for 12 hours. Prior to the treatment phases, subjects underwent ECG monitoring for 12 hours to calculate individualized heart rate-corrected QT intervals (QTcI).
Results
Fifteen subjects completed all study phases. Maximum serum ibutilide concentrations in the progesterone and placebo phases were similar (1247±770 vs 1172±709 pg/mL, p=0.43). Serum progesterone concentrations were higher during the progesterone phase (16.2±11.0 vs 1.2±1.0 ng/mL, p<0.0001), while serum estradiol concentrations in the two phases were similar (89.3±62.8 vs 71.8±31.7 pg/mL, p=0.36). Pre-ibutilide lead II QTcI was significantly lower in the progesterone phase (412±15 vs 419±14 ms, p=0.04). Maximum ibutilide-associated QTcI (443±17 vs 458±19 ms, p=0.003), maximum percent increase in QTcI from pretreatment value (7.5±2.4 vs 9.3±3.4%, p=0.02) and area under the effect (QTcI) curve during the first hour post-ibutilide (497±13 vs 510±16 ms-hr, p=0.002) were lower during the progesterone phase. Progesterone-associated adverse effects included fatigue/malaise and vertigo.
Conclusions
Oral progesterone administration attenuates drug-induced QTcI lengthening.
Keywords: QT interval, Torsades de pointes, Electrocardiography, Hormonal therapy, Preventive
Torsades de pointes (TdP) is a polymorphic ventricular tachycardia associated with QT interval prolongation (1), which may be induced by more than 100 medications available in the Unites States (2). TdP can be catastrophic, often degenerating into ventricular fibrillation causing sudden cardiac arrest (3). The risk for TdP increases as the heart rate-corrected QT (QTc) interval increases (4,5), particularly exceeding 500 ms (6,7). Consequently, QTc interval prolongation is used commonly as a marker of increased risk of TdP.
Female sex is an independent risk factor for TdP in patients with acquired or congenital long-QT syndrome (LQTS) (6,8-10). QTc intervals are longer in women than men (11), a difference which becomes apparent only after puberty (12), suggesting that sex hormones may be responsible. Post-pubertal differences in QTc intervals may be partially due to reduction in QTc intervals in males as a result of testosterone and dihydrotestosterone production (11). However, other factors may also contribute to the difference in risk of TdP. Some studies have reported that hormone replacement therapy with estrogen resulted in QTc interval lengthening (13,14).
Progesterone is a testosterone precursor (15) and has a similar androgenic structure (16). Higher serum progesterone concentrations are associated with shorter QTc intervals (17) and may exert protective effects against lengthening of ventricular repolarization (18). Preclinical data suggest that exogenous progesterone administration may protect against drug-induced prolongation of ventricular repolarization (19-21), ventricular early afterdepolarizations (21) and arrhythmias (22,23). However, the influence of exogenous progesterone administration on response to QTc interval-prolonging drugs in humans has not been determined.
Few effective strategies have been developed to reduce the risk of drug-induced QTc interval prolongation and TdP. We tested the hypothesis that oral administration of progesterone attenuates drug-induced QT interval lengthening in young healthy women.
Methods
Study Subjects
Healthy, premenopausal female volunteers age 21-40 years were enrolled. Exclusion criteria included: serum potassium <4.0 mEq/L; serum magnesium <1.8 mg/dL; hemoglobin <9.0 mg/dL; hematocrit <26%; history of hypertension, coronary artery disease, heart failure, liver or kidney disease; serum creatinine >1.5 mg/dL; use of hormonal contraceptives; baseline Bazett's-corrected QT interval > 450 ms; personal or family history of LQTS, arrhythmias, or sudden cardiac death; concomitant use of any QT interval-prolonging drugs; pregnancy; weight < 45 kg; unwillingness to use non-hormonal forms of birth control during the study period. This study was approved by the Institutional Review Board at Indiana University (IU) Purdue University Indianapolis. All subjects provided written informed consent.
Study Procedures
This was a prospective, randomized, double-blind, placebo-controlled, crossover study conducted in the Indiana Clinical Research Center (ICRC). Subject recruitment began in April, 2013 and study procedures were completed on the last enrolled subject in February, 2014. The study consisted of three phases: a pre-randomization phase to determine each subject's individual QT interval heart rate-correction, and the randomized, double-blind progesterone and placebo phases. Prior to inclusion, all subjects underwent a screening physical examination and blood was obtained for determination of serum potassium, magnesium, creatinine, transaminases, hemoglobin, and hematocrit. A urine human chorionic gonadotropin (HCG) test was performed to rule out pregnancy, and a 12-lead electrocardiogram (ECG) was obtained. Each study phase was conducted during the menses phase of the menstrual cycle (defined as 24-60 hours after menses onset), when serum estradiol and progesterone concentrations are at their lowest, to minimize the effects of endogenous sex hormones (18).
During the pre-randomization phase, subjects underwent a 12-hour stay in the ICRC. Each subject underwent three 12-lead ECGs (Marquette Mac 5500, GE Healthcare Bio-Sciences, Pittsburgh, PA) one minute apart, at 0, 15 & 30 minutes, and 1, 2, 4, 6, 8 & 12 hours. ECGs were initiated between 7:00 am and 9:00 am, and were completed between 7:00 pm and 9:00 pm. QT and RR intervals were used to determine each subject's individual heart rate-corrected QT interval (QTcI) using the parabolic model β•RRα (24), where RR is the interval between adjacent QRS complexes, β is the regression coefficient and α is the slope.
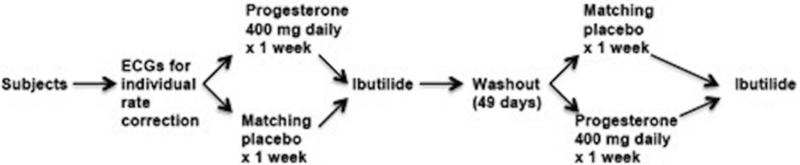
The study design is presented in Figure 1. Following the pre-randomization phase, subjects were randomized in double-blind fashion to receive oral progesterone 400 mg (2 × 200 mg capsules, Teva Pharmaceuticals, North Wales, PA) or matching placebo orally once daily at bedtime for 7 days. Matching placebo was prepared by the IU Health Investigational Drug Service (IDS). Randomization was performed by the IDS using a computerized random number generator and recorded on the IDS randomization log. Participants were assigned to progesterone or placebo in each phase by IDS personnel. Progesterone or matching placebo were delivered to the ICRC by IDS personnel; investigators, study subjects, and ICRC personnel were blinded to treatment assignments during data collection and analysis. The minimum desired washout period was 28 days; after 28 days, we initiated dosing 7 days prior to the next menstrual cycle, resulting in a total washout period of 49 days.
Figure 1.
Study Design
On the morning after the last dose of oral progesterone or placebo, subjects presented to the ICRC for an approximately 13-hour stay. Each subject underwent another urine HCG test to assure absence of pregnancy. Three ECGs, one minute apart, were obtained for baseline measurements. If the urine HCG test was negative and the Bazett's-corrected QT interval was < 450 ms, subjects were placed on a continuous ECG monitor (Heal Force model PC 80B, Heal Force Bio-Meditech, Shanghai, China) and one peripheral indwelling intravenous catheter was inserted into each arm. Blood (4.5 mL) for determination of serum estradiol and progesterone concentrations was collected in gold-top serum separator tubes (Vacutainer, Becton Dickinson, Franklin Lakes, NJ).
Subjects then received a single intravenous dose of ibutilide 0.003 mg/kg diluted in 20 mL normal saline and infused over 10 minutes (18). Three 12-lead ECGs were obtained one minute apart immediately at the end of infusion and at 5, 10, 15, 20, 30, 45 minutes and 1, 2, 4, 6, 8 & 12 hours post-infusion. Pre-ibutilide ECGs were initiated between 7:00 am and 9:00 am, and ibutilide was administered immediately following baseline ECGs. ECGs were completed between 7:00 pm and 9:00 pm. These times correspond to the same times of day as those during which ECGs were obtained for determination of individualized QT interval corrections. Blood (10 mL) for determination of serum ibutilide concentrations was obtained from the indwelling catheter in the arm contralateral to that into which ibutilide was infused and collected in red-top tubes (Vacutainer, Becton Dickinson, Franklin Lakes, NJ) at the same times as ECGs were obtained. Subjects underwent continuous ECG monitoring for 6 hours post-ibutilide. Subjects were discharged after the 12-hour ECG and blood sample, providing that their Bazett's-corrected QTc interval was < 450 ms.
QT Interval Measurements
QT intervals were measured from leads II, V1, and V5 by one investigator (HJ) who was blinded to subjects’ assigned groups. QT intervals were measured using the MUSE automated system (GE Healthcare Bio-Sciences, Pittsburgh, PA) using electronic calipers. QT and RR intervals were averaged over ≥ 5 consecutive beats and the average of three QT intervals at each time point for each lead was determined. Only clearly discernible QT intervals were measured. Determination of the QTcI for each subject was performed as described. QT intervals were also corrected using the Fridericia method (QTF) (25).
Determination of Serum Hormone and Ibutilide Concentrations
Serum estradiol and progesterone concentrations were determined in the IU Health Pathology laboratory using chemiluminescence immunoassays (26,27). Serum ibutilide concentrations were determined in the IU Clinical Pharmacology Analytical Core Laboratory using reverse-phase high-performance liquid chromatography with mass spectrometry detection. Additional detail regarding this assay is provided in the online Appendix in the text, in Appendix Table 1, and in Appendix Figures 1 and 2.
Study Outcome Measures
Outcome measures compared in the progesterone and placebo phases were: 1) Baseline (pre-ibutilide) QTcI and QTF intervals, 2) Maximum QTcI and QTF intervals following ibutilide administration, 3) Maximum ibutilide-induced % change in QTcI and QTF intervals, and 4) Area under the QTcI and QTF interval-time curves from 0-1 hour following ibutilide administration.
Data Analysis
Area under the QTcI and QTF interval-time curves were calculated using the linear trapezoidal rule. Maximum serum ibutilide concentration was determined via visual inspection of serum concentration data.
Sample Size and Statistical Analysis
A sample size of 16 subjects was determined to be sufficient to detect a difference in maximum QTcI of 12 ms (19% reduction), assuming a QTcI prolongation of 63±13 ms associated with ibutilide in the placebo group and a power of 0.80 (18). Analyses were performed using SAS 9.2 (SAS, Cary, North Carolina). Normality of outcome measures data was determined using the Kolmogorov-Smirnov test. Comparisons of the outcome measures during progesterone and placebo phases were performed using paired Student's t-tests. Differences in adverse event proportions were analyzed using the Fisher's Exact Test. Potential treatment-period interactions were tested by comparing the mean within-individual differences for the progesterone-placebo sequence versus those in the placebo-progesterone sequence for each outcome measure using t-tests for paired samples. All comparisons were performed utilizing a two-sided α level of 0.05.
Results
Subjects
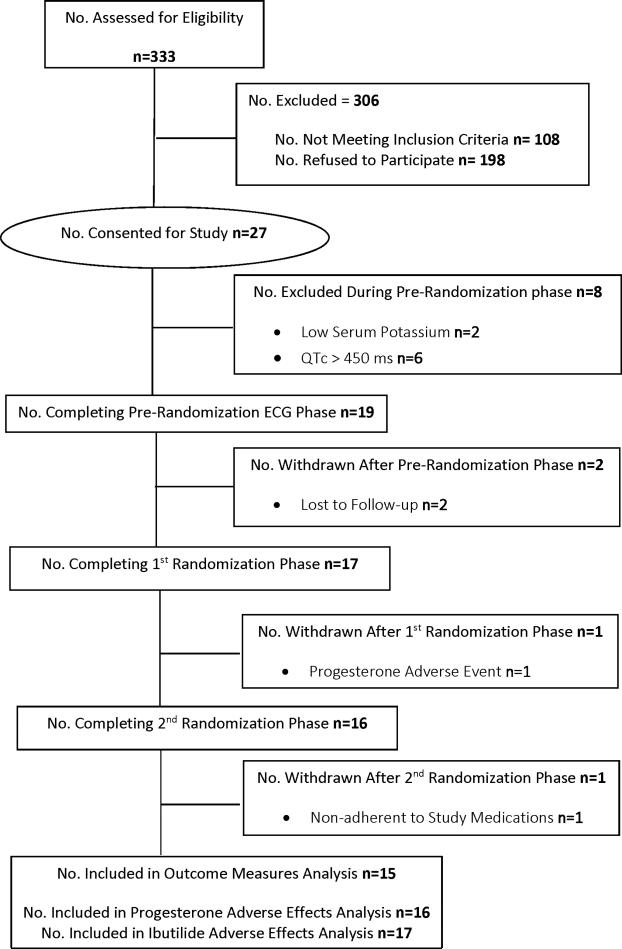
Nineteen subjects were enrolled (Figure 2). Four subjects were excluded from the outcome measures analysis because they did not complete all treatment phases (n=3) or were determined to have been nonadherent to study medications after completion of all study phases (n=1). Therefore, n=15 subjects comprise the final sample size for analysis of outcome measures. For analysis of adverse effects, n=16 received progesterone and n=17 received placebo; n=15 received ibutilide during the progesterone phase, n=17 received ibutilide during the placebo phase. The subjects’ mean age was 29±5 years. Nine subjects were white, 5 were black, and 1 was of Middle Eastern descent. Mean weight was 83±20 kg, and the mean ibutilide dose was 0.24±0.06 mg.
Figure 2.
Recruitment and Enrollment for Randomized, Crossover Placebo-Controlled Study of Influence of Oral Progesterone on Drug-Induced QTc Interval Lengthening
Serum Ibutilide and Hormone Concentrations
There was no significant difference between the progesterone and placebo phases in maximum serum ibutilide (1247±770 vs 1172±709 pg/mL, p=0.43) or serum estradiol concentration (89.3±62.8 vs 71.8±31.7 pg/mL, p=0.36). Serum progesterone concentrations were significantly higher during the progesterone phase (16.2±11.0 vs 1.2±1.0 ng/mL, p<0.0001), as was the serum progesterone: estradiol concentration ratio (205±40 vs 18±16, p=0.001).
Individualized QT Interval Heart Rate Correction – Heart Rates
There was no significant difference in minimum heart rate between the pre-randomization (development of QTcI) phase and on ibutilide administration days in the placebo and progesterone phases (60±7 vs 60±9 vs 61±9 bpm, respectively, p=0.58). There was no significant difference in maximum heart rate between the pre-randomization phase and ibutilide administration days in the placebo and progesterone phases (78±8 vs 79±9 vs 80±11 bpm, respectively, p=0.70). There was no significant difference in average heart rate between the pre-randomization phase and on ibutilide administration days in the placebo and progesterone phases (68±7 vs 68±8 vs 70±9 bpm, respectively, p=0.51). Heart rates in the three study phases are presented in Appendix Figure 3.
Baseline (Pre-Ibutilide) QTcI and QTF Intervals
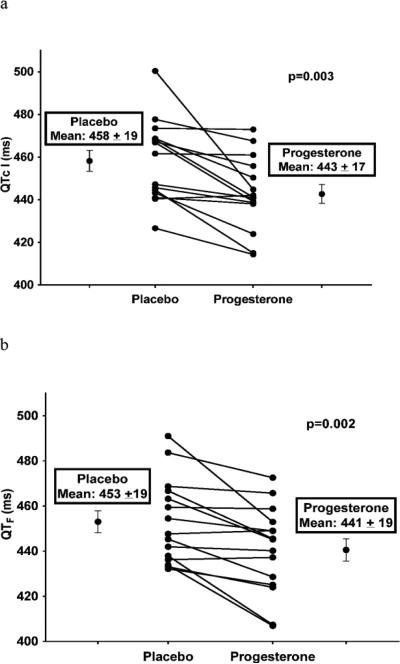
Baseline (pre-ibutilide) QTcI and QTF intervals in ECG lead II were significantly lower during the progesterone phase than during the placebo phase (Figure 3), indicating that oral progesterone administration affected ventricular repolarization in the absence of a QT-lengthening drug. Similar results were demonstrated from ECG leads V1 and V5 (Appendix Figures 4 and 5).
Figure 3.
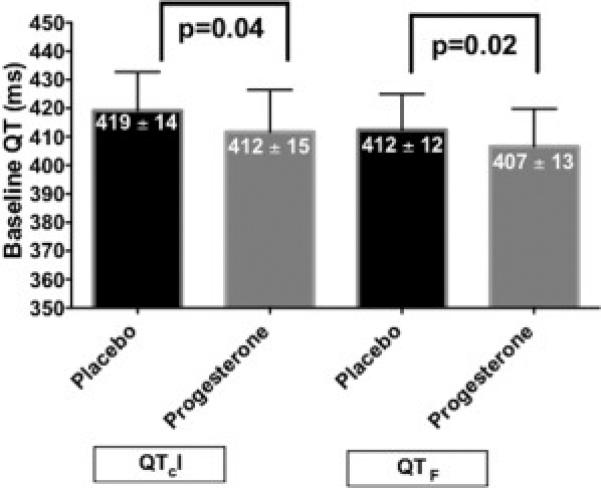
Influence of Oral Progesterone Administration on Baseline (Pre-Ibutilide) QTcI and QTF Intervals (from ECG lead II)
ECG = Electrocardiogram
QTcI = Individually-corrected QT intervals
QTF = Fridericia-corrected QT intervals
Data are presented as mean ± SD
QTcI and QTF Intervals Following Ibutilide Administration
Lead II QTcI and QTF intervals prior to and during the first hour following ibutilide administration in the progesterone and placebo groups are presented in Figure 4. Similar results were demonstrated in leads V1 and V5 (Appendix Figures 6 and 7). The maximum lead II QTcI and QTF following ibutilide administration during the progesterone and placebo phases are presented in Figure 5. Maximum QTcI and QTF intervals were significantly lower during the progesterone phase than during the placebo phase. Similar results were demonstrated in leads V1 and V5 (Appendix Figures 8 and 9).
Figure 4.
QTcI (4a) and QTF (4b) Intervals During and Following a 10-Minute Infusion of Ibutilide 0.003 mg/kg (from ECG lead II) During Progesterone and Placebo Phases
ECG = Electrocardiogram
QTcI = Individually-corrected QT intervals
QTF = Fridericia-corrected QT intervals
Data presented as mean ± SEM
Figure 5.
Maximum QTcI (5a) and QTF (5b) Intervals (from ECG lead II) Following Ibutilide 0.003 mg/kg During Progesterone and Placebo Phases
QTcI = Individually-corrected QT intervals
QTF = Fridericia-corrected QT intervals
Data are presented as mean ± SD
Maximum % Change from Baseline in QTcI and QTF Intervals Following Ibutilide Administration
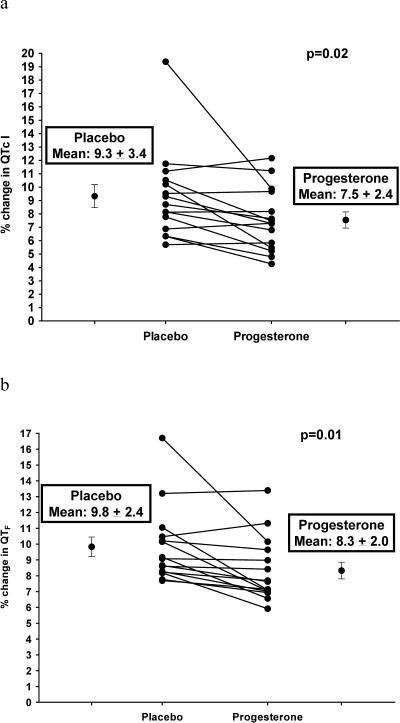
Maximum ibutilide-associated % change in lead II QTcI and QTF intervals from baseline values is presented in Figure 6. There were significantly smaller ibutilide-associated % changes in QTcI and QTF intervals in the progesterone phase compared with the placebo phase. Similar results were shown in leads V1 and V5 (Appendix Figures 10 and 11).
Figure 6.
Maximum % Change from Baseline in QTcI (6a) and QTF (6b) Intervals (from ECG lead II) Following Ibutilide 0.003 mg/kg During Progesterone and Placebo Phases
QTcI = Individually-corrected QT intervals
QTF = Fridericia-corrected QT intervals
Data are presented as mean ± SD
Area Under the QTcI and QTF Interval Versus Time (0-1 hour) Following Ibutilide Administration
Area under the lead II QTcI interval versus time (0-1.17 hours, 1 hour after completion of ibutilide infusion) curve was significantly lower during the progesterone phase compared with the placebo phase (497±13 vs 510±16 ms hr, p=0.002). Similarly, area under the lead II QTF interval versus time (0-1.17 hours) curve after ibutilide administration was significantly lower during the progesterone phase compared with the placebo phase (499±17 vs 506±15 ms hr, p=0.013). Similar results were demonstrated with leads V1 and V5 (Appendix Table 2).
Adverse Effects Associated with Progesterone
Progesterone-associated adverse effects were generally mild, including fatigue/general malaise [progesterone 6/16 (38%) vs placebo 1/17 (6%), p=0.04], headache [2/16 (13%) vs 1/17 (6%), p = 0.60], mood changes [2/16 (13%) vs 0, p=0.23], breast tenderness [2/16 (13%) vs 0, p=0.23], hypotension [1/16 (6%) vs 0, p=0.48], and vertigo 1/16 (6%) vs 0, p=0.48]. The subject who experienced vertigo and hypotension during progesterone therapy withdrew from the study as a result.
Adverse Effects Associated with Ibutilide
There were no differences between the progesterone and placebo phases in ibutilide-associated adverse effects, which included bradycardia [heart rate < 60 bpm; progesterone phase 3/15 (20%) vs placebo phase 2/17 (12%), p=0.65] and burning at the infusion site [1/15 (7%) vs 1/17 (6%), p>0.99]. In one subject (during the placebo phase), Bazett's-corrected QT interval transiently prolonged to > 500 ms.
Potential Treatment-Period Interactions
There were no significant treatment-period interactions for any of the study outcome measures.
Discussion
This is the first study to investigate the effect of oral progesterone administration on drug-induced QT interval lengthening in humans. In this initial proof-of-concept study, we found that oral progesterone 400 mg administered daily for one week significantly reduced non-drug associated QTcI and QTF intervals during the menses phase in young healthy women. In addition, oral progesterone attenuated the QTcI and QTF interval response to low-dose ibutilide. These findings suggest that oral progesterone could be effective for reducing the risk of drug-induced QTc interval prolongation in patients requiring therapy with QTc interval-prolonging drugs, and provide support for further investigation of the effect of oral progesterone in targeted populations requiring QTc interval-prolonging drug therapy.
Previous studies have suggested that progesterone may be protective against lengthening of ventricular repolarization and/or drug-induced arrhythmias. In studies in which hormone replacement therapy with estrogen alone prolonged QTc interval, regimens that included progesterone did not (13,14,28,29). In women with congenital LQTS, the risk of TdP is low during pregnancy, but increases immediately post-partum, when serum progesterone concentrations abruptly decline (30). The QTc interval is significantly shorter during the luteal phase of the menstrual cycle, when serum progesterone concentrations are highest, compared with the follicular phase (17). In healthy volunteers, drug-induced QTc interval lengthening is greatest during the menses and ovulation phases and least during the luteal phase. There was a significant inverse correlation between serum progesterone concentrations and degree of ibutilide-associated QT interval lengthening (18). Progesterone shortens ventricular action potential duration in guinea pig ventricular myocytes, effects that were reversed by mifepristone, a progesterone receptor inhibitor (20). Progesterone and dihydrotestosterone protected against sudden cardiac death in a transgenic rabbit model of LQTS type 2, whereas estradiol promoted sudden cardiac death (23).
Potential mechanisms by which progesterone exerts protective effects against lengthening of ventricular repolarization and ventricular arrhythmias have been investigated. Nakamura et al (20) reported that progesterone enhances the slow component of the delayed rectifier current (IKs) and inhibits L-type Ca2+ currents (ICa,L) under cyclic adenosine monophosphate-stimulated conditions in isolated guinea pig myocytes. These effects were found to be mediated by nitric oxide release through nongenomic activation of endothelial nitric oxide synthase (21). Odening et al (23) found that progesterone decreases the density of ICa,L in rabbit cardiomyocytes and increases expression of sarcoplasmic reticulum calcium ATPase2a, which may contribute to increasing sarcoplasmic reticular Ca2+ uptake, thus shortening Ca2+ transient duration (23). Whether the effects of oral progesterone on attenuation of drug-induced QTc interval lengthening are maintained during longer-term progesterone therapy or whether there is compensatory lengthening of the QTc interval after longer-term progesterone exposure requires additional study.
We selected a progesterone dose of 400 mg daily as this dose is used commonly for management of polycystic ovary syndrome (31) and for prevention of preterm birth (32). Oral progesterone 400 mg once daily led to a significant reduction in baseline, non-drug-associated QTcI and QTF intervals. This was an important contributor to the overall effect of progesterone-associated reduction in maximum ibutilide-associated QTcI and QTF intervals and areas under the QTcI interval and QTF interval versus time curves during the first hour following ibutilide administration. However, the effects of progesterone were not solely attributable to a reduction in baseline QT intervals. Oral progesterone also exerted a protective effect against ibutilide-associated QTcI and QTF interval lengthening, as manifested by a reduction in % change in maximum ibutilide-associated QTcI and QTF interval from pretreatment values.
Progesterone 400 mg once daily was associated with adverse effects, most of which were mild. Additional study is necessary to determine whether a lower dose of oral progesterone resulting in proportionately lower serum concentrations is effective for attenuation of drug-induced QTc interval lengthening. The long-term incidence of adverse effects associated with oral progesterone 400 mg daily has not been well-studied. The incidence of adverse effects associated with oral progesterone 300 mg daily for 12 weeks was not significantly different than that associated with placebo (33). Oral progesterone 400 mg daily administered for 18 weeks was associated with no reported adverse effects in pregnant women (34). The incidence of adverse effects associated with longer-term administration of oral progesterone 400 mg daily requires further study.
Ibutilide prolongs the QT interval in a dose-dependent fashion via inhibition of the rapid component of the delayed rectifier potassium current (35), as well through activation of a slow inward sodium current (36). Ibutilide was an appropriate probe drug for this investigation since serum concentrations peak and decline rapidly after intravenous administration (18). We administered a mean dose of 0.24±0.06 mg, 24% of the lowest therapeutic dose (1 mg) and 12% of the highest total therapeutic dose (2 mg). This subtherapeutic dose was selected based on previous investigations in which ibutilide 0.003 mg/kg provoked a modest, but not excessive, lengthening of the QTc interval in healthy volunteers (18). In our subjects, QTcI and QTF intervals generally returned to baseline values within 60-90 minutes of ibutilide administration. There was no significant difference in maximum serum ibutilide concentration between the progesterone and placebo phases; therefore, differences in QTcI and QTF intervals in the two phases were not attributable to differences in serum ibutilide concentration.
Limitations of this study include the fact that it was conducted in young healthy women during the menses phase, when endogenous serum progesterone and estradiol concentrations are lowest. It remains unknown whether the effects of oral progesterone would be similar if administered during different phases of the menstrual cycle, or to postmenopausal women. The range of heart rates in our healthy subjects was relatively narrow during the phase when the heart rate-correction factors for the individualized QT interval corrections were determined. However, there were no differences in heart rates during the pre-randomization phase versus those in the placebo or progesterone phases; therefore, the individualized heart rate corrections were derived using a similar range of heart rates during each phase of the study. In addition, we also report our results using the Fridericia heart rate correction for QT interval, and the QTF results mirror those of our QTcI analysis. The period of progesterone administration was relatively short (7 days); the effect of longer periods of progesterone administration on naturally occurring QTc interval and drug-induced QTc interval lengthening, as well as the safety of long-term oral progesterone, require further study.
Conclusions
Oral progesterone 400 mg daily reduces baseline QTcI intervals and attenuates drug-induced QTcI interval lengthening. These findings provide support for additional studies investigating the efficacy, safety and clinical feasibility of oral progesterone administration for reducing the risk of drug-induced QTc interval prolongation and TdP in patients with risk factors who require therapy with QT interval-prolonging drugs.
Supplementary Material
Perspectives.
Competency in Medical Knowledge: Torsades de pointes is a potentially life-threatening polymorphic ventricular tachycardia associated with QT interval prolongation, which may be induced by more than 70 medications available in the Unites States. Torsades de pointes can be a catastrophic occurrence, as it may degenerate into ventricular fibrillation and cause sudden cardiac arrest. Methods for reducing the risk of drug-induced QT interval prolongation may result in improved medication safety. However, few effective strategies have been developed to reduce the risk of drug-induced QTc interval prolongation and torsades de pointes.
Translational Outlook 1: Administration of oral progesterone at a dose of 400 mg daily reduces baseline QTcI intervals and attenuates drug-induced QTcI interval lengthening during the menses phase of the menstrual cycle in young healthy female subjects.
Translational Outlook 2: These data provide support for additional studies investigating the efficacy, safety and clinical feasibility of oral progesterone administration for reducing the risk of drug-induced QTc interval prolongation and TdP in patients with risk factors who require therapy with QT interval-prolonging drugs.
Acknowledgements
The authors gratefully acknowledge the assistance of David Jones, PhD, Director, Clinical Pharmacology Analytical Core Laboratory, School of Medicine, Indiana University, for performance of the HPLC-MS analysis of serum ibutilide concentrations. The authors also acknowledge Andi Corya, PharmD, College of Pharmacy, Purdue University for her assistance with the study.
Grant Support
Supported by a grant from the American Heart Association Midwest Affiliate (12GRNT12060187). This investigation was also supported in part with support from the Indiana Clinical and Translational Sciences Institute funded, in part, by grant number UL1TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. In addition, this investigation was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 RR020128-01 from the National Center for Research Resources, National Institutes of Health. Dr. Overholser was supported in part by National Institutes of Health grant K08 HL095655. Analytical work was performed by the Clinical Pharmacology Analytical Core Laboratory, a core laboratory of the Indiana University Melvin and Bren Simon Cancer Center, supported by the National Cancer Institute grant P30 CA082709.
Abbreviations
- bpm
Beats per minute
- ECG
Electrocardiogram
- HCG
Human chorionic gonadotropin
- ICRC
Indiana Clinical Research Center
- IDS
Investigational Drug Service
- LQTS
Long QT syndrome
- QTc interval
Bazett's-corrected QT interval
- QTcI
Individualized heart rate-corrected QT interval
- QTF
Fridericia-corrected QT interval
- TdP
Torsades de pointes
- IU
Indiana University
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Dr. Kovacs serves as a consultant for Eli Lilly & Company, and is a member of Data Safety Monitoring Boards for Biotie Therapies and Teva Pharmaceuticals
Clinical Trial Registration: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01929083.
References
- 1.Gupta A, Lawrence AT, Krishnan K, Kavinsky CJ, Trohman RG. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am Heart J. 2007;153:891–9. doi: 10.1016/j.ahj.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 2.Tisdale JE. Ventricular arrhythmias. In: Tisdale JE, Miller DA, editors. Drug-Induced Diseases. Prevention, Detection and Management. 2nd ed. American Society of Health-System Pharmacists; Bethesda, MD: 2010. pp. 485–515. [Google Scholar]
- 3.Drew BJ, Ackerman MJ, Funk M, et al. on behalf of the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology, the Council on Cardiovascular Nursing, and the American College of Cardiology Foundation Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2010;55:934–47. doi: 10.1016/j.jacc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moss AJ, Schwartz PJ, Crampton RS, et al. The long QT syndrome. Prospective longitudinal study of 328 families. Circulation. 1991;84:1136–44. doi: 10.1161/01.cir.84.3.1136. [DOI] [PubMed] [Google Scholar]
- 5.Zareba W, Moss AJ, Schwartz PJ, et al. Influence of genotype on the clinical course of the long-QT syndrome. International Long-QT Syndrome Registry Research Group. N Engl J Med. 1998;339:960–5. doi: 10.1056/NEJM199810013391404. [DOI] [PubMed] [Google Scholar]
- 6.Woosley RL, Chen Y, Freiman JP, Gillis RA. Mechanism of the cardiotoxic actions of terfenadine. JAMA. 1993;269:1532–6. [PubMed] [Google Scholar]
- 7.De Bruin ML, Langendijk PN, Koopmans RP, Wilde AA, Leufkens HG, Hoes AW. Inhospital cardiac arrest is associated with use of nonantiarrhythmic QTc-prolonging drugs. Br J Clin Pharmacol. 2007;63:216–23. doi: 10.1111/j.1365-2125.2006.02722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–7. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 9.Lehmann MH, Hardy S, Archibald D, Quart B, MacNeil DJ. Sex difference in risk of torsade de pointes with d.l-sotalol. Circulation. 1996;94:2535–41. doi: 10.1161/01.cir.94.10.2535. [DOI] [PubMed] [Google Scholar]
- 10.Locati EH, Zareba W, Moss AJ, et al. Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the International LQTS Registry. Circulation. 1998;97:2237–44. doi: 10.1161/01.cir.97.22.2237. [DOI] [PubMed] [Google Scholar]
- 11.Pham TV, Rosen MR. Sex, hormones, and repolarization. Cardiovasc Res. 2002;53:740–51. doi: 10.1016/s0008-6363(01)00429-1. [DOI] [PubMed] [Google Scholar]
- 12.Rautaharju PM, Zhou SH, Wong S, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–5. [PubMed] [Google Scholar]
- 13.Carnethon MR, Anthony MS, Cascio WE, et al. A prospective evaluation of the risk of QT prolongation with hormone replacement therapy: the atherosclerosis risk in communities study. Ann Epidemiol. 2003;13:530–6. doi: 10.1016/s1047-2797(03)00050-4. [DOI] [PubMed] [Google Scholar]
- 14.Gökçe M, Karahan B, Yilmaz R, Örem C, Erdöl C, Özdemir S. Long term effects of hormone replacement therapy on heart rate variability, QT interval, QT dispersion and frequencies of arrhythmia. Int J Cardiol. 2005;99:373–9. doi: 10.1016/j.ijcard.2003.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Ye L, Su ZJ, Ge RS. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules. 2011;16:9983–10001. doi: 10.3390/molecules16129983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guyton AC, Hall JE. Textbook of Medical Physiology. 11th ed. Elsevier, Inc; Philadelphia: 2006. [Google Scholar]
- 17.Nakagawa M, Ooie T, Takahashi N, et al. Influence of menstrual cycle on QT interval dynamics. Pacing Clin Electrophysiol. 2006;29:607–13. doi: 10.1111/j.1540-8159.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez I, Kilborn MJ, Liu X-K, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–6. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 19.Tisdale JE, Overholser BR, Wroblewski HA, Sowinski KM. The influence of progesterone alone and in combination with estradiol on ventricular action potential duration and triangulation in response to potassium channel inhibition. J Cardiovasc Electrophysiol. 2011;22:325–31. doi: 10.1111/j.1540-8167.2010.01869.x. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura H, Kurokawa J, Bai CX, et al. Progesterone regulates cardiac repolarization through a nongenomic pathway: an in vitro patch-clamp and computational modeling study. Circulation. 2007;116:2913–22. doi: 10.1161/CIRCULATIONAHA.107.702407. [DOI] [PubMed] [Google Scholar]
- 21.Cheng J, Zhang J, Ma X, Su D. Frequency-dependent acceleration of cardiac repolarization by progesterone underlying its cardiac protection against drug-induced proarrhythmic effects in female rabbits. Eur J Pharmacol. 2012;689:172–8. doi: 10.1016/j.ejphar.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 22.Cheng J, Su D, Ma X, Li H. Concurrent supplement of estradiol and progesterone reduces the cardiac sensitivity to D,L-sotalol-induced arrhythmias in ovariectomized rabbits. J Cardiovasc Pharmacol Ther. 2012;17:208–14. doi: 10.1177/1074248411418972. [DOI] [PubMed] [Google Scholar]
- 23.Odening KE, Choi B-R, Liu GX, et al. Estradiol promotes sudden cardiac death in transgenic long QT type 2 rabbits while progesterone is protective. Heart Rhythm. 2012;9:823–32. doi: 10.1016/j.hrthm.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malik M, Färbom P, Batchvarov V, Hnatkova K, Camm AJ. Relation between QT and RR intervals is highly individual among healthy subjects: implications for heart rate correction of the QT interval. Heart. 2002;87:220–8. doi: 10.1136/heart.87.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fridericia LS. Die systolendauer im elektrokardiogramm bei normalen menchen und bei herzkranken. Acta Medica Scandinavica. 1920;53:469–86. [Google Scholar]
- 26.Bryant C, Moore J, Curry TE., Jr. Determination of serum estradiol levels by radiometric and chemiluminescent techniques. Methods Mol Biol. 2009;590:21–32. doi: 10.1007/978-1-60327-378-7_2. [DOI] [PubMed] [Google Scholar]
- 27.Ren S, Wang X, Lin Z, et al. Development of a high-throughput, indirect antibody immobilization format chemiluminescence immunoassay (CLEIA) for the determination of progesterone in human serum. Luminescence. 2008;23:175–81. doi: 10.1002/bio.1031. [DOI] [PubMed] [Google Scholar]
- 28.Haseroth K, Seyffart K, Wehling M, Christ M. Effects of progestin-estrogen replacement therapy on QT-dispersion in postmenopausal women. Int J Cardiol. 2000;75:161–5. doi: 10.1016/s0167-5273(00)00317-x. [DOI] [PubMed] [Google Scholar]
- 29.Kadish AH, Greenland P, Limacher MC, Frishman WH, Daugherty SA, Schwartz JB. Estrogen and progestin use and the QT interval in postmenopausal women. Ann Noninvas Electrocardiol. 2004;9:366–74. doi: 10.1111/j.1542-474X.2004.94580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seth R, Moss AJ, McNitt S, et al. Long QT syndrome and pregnancy. J Am Coll Cardiol. 2007;49:1092–8. doi: 10.1016/j.jacc.2006.09.054. [DOI] [PubMed] [Google Scholar]
- 31.Setji TL, Brown AJ. Polycystic ovary syndrome: update on diagnosis and treatment. Am J Med. 2014;127:912–9. doi: 10.1016/j.amjmed.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Dodd JM, Jones L, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth in women considered to be at risk of preterm birth. Cochrane Database Syst Rev. 2013;7:CD004947. doi: 10.1002/14651858.CD004947.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hitchcock CL, Prior JC. Oral micronized progesterone for vasomotor symptoms – a placebo-controlled randomized trial in healthy postmenopausal women. Menopause. 2012;19:886–93. doi: 10.1097/gme.0b013e318247f07a. [DOI] [PubMed] [Google Scholar]
- 34.Glover MM, McKenna DS, Downing CM, Smith DB, Croom CS, Sonek JD. A randomized trial of micronized progesterone for the prevention of recurrent preterm birth. Am J Perinatol. 2011;28:377–81. doi: 10.1055/s-0031-1274509. [DOI] [PubMed] [Google Scholar]
- 35.Yang T, Snyders DJ, Roden DM. Ibutilide, a methanesulfonanilide antiarrhythmic, is a potent blocker of the rapidly activating delayed rectifier K+ current (IKr) in AT-1 cells. Concentration-, time-, voltage-, and use-dependent effects. Circulation. 1995;91:1799–1806. doi: 10.1161/01.cir.91.6.1799. [DOI] [PubMed] [Google Scholar]
- 36.Lee KS. Ibutilide, a new compound with potent class III antiarrhythmic activity, activates a slow inward Na+ current in guinea pig ventricular cells. J Pharmacol Exp Ther. 1992;262:99–108. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.