Abstract
Bright light therapy (BLT) is considered among the first-line treatments for seasonal affective disorder (SAD), yet a growing body of literature supports its use in other neuropsychiatric conditions including non-seasonal depression. Despite evidence of its antidepressant efficacy, clinical use of BLT remains highly variable internationally. In this article, we explore the autonomic effects of BLT and suggest that such effects may play a role in its antidepressant and chronotherapeutic properties. After providing a brief introduction on the clinical application of BLT, we review the chronobiological effects of BLT on depression and on the autonomic nervous system in depressed and non-depressed individuals with an emphasis on non-seasonal depression. Such a theory of autonomic modulation via BLT could serve to integrate aspects of recent work centered on alleviating allostatic load, the polyvagal theory, the neurovisceral integration model and emerging evidence on the roles of glutamate and gamma-hydroxybutyric acid (GABA).
Keywords: Autonomic nervous system, bright light therapy, chronotherapy, major depressive disorder, seasonal affective disorder, sympathovagal balance
INTRODUCTION
According to the World Health Organization, major depressive disorder (MDD) is a leading cause of disability worldwide. In 2010, the Global Burden of Disease Study found that major depression was the second greatest cause of disability worldwide based on a composite measure of productive years lost due to disability, and it affected an estimated 300 million people worldwide (Vos et al., 2012). In the United States alone, the U.S. National Institutes of Mental Health estimates that MDD afflicts 20 million individuals each year (Kessler et al., 2005). A recent cost analysis of modifiable health risk factors found that being at high risk for depression was the single greatest factor associated with increased per capita annual medical costs – greater than the increased costs associated with elevated blood sugar, high blood pressure, obesity, tobacco use or physical inactivity (Goetzel et al., 2012). Healthcare spending was increased by nearly 50% for those at high risk for depression, which amounted to more than $2000 a year per person (Goetzel et al., 2012).
Lamentably, the most common treatments recommended for non-seasonal MDD (NS-MDD1) require at least 2 weeks for hints of efficacy although response of core symptoms of depression and anhedonia generally require a month or longer (American Psychiatric Association, 2010). While research seeks effective, sustainable, affordable, faster-acting, relapse-preventing antidepressant strategies (Wirz-Justice et al., 2005), a recent review of bright light therapy’s (BLT’s) efficacy in mood disorders sponsored by the American Psychiatric Association found statistical treatment responses were evident within a week (Golden et al., 2005). More broadly, combination chronotherapeutic treatments – including BLT, wake therapy (the preferred term for total sleep deprivation) and sleep phase advance therapy – have demonstrated efficacy within days yet remain broadly underappreciated. Recent evidence of the rapid onset of ketamine’s antidepressant effect has garnered attention; however, evidence of rapid response to chronotherapeutics has been demonstrated for decades (Wirz-Justice, 2008; Wirz-Justice et al., 2009).
Efficacy of BLT for depression
BLT has been shown to be efficacious for the treatment of seasonal and non-seasonal depression with effect sizes of 0.84 and 0.53, respectively (Golden et al., 2005), yet it is often excluded from discussions of management of major depression or, if included, relegated to a status beneath that of psychopharmacological (and even psychotherapeutic) modalities (Wirz-Justice et al., 2005). These meta-analytic effect sizes are on the order of those found with antidepressant medications and, more compellingly, a range of currently available general psychiatric and general medicine prescription medications (Leucht et al., 2012).
Despite such an impressive array of potential benefits and its remarkable safety profile and therapeutic index, the use of BLT for NS-MDD remains limited and, at the very least, incommensurate with the data in support of its safety and efficacy (Tables 1–3). For instance, these authors are aware of only two surveys of “prescribing patterns”2 related to BLT (both of which were performed by the same European group; Fischer et al., 2012; Kasper et al., 1994), a fact itself likely reflective of such limited interest throughout the international psychiatric community.
TABLE 1.
Potential benefits of BLT versus antidepressant medications.
| 1. | Greater effect size in patients with SAD and comparable effect size in NS-MDD relative to psychotropic medications (Golden et al., 2005). |
| 2. | More rapid depression response than psychotropics relative to placebo in SAD (Lam et al., 2006; Ruhrmann et al., 1998) and depression responses in NS-MDD within a week (Golden et al., 2005). |
| 3. | Near absence of systemic adverse events (e.g. sexual dysfunction or weight gain). |
| 4. | Non-dependence on absorptive capacity of the gastrointestinal system, which also avoids the need – as with certain medications – to take either with meals or on an empty stomach to enhance absorption. |
| 5. | Non-dependence on metabolic or elimination processes (i.e. no need for renal or hepatic dosing). |
| 6. | Absence of medication interactions. |
| 7. | No conceivable means of overdose. |
| 8. | No documented discontinuation syndrome (although clinical effect abates within days of BLT discontinuation). |
| 9. | Absence of recurring monthly costs (save for the nominal power consumption required to illuminate the light-delivery device). |
| 10. | Absence of teratogenic effects (Wirz-Justice et al., 2011). |
| 11. | Clinical acceleration of treatment response in NS-MDD when employed as an adjunct to antidepressant medication (Benedetti et al., 2003a; Martiny, 2004). |
TABLE 3.
Limitations common to BLT and antidepressant medication.
| 1. | Dose-dependent idiosyncratic side effects (e.g. BLT may cause mild headache, eyestrain, nausea or agitation). |
| 2. | Potential for manic overshoot or transition into hypomanic, manic, or mixed episodes reported to be comparable to the rates due to selective-serotonin reuptake inhibitors |
| 3. | New-onset suicidal ideation has been reported after initiation of BLT (Terman & Terman, 2005) and antidepressant medication. |
Biological rationale
Throughout most its history, psychiatry has wrestled with questions related to the relative balance of psychosocial and biological contributors to mental illness, and now, in the era of genetics, proteomics and evermore-sophisticated brain imaging and biological assays, many are turning to explain mental illness and its various therapeutic modalities in the context of neuroscience while exploring novel treatments (Campbell, 2010; Insel, 2009). For instance, Bunney & Bunney (2012) have highlighted several clinical and proposed neurobiological parallels between ketamine qua antidepressant and chronotherapeutics for affective disorders. Perhaps, the recent demonstration of rapid synaptogenesis in response to ketamine (Duman & Aghajanian, 2012) may fuel further developments in understanding chronotherapeutic mechanisms of action. Although we acknowledge the pivotal role that reimbursement models and funding play in treatment selection and research, we propose that a more cogent neurobiological framework for the efficacy of chronotherapeutics, particularly BLT, in conjunction with ongoing trials of efficacy and effectiveness may enhance clinical implementation, providing a much-needed, safe, rapid-onset antidepressant treatment in psychiatry’s armamentarium against MDD.3
In short, the lack of a sufficiently cogent biological rationale for the efficacy of BLT may substantiate a sense that it is “not biological enough.” In concert with this, there is a general perception that limitations in the literature on BLT “may have created the unsubstantiated impression that the treatment itself has limitations in terms of its efficacy” (Golden et al., 2005). In this review, we explore the potential role of the autonomic nervous system (ANS) in the antidepressant effects of BLT in support of our hypothesis articulated below. What follows is a brief introduction to chronobiology (CB) and the ANS in both non-depressed and non-seasonally depressed individuals, a review of the current literature in support of various known biological correlates of BLT, and finally a review the literature on the chronobiological effects of BLT on the ANS in both non-depressed and non-seasonally depressed individuals, particularly in relation to antidepressant properties.
HYPOTHESIS
We propose that the chronobiological effects of BLT on the ANS may be integral to its antidepressant properties. The evidence compiled below (see Table 4 for overview) suggests that BLT may have the potential to restore sympathovagal balance in patients with MDD (for a review of the content, see Streeter et al., 2012), thus alleviating allostatic load (McEwen, 1998). This understanding would bridge CB with recent advances in the polyvagal theory (Porges, 2001), the neurovisceral integration model (Thayer & Lane, 2009) and emerging evidence on the glutamatergic and GABA-ergic systems (Thayer & Brosschot, 2005). BLT may thus represent, in part, a non-invasive variant of vagal nerve stimulation (VNS). Improvements in sympathovagal balance as measured by enhanced heart rate variability (HRV) may also have implications for cardiovascular outcomes (Carney & Freedland, 2009; Thayer & Brosschot, 2005).
TABLE 4.
Overview of rationale.
| General discussion
|
Effects of BLT
|
||||
|---|---|---|---|---|---|
| Non-depressed | Non-seasonal major depressive sisorder (NS-MDD) | Neurobiology | Non-depressed | NS-MDD | |
| Chronobiology (CB) | CB | CB in NS-MDD | Monoamines CB The ANS Miscellany |
Effects on CB | Effects on CB in NS-MDD |
| The autonomic nervous system (ANS) | The ANS | The ANS in NS-MDD | Effects on the ANS | Effects on the ANS in NS-MDD | |
| CB aspects of the ANS | CB aspects of the ANS | CB aspects of the ANS in NS-MDD | Effects on CB aspects of the ANS | Effects on CB aspects of the ANS in MS-MDD | |
CB AND THE ANS
Chronobiology
Nearly all aspects of human physiology are modulated by the 24-h circadian rhythm (Kraft & Martin, 1995). Regular periodicity is seen in most – if not all – serological values (e.g. melatonin and cortisol) and physiological parameters (e.g. core body temperature (Tcore) and brain waves), including measures of autonomic function. Notably, each of the various electrophysiological measures used to evaluate moment-by-moment ANS function – including heart rate (HR), HRV, QTc and QTc dispersion – demonstrate regular circadian rhythms (Guo & Stein, 2002; Ishida et al., 1997; Portaluppi & Hermida, 2007).
The ANS
The ANS is composed of two complementary divisions: the sympathetic and parasympathetic nervous systems. The parasympathetic nervous system maintains a default state, contributing to baseline repair (rest) and nutrient storage (digest) functions, whereas the sympathetic system includes both neurological and humoral elements and allows for real-time reactions to stress by initiating the fight, flight or fright response. The parasympathetic system is mediated largely by the vagus nerve (also known as cranial nerve X) with a lesser contribution within the calvarium from other cranial nerves and distally in the spinal cord from the sacral plexus. In the absence of external perturbation, the body is under tonic inhibitory control via parasympathetic innervation (i.e. a state of relatively low sympathovagal tone). This state of limited sympathetically mediated arousal provides for the highest degree of repair and energy conservation, which is the preferred resting homeostasis (McEwen, 1998). Additionally, given that the parasympathetic system can “turn off” in response to stressors on the order of milliseconds, this state also allows for the greatest degree of dynamic flexibility (Thayer & Brosschot, 2005).
Although a comprehensive review of clinical indices employed to evaluate autonomic function is beyond the scope of this article, the more commonly investigated indices are briefly discussed below.
Tcore generally reflects sympathetic activity as thermoregulation occurs largely via sympathetically mediated peripheral vascular tone, sweating via sudomotor activity and overall metabolic rate with a much lesser contribution by the vagus via metabolic processes (Székely, 2000). Peripheral vasoconstriction prevents heat loss through the skin, whereas decreases in peripheral vascular tone promote elevated peripheral skin temperature allowing for dissipation of heat into the environment and subsequent lowering of Tcore (Okamoto-Mizuno & Mizuno, 2012).
Resting HR and diurnal variation (DV) of HR are under predominantly parasympathetic control, although changes in HR in response to bright light appear sympathetically mediated (Scheer et al., 2003).
- HRV is a broad category of measuring aspects of change in HR. Four broad domains of HRV have been defined (Task Force, 1996) and reviewed recently (Xhyheri et al., 2012):
- Time domain (linear) measures such as standard deviation of normal-to-normal (SDNN) beats or root mean square of successive R–R-interval differences, which must be standardized based on recording length, provide an index of the total variance of HRV over that period reflecting sympathetic and parasympathetic tone, circadian rhythm and physical activity.
- Frequency domains, which require specialized computational analysis, include the following:
- High frequency (HF [0.15–0.40 Hz]; a common measure for respiratory sinus arrhythmia [RSA]): largely reflects vagal activity. RSA in particular refers to the natural variation of HR that occurs in phase with respirations: inspiration is associated with HR acceleration and expiration with deceleration.
- Low frequency (LF [0.04–0.15 Hz]): although reflecting primarily sympathetic thermoregulatory mechanisms including both neural and endocrine processes it is also affected by the overall autonomic balance.
- Very low frequency (0.003–0.04 Hz): unclear utility.
- LF/HF ratio: reflects sympathovagal balance.
- Non-linear measures involve complex mathematical calculations. For example, indices here subsumed include the de-trended fluctuation analysis, delta entropy analysis and standard deviation of the Poincare plot (pcSD-1), each of which provides an index of parasympathetic tone.
- Pattern analysis, the fourth domain, appears to have limited research application.
Mean arterial pressure (MAP) estimates sympathetic tone via neural and humoral factors. That is, in addition to affecting HR and vascular tone directly by way of post-synaptic noradrenergic neurotransmission, the sympathetic nervous system also indirectly modulates MAP indirectly by humoral factors such as adrenal release of catecholamines and glucocorticoids.
Blood pressure variation (BPV) measures changes in blood pressure, typically in response to maneuvers or interventions including pressor agents, tilt table testing, Valsalva, postural changes or traditional orthostatic vital signs, and it estimates sympathetic tone via neural and humoral factors. BPV broadly describes baroreflex sensitivity, the ability for the body to compensate for changes in blood pressure as sensed by the carotid baroreceptors and mediated by the ANS.
Pre-ejection period, which is derived from noninvasive thoracic impedance cardiography, is the time between onset of ventricular depolarization and semilunar valve opening. It provides a specific index of cardiac sympathetic activity as reflected in β-adrenergic control over left ventricular contraction (Licht et al., 2012).
Pupillary light reflex (PLR), as measured by pupillometry, can assess both sympathetic and parasympathetic tone owing to the nuanced autonomic innervation of pupillary tone. Various features of pupil size and reactivity such as diameter, latency to constriction (in response to a light stimulus) and relative amplitude of constriction may be assessed (Bär et al., 2004). The PLR deserves particular attention in the evaluation of BLT’s autonomic effects as it represents an integrated autonomic response to a light stimulus. The reflex involves the non-visual intrinsically photosensitive retinal ganglion cells (ipRGCs) (see Circadian rhythm of the ANS), which prompt the Edinger–Westphal nuclei bilaterally to propagate a signal to parasympathetic ciliary ganglia leading ultimately to bilateral pupillary constriction.
Muscle sympathetic nerve activity, a direct measure of peripheral sympathetic activity, can be measured using microneurography with the insertion of microelectrodes (Scalco et al., 2009). The peroneal nerve is commonly evaluated for this purpose.
Galvanic skin conductance level and response are indices of skin sympathetic nerve activity (SSNA) and are elevated during states of emotional arousal (Lindsey et al., 2011). Although surface skin conductance has been used historically (i.e. sympathetically-mediated sweat release increases skin conductance due to its electrolytes), modern investigations have used microneurography to evaluate SSNA directly (Brown et al., 2012).
Circadian rhythm of the ANS
The intrinsic 24-hour circadian rhythm in humans is governed by zeitgebers (Ger. time givers), the most potent of which is light from the sun (Czeisler et al., 1986), and when one’s sleep–wake cycle is in phase with day–night a person’s circadian rhythm is said to be “entrained” with the light–dark cycle. Photic stimuli activate nonimage-forming (NIF) ipRGCs principally by means of attached melanopsin photoreceptors but also, to a lesser degree, by adjacent rhodopsin-containing rods or photopsin-containing cones by way of ancillary neuronal networks in the retina (Packard & Sollars, 2012). Once activated, ipRGCs send their chief projections to the suprachiasmatic nucleus (SCN) in the anterior hypothalamus via the retinohypothalamic tract; other recipients include the intergeniculate leaflet of the thalamus, olivary pretectal nucleus, lateral habenula, superior colliculus, periaqueductal gray and both the bed nucleus of the stria terminalis and medial amygdala, each a part of the extended amygdala (Warthen & Provencio, 2012). However, ipRGCs do not project to the dorsal raphe or dorsal lateral geniculate nuclei, which relay photic stimuli to the primary visual cortex for sight (Hattar et al., 2006) (see Figures 1 and 2).
FIGURE 1.
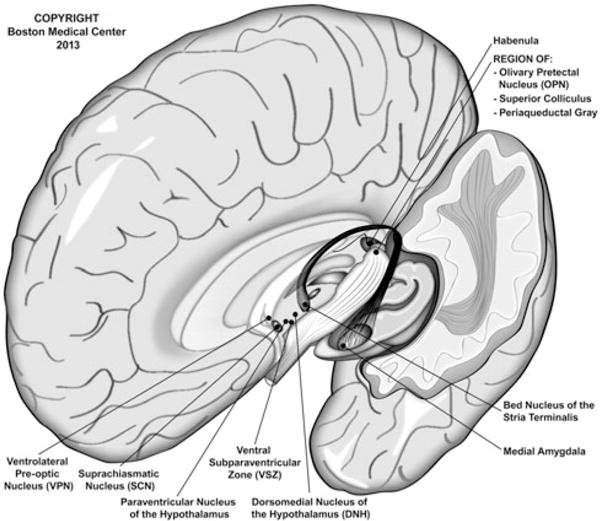
Central projections of intrinsically photosensitive retinal ganglion cells (ipRGCs). Photic stimuli are transmitted via a NIF route from the ipRGCs to several central nuclei. Although native ipRGCs contain the chromophore melanopsin, whose peak activation occurs in response to light of wavelength ~480 nm, collateral neural networks allow for adjacent rhodopsin-containing rods and cones to active ipRGCs in the absence of melanopsin (e.g. those with absent or nun-functioning Opn4; see Altimus et al., 2008). Note that there are no direct projections to the dorsal lateral geniculate nucleus and, hence, very limited stimulation of the visual pathway beyond “brightness” detection. The primary target of ipRGC projections is the SCN by direct innervation; however, an indirect route via the intergeniculate leaflet has also been described. Autonomic modulation via ipRGCs is thought to occur primarily by way of projections from the SCN to the paraventricular nucleus of the hypothalamus (PVN). Although not depicted here, neural transmission to the pineal gland occurs by way of the PVN → medial forebrain bundle → spinal cord → superior cervical ganglion (of the sympathetic nervous system) pathway. This figure is a compilation of data derived from several sources (Cajochen, 2007; Gooley et al., 2003; Hattar et al., 2006; Lockley & Gooley, 2006; McCarthy & Welsh, 2012; Scheer et al., 2003; Warthen & Provencio, 2012).
FIGURE 2.
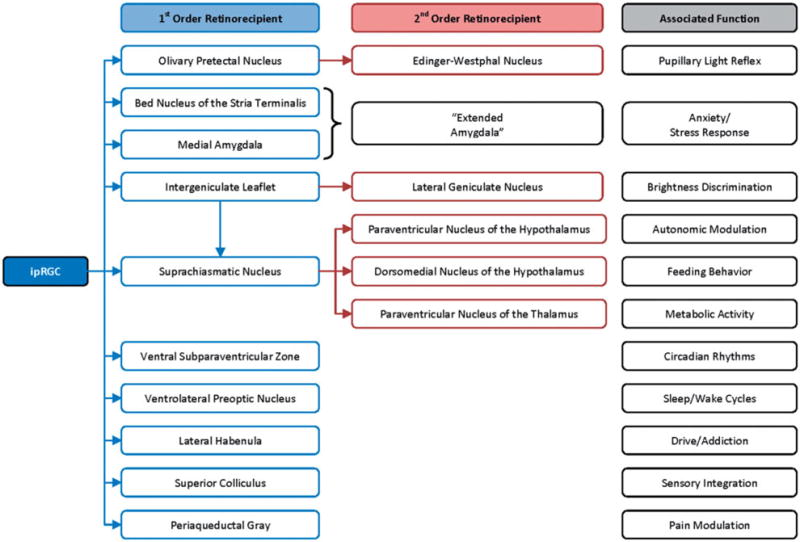
Neurological projections from ipRGCs.
SCN activation dictates clock gene expression within the SCN, which serves as the biological master clock (Thapan et al., 2001). The SCN, in turn, projects to the paraventricular nucleus of the hypothalamus (PVN), the dorsomedial nucleus of the hypothalamus and the paraventricular nucleus of the thalamus (Dai et al., 1998). Light appears to exert autonomic control via the SCN’s projections to the PVN (Scheer et al., 2003), which modulate sympathetic control of cardiovascular state and cortisol release (Hatanaka et al., 2008). Interestingly, though bright light produces sympathomimetic effects on the heart, it inhibits sympathetically driven release of cortisol from the adrenal glands (that is, independent of adrenocorticotropin hormone signaling; Jung et al., 2010) and suppresses β-adrenergic activity in the pineal gland prompting melatonin release (Scheer et al., 2003; Simmoneaux & Ribelayga, 2003). In view of the central role that the PVN plays in activating the ANS, modulation of this pathway is hypothesized to mediate a part of the antidepressant and antiinflammatory properties of VNS (Bonaz et al., 2013) and, as we have suggested, may also provide a biological substrate for BLT’s mechanism of action.
The ANS and the related sympatho-adrenal system exhibit clear circadian rhythmicity as demonstrated by several lines of evidence. Various measures of cardiac activity (e.g. HR, BP and HRV), susceptibility to arrhythmias and heart attacks, circulating catecholamine levels, etc. follow 24-hour cycles (see Fabbian et al., 2013; Portaluppi & Hermida, 2007). Further support of the link between the chronobiological neural circuitry and the ANS is derived from evidence implicating polymorphisms in PER3 (short for “period”, of the clock gene machinery) in altered sympathovagal balance (Viola et al., 2008). Similarly, Chellappa et al. (2012) have demonstrated that subjects homozygous for PER35/5 allele exhibited a significantly more pronounced alerting response to blue-enriched light (2500 K) than PER34/4 homozygotes; however, it should be noted that this study did not directly investigate indices of autonomic tone in response to light exposure.
CB AND THE ANS IN NS-MDD
CB of NS-MDD
Disruption of circadian rhythms and sleep cycles in mood disorders tends to be the rule rather than an exception (Germain & Kupfer, 2008), and evidence suggests that this is not simply epiphenomenal (see “BLT: effects on CB in NS-MDD” and “BLT: effects on the ANS in NS-MDD” below). In fact, patients with major depression often exhibit significant alterations in CB and circadian rhythms relative to non-depressed controls (for a multifaceted discussion see European Neuropsychopharmacology, 2011 [Suppl. 4]:S669–S716). Distinct rapid eye movement (REM) abnormalities on electroencephalography (EEG) are among the most consistent biomarkers for depression to date. As reviewed by Germain & Kupfer (2008), circadian disturbances in depression are suggested by a host of findings such as
Tcore in depressed patients tends to be increased on average and exhibits a decrease in circadian amplitude.
Circadian fluctuations in cortisol and norepinephrine tend to be phase-advanced in depression with a greater degree of irregularity in cortisol levels.
Initial insomnia and early morning awakenings occur in over half of depressed patients.
Insomnia increases the risk of either new-onset or relapse into depression.
Suicide rates vary diurnally and seasonally.
Changes in sleep architecture in depressed patients include shortened REM latency, increased REM density and overall duration and diminished slow-wave sleep.
Normalization of sleep architecture in depression often corresponds with treatment response.
Shortened REM latency and increased REM and REM density predict depression and may serve as an endophenotype for depression in first degree relatives.
Patterns in individuals’ sleep–wake cycles, behavioral patterns and physiological rhythms often reflect a preference for mornings (morningness or “morning larks”) or evenings (eveningness or “night owls”). Such distinctions are well-established and often described as either early (or advanced) or late (or delayed) chronotypes. Early chronotypes exhibit greater activation early in the day and tend to be phase advanced, whereas the sleep–wake cycle of late chronotype is delayed relative to the 24-hour day–night cycle (Selvi et al., 2010). As reviewed by Wirz-Justice (2008), several features of major depression suggest a link with chronotypology:
Non-seasonal depression is often characterized by some degree of DV in affect.
Positive affect in patients with major depression tends to correlate with Tcore.
Being a late chronotype (relative to early chronotypes) and DV of mood portend a better response to chronotherapeutic interventions (Bunney & Bunney, 2012).
Several neuroimaging studies have demonstrated correlations between depression and chronobiological interventions (Bunney & Bunney, 2012). For example, regional glucose utilization may predict response to wake therapy and correspond to chronotypology.
Major depression tends to be more commonly associated with phase delays, although phase advance variants have also been reported (Robillard et al., 2013). Further evidence of circadian disruption in major depression is derived from calculating the time from dim light melatonin onset to sleep onset. In healthy controls, this time period – known as the phase angle delay – is roughly 2 h in both early and late chronotypes (Sletten et al., 2010); however, the degree of phase angle delay in depression may be associated with depression severity. For example, a pilot study of non-seasonally depressed women demonstrated a significant correlation between phase angle delay and depression scores on Hamilton Depression Rating Scale (HDRS) even after sleep items were omitted (Emens et al., 2009).
The ANS in NS-MDD
A robust body of literature has demonstrated the involvement of the ANS in MDD and other mental illness although psychotropics may mediate a significant portion of these observations (Licht et al., 2008). In the largest study of its type, the Netherlands Study of Depression and Anxiety was a cohort study that enrolled 524 controls, 774 individuals with remitted MDD and 1075 with current MDD; all were assayed for HRV using the time domain value of SDNN beats and RSA (Licht et al., 2008). Multivariate analysis demonstrated reduced parasympathetic tone as evidenced by decreased RSA in non-remitted depressed subjects relative to controls and to those with remitted depression. Despite these statistically significant findings, psychotropics were found to mediate a large portion of this effect.
A few studies in drug-naïve depressed individuals have found a significantly higher sympathovagal tone among depressed patients relative to demographically matched healthy controls (Berger et al., 2012; Chang et al., 2012; Udupa et al., 2007). Such autonomic allostasis appears to be more pronounced in subjects with current or historical suicidal ideation (Chang et al., 2012) and preferentially expressed in cardiac indices of autonomic function rather than in respiratory indices (Berger et al., 2012). A select few other trials have investigated the link between MDD and ANS activity in drug-naïve subjects: one using cognitive-behavioral therapy (Carney et al., 2000) and two using acupuncture (Chambers & Allen, 2002; Rottenberg et al., 2007). We expect that evaluations of autonomic function in drug-naïve depressed patients treated with BLT would also circumvent the confounding effect of psychotropics’ autonomic effects.
Circadian rhythm of the ANS in NS-MDD
The CB of the ANS is disrupted in MDD. For example, DV of depressive signs and symptoms is a common feature of MDD, and, as described above, both DV (Bunney & Bunney, 2012) and atypical features characterized by reverse DV (Stinson & Thompson, 1990) positively predict a favorable response to BLT.
Rechlin et al. (1995a) have demonstrated that depressed patients on amitriptyline monotherapy whose symptoms improve significantly toward the evening experienced a concurrent increase in several measures of evening parasympathetic activity toward the evening including decreased resting HR, enhanced sinus arrhythmia and greater high-frequency HRV. Depressed patients without evening improvement of symptoms exhibited only mild enhancement in sinus arrhythmia in the evening relative to the morning (Rechlin et al., 1995a). In addition, a Lithuanian cohort trial by Andruškevičius (2009) demonstrated consistent circadian variations in sympathetic and parasympathetic tone in depressed patients as well as an overall elevation in sympathetic tone among depressed individuals.
A separate body of literature expounds upon the link among dysregulated neurotransmitters, neuroendocrine function and autonomic balance in depression with a focus on the hypothalamic–pituitary–adrenal axis (Trestman et al., 1995). These studies have demonstrated altered adrenergic tone and responses to anticholinergic and antiadrenergic (e.g. α2 antagonists such as clonidine) agents (Dilsaver, 1989).
NEUROBIOLOGY OF BLT
MDD is characterized by a diverse heterogeneity of phenotypes. The underlying “etiology” of depression remains elusive; instead, data have implicated a multifactorial array of potential contributors leading to the recent proposal of disorder-specific “causal signatures” (Kendler, 2012). The mechanism whereby BLT exerts its antidepressant effects is unclear but appears to involve several interrelated systems and, as such, several theories have been proffered to explain how BLT improves depressive symptoms (for reviews see Pail et al., 2011; Parry & Maurer, 2003; Rosenthal & Wehr, 1992). We provide an overview of the more commonly proposed mechanisms below but emphasize that any such explanation may harbor any number of relationships with autonomic modulation. For example, the presumed role of serotonin in the efficacy of BLT is mutually compatible with descriptions of various other biological effects of BLT, namely those of an autonomic and chronobiological nature.
Neurobiology of BLT: monoamines
Two distinct classes of monoamines have been implicated in depression and the efficacy of BLT: the tryptamines serotonin and melatonin, both being derived from the amino acid L-tryptophan and containing the indole structure, and the catecholamines norepinephrine and dopamine, which share the catechol moiety.
Serotonin
The prevailing biological model of depression centers on the role of serotonin (5-hydroxytryptamine or 5-HT), a notion bolstered by perhaps nothing greater than the preponderant role of pro-serotonergic psychotropic agents for the management of depression. In fact, several lines of study would suggest that serotonin modulation may be instrumental in the efficacy of BLT for depression such that the 2010 American Psychiatric Association Practice Guidelines for MDD cite serotonergic activity as the presumed mechanism of action for BLT.
Numerous trials have demonstrated fluctuations of serotonin transporters (SERTs) by up to 40% in response to seasonal changes (Pail et al., 2011). Light has been shown to enhance serum serotonin levels throughout the day in NS-MDD (Rao et al., 1990). Given the presence of SERTs on platelets, binding of agents with serotonin reuptake inhibition (e.g. paroxetine and imipramine) to platelet SERTs has been assayed as a measure of serotonergic activity. The most consistent positive finding of these studies related to BLT has been the correlation between depression response to BLT and decreased paroxetine (Smedh et al., 1999) or imipramine (Szádóczky et al., 1991) platelet binding. The levels of mononuclear leukocyte Gs alpha and Gi alpha proteins are known to be decreased in patients with seasonal and non-seasonal depression relative to non-depressed controls. These differences in patients with SAD dissipate in the summertime and in parallel with efficacious BLT (Avissar et al., 1999). In sum, these assays of peripheral serotonergic modulation provide evidence implicating serotonin neurotransmission in the salubrious effects of BLT on mood.
Acute depletion of L-tryptophan, a serotonin precursor, has been shown to reverse previous depression response to BLT in SAD (Neumeister et al., 1997, 1998); however, an attempt to replicate these results was unsuccessful (Lam et al., 2000). Recent genetic evidence also links SERT activity with BLT efficacy. In one study, depressed subjects were treated with wake therapy and continued on BLT to sustain treatment response (Benedetti et al., 2003b). The sustained efficacy of BLT was found to be greater in patients homozygous for the long variant of the SERT promoter region than those homozygous for the short variant.
Melatonin
Melatonin release is intimately related to light–dark circadian cycles. Patients with SAD have been found to have higher daytime melatonin levels during wintertime depression than non-depressed controls, a difference that normalized when these patients experienced summertime euthymia and after efficacious treatment of wintertime depression with BLT (Danilenko et al., 1994). A correlation between melatonin suppression and depression response to BLT has been shown in some (Lieberman et al., 1985) but not all (Rao et al., 1990) studies. A postmortem study has also demonstrated a greater density of MT1 receptors in the SCN of depressed subjects relative to healthy controls and that the MT1 receptor prevalence was inversely proportional to age of depression onset (Wu et al., 2013).
Catecholamines
Researchers have entertained the role of catecholamines in depression for half a century – the two principal catecholamine neurotransmitters being norepinephrine and dopamine. Although a large body of research has explored the role of catecholamines in depression, a limited body has investigated the role of catecholamines in response to BLT. Decreases in urinary norepinephrine excretion may parallel improvements in depression in response to BLT (Anderson et al., 1992), and catecholamine depletion has been shown to reverse previous BLT response (Neumeister et al., 1998). Nevertheless, early studies such as that by Rudorfer et al. (1993) investigating norepinephrine and its metabolites in serum and cerebrospinal fluid before and after BLT (albeit using various, unconventional dosing regimens by current standards) have been unable to yield sizeable support for the role of catecholamines in treatment response.
Neurobiology of BLT: CB
Circadian phase-shift
Bright light serves as the primary zeitgeber to entrain sleep–wake cycles and tends to push sleep periods away from it temporally, which is described by the phase-response curve (Khalsa et al., 2003). That is, morning bright light tends to “push” the sleep phase earlier causing a phase advance, whereas evening bright light tends to “push” sleep later causing a phase delay. Patients with SAD tend to exhibit phase delay in melatonin release (Dahl et al., 1993), and it was Lewy et al. who initially suggested that phase advances due to morning BLT may play a role in the antidepressant effects in SAD (Lewy et al., 1987). The most often-cited evidence suggesting that morning BLT is more efficacious than evening BLT for depression is derived from three trials published in the same issue of Archives of General Psychiatry (Wirz-Justice, 1998), all of which were in winter depression. Inconsistent data suggest that timing of BLT relative to an individual’s circadian rhythm may significantly affect antidepressant response such that each person has a maximally photosensitive window relative to the location in the circadian rhythm (Terman & Terman, 2005). One naturally wonders to what degree these data, obtained from patients with SAD who also tend to have phase delays, may generalize to those with NS-MDD, particularly in those with either no phase delay or rather phase advances.
The phase-shift theory may also explain the finding that the presence of atypical features is a positive predictive feature of response to BLT; however, only one study to date has demonstrated that the size of phase advance correlates with the magnitude of depression improvement (Terman et al., 2001). Whereas the most common circadian phase disturbance of “typical” NS-MDD (i.e. without atypical features) is phase delay (see CB of NS-MDD), it is unclear whether such patients may respond preferentially to evening BLT. For example, certain patients with SAD do appear to respond preferentially to evening BLT (Lewy et al., 1998).
Circadian amplitude
Diminished amplitude of diurnal fluctuations in several biological correlates (including Tcore, HR, melatonin, cortisol, etc.) represents a common disruption in depressed patients (Lanfumey et al., 2013). Moreover, depression is often accompanied by an overall attenuation of physiological reactivity to active stressors (Schwerdtfeger & Rosenkaimer, 2011). BLT has been shown to enhance the amplitude of circadian rhythms in Tcore in patients with SAD during wintertime depression (Rosenthal et al., 1990). Normalization in amplitude of such a core biorhythm implies an effect on temporal aspects of thermoregulation, principally on the sympathetic nervous system.
Neurobiology of BLT: the ANS
That the ANS is dysregulated in MDD is well-established; depressed individuals tend to exhibit an elevated sympathovagal balance relative to euthymic controls (see The ANS in NS-MDD), and, as we have described, research to date may suggest a role for the ANS in mediating the efficacy of BLT in depression.
VNS, which has been used in the management of treatment-resistant depression, is an invasive means of directly enhancing vagal tone, an intervention strongly suggesting that parasympathetic modulation is associated with antidepressant effects (Rush & Siefert, 2009). Given the established antidepressant effects of BLT, evidence that BLT modulates the parasympathetic nervous system (see BLT: effects on the ANS in non-depressed and BLT: effects on the ANS in NS-MDD) raises questions about a potential role – whether directly or indirectly – for the ANS antidepressant effects. Evidence for a vagally mediated cholinergic antiinflammatory pathway provides a further bridge of autonomic function and inflammatory mediators in depression such as tumor necrosis factor α and interleukin 1 (Bonaz et al., 2013; Borovikova et al., 2000).
In a prospective cohort trial, Rechlin et al. (1995b) compared non-seasonally depressed inpatients (n=30) and demographically matched euthymic controls (n = 18). All subjects were started on a tricyclic antidepressant (TCA), and adjunctive BLT was added (>2500 lux each morning from 6:00 to 7:30 AM for 14 days followed by dim light placebo of <200 lux over the same morning period for 5 days) after TCA dose titration. Non-medicated controls received BLT on the same administration schedule. Inpatients were then stratified by clinical antidepressant response based on either clinical global impression (CGI) or visual analog scale (VAS) on day 4. Those with a clinical response were found to be more likely to have enhanced high-frequency HRV after BLT than after dim-light exposure, suggesting enhanced parasympathetic activity associated with BLT (Rechlin et al., 1995b).
More recently, Choi et al. (2011) showed that among a cohort of healthy Korean adults without diagnosable mental illness red light (wavelength range 620–780, 0.4 lux) decreased two measures of HRV associated with parasympathetic activity among subjects with state anxiety or depression suggesting that light may have mood-dependent, wavelength-specific effects. In particular, this study implemented very dim light, whereas the antidepressant effects of light therapy have been demonstrated with bright light (in the range of several thousand lux); it is also notable that only short-term measures of HRV immediately before and after light exposure were evaluated. In view of these data, we imagine that studies systematically investigating the variable effects of time of day exposure, duration of exposure, and light wavelength(s) and intensity used would provide valuable information to guide the clinical use of BLT.
Neurobiology of BLT: other proposed mechanisms
Several other proposed or hypothesized biological explanations of BLT’s efficacy have garnered very limited evidence such as bilirubin qua photoreceptor, cortisol, EEG activation patterns and thermoregulation (for a review see Parry & Maurer, 2003). It is unclear to what degree the direct effect that bright light has on pupillary fluctuations or visual contrast sensitivity may play in treatment response; however, as described under CB and the ANS: the ANS, the PLR may itself be a measure of parasympathetic activity. Other potential mechanisms of action yet to be explored include the glutamatergic system (Sanacora et al., 2012) and neuroactive gases such as nitric oxide via inhibition of the neuronal nitric oxide synthase (Zhou & Zhu, 2009).
BLT: EFFECTS ON CB AND THE ANS IN NON-DEPRESSED
BLT: effects on CB in non-depressed
Light is the primary zeitgeber, and shifts in circadian rhythm are reflected in a host of biomarkers including melatonin, cortisol (Jung et al., 2010), Tcore and subjective measures of alertness (Rüger et al., 2006).
BLT: effects on the ANS in non-depressed
In trials of healthy, non-depressed patients, bright light has been shown to exert significant effects on ANS function. Saito et al. (1996) were the first to demonstrate a link between bright light and the ANS by showing that bright light (6000 lux for 20 min) enhanced muscle sympathetic nerve activity, itself a measure of post-ganglionic sympathetic nervous system activity. Bright light via the SCN has been shown to regulate HR via autonomic modulation (Scheer et al., 2001), and, more recently, a series of genetic and surgical investigations in mice has sought to map out the association between light activation of the SCN and activation of clock genes in the adrenal glands (namely Per1 and Per2) via sympathetically mediated neuronal input (Ishida et al., 2005).
Further evidence that bright light modulates autonomic function is derived from two related articles published by researchers from the Hokkaido University School of Medicine in Japan, who assessed the effects of evening bright light on HRV in a cohort of healthy women in their 20s (Kohsaka et al., 2001; Sakakibara et al., 2000). In the first of these cross-over investigations, white light (5000 lux for 2 h) delivered from 6 to 8 PM daily for five consecutive evenings led to an increase in the low-frequency (LF) band of HRV immediately after administration relative to dim-light control conditions, suggesting enhancements in sympathetic tone (Sakakibara et al., 2000). No changes in high-frequency (HF) band, LF:HF ratio or the coefficient of variance relative to dim-light control conditions were found, which suggests no immediate post-exposure parasympathetic effects (Sakakibara et al., 2000). Such enhanced sympathetic tone is consistent with findings that bright light immediately enhances alertness and performance (Myers & Badia, 1993). In the second report, following the same healthy young women, polysomnographic results revealed that bright light caused decreased LF HRV and LF:HF ratio during slow-wave sleep relative to dim-light controls (Kohsaka et al., 2001); no changes were found in HF HRV. Accordingly, it appears that the immediate enhancement of sympathetic activity is relatively short-lived, giving way to a subsequent decrease in sympathovagal tone. That recurrent bright light exposure may lead to enhanced parasympathetic activity would provide some explanation of the findings of Rechlin et al. (1995b) cited under Neurobiology of BLT: the ANS.
Further support for this interpretation was demonstrated by Ono et al. who investigated the effects of white light in post-esophagectomy patients in the intensive care unit (2011). White light delivered in an unconventional dosing pyramid (2500 lux for 15 min., then 4000 lux for 15 min., then 5000 lux for 1 h., then 4000 lux for 15 min., then 2500 lux for 15 min.) for four consecutive days led to a decrease in the LF:HF ratio at night, suggestive of deeper sleep (Ono et al., 2011). Taken together, this suggests that although bright light may enhance sympathetic tone immediately (i.e. a state change), the body subsequently compensates by decreasing sympathovagal balance (i.e. a trait change). One wonders what autonomic effects may be seen with varied exposure timing, exposure duration or recurrent exposure. Research on the ANS in response to exercise, which causes exercise-related elevations in sympathetic activity, would suggest that those who are younger (Trevizani et al., 2012) or better conditioned (Shin et al., 1995) have enhanced parasympathetic activity at baseline as revealed by enhanced measures of HRV and more responsive post-exercise HR recovery. Perhaps, then, as an application of the allostatic load theory in which depression represents a state of sub-optimal homeostasis, recurrent challenges of the sympathetic nervous system via BLT may condition the ANS, leading to enhanced parasympathetic tone and a more responsive autonomic baseline.
More recently, research has sought to clarify the roles of different wavelengths of light on the ANS, although data are not always consistent (for brief reviews see the introductions to Choi et al., 2011; Rajae-Joordens, 2011). Studies that investigate wavelength-dependent effects on arousal, affective valence and autonomic indices often vary widely in their characterization of light properties (e.g. hue, chroma/saturation and value/lightness versus monochromatic or narrow-band light measured in lux at the level of the cornea) and consequently light intensity experienced by the observer, exposure duration (from a minute to hours), time of day (some fail to specify) and operationalized outcome measures particularly including the temporal course of effects (e.g. HRV over 5 min. during or immediately following exposure versus 24-hour monitoring). Evidence suggests that each of these variables modulates the resultant effects of light on autonomic tone. For example, variations in illumination within the spectrum of standard ambient room lighting (90–180 lux) significantly affect physiological responses (Cajochen et al., 2000). Furthermore, lower- and higher-order cognitive processes including learned associations, domain-specific tasks and associated motivational constructs (Mehta & Zhu, 2009) and even transient mood states (Choi et al., 2011) significantly modulate physiological and neurocognitive responses to colored light exposure.
Studies that evaluate autonomic effects of colored light tend to measure autonomic indices either during or immediately following colored light exposure (see An et al., 2009; Laufer et al., 2009; Schäfer & Kratky, 2006); hence, it is clear that changes in autonomic function in response to colored illumination are detectable on the order of minutes, and many studies do find wavelength-specific effects despite the wide variety of protocols. Perhaps the most consistent wavelength-specific effects have been found with short-wavelength light ~480 nm (blue light). The ipRGCs principally responsible for transmitting photic stimuli to the SCN bear the photopigment melanopsin whose peak absorption is ~480 nm (Enezi et al., 2011), and studies have shown that this wavelength of light has particularly activating effects (Huang et al., 2009) and increases both HR and Tcore (Cajochen et al., 2005).
BLT: circadian rhythm of the ANS in non-depressed
Current research continues to elaborate the central role of the SCN in synchronizing systemic circadian rhythms via the ANS (Scheer et al., 2003). The two divisions of the ANS appear to serve complementary roles in the DV of HR. The underlying circadian rhythm of HR appears to be regulated predominantly by the inhibitory influence of the parasympathetic nervous system, whereas bright light causes a largely sympathetically driven, phase-dependent increase in HR with greater effects in the morning (Scheer et al., 2004).
The ability of light during the day to synchronize DV in the ANS has been demonstrated most clearly by forced desynchrony protocols (FDPs) in which sleep– wake/behavior cycles have been uncoupled from the endogenous circadian cycle (i.e. repeating sleep–wake cycles lasting longer than 24 h in the presence of constant lighting and the absence of external zeitgebers). Although the ANS is naturally entrained to the sleep–wake and light–dark cycles, such FDPs investigating linear time domains of HRV have demonstrated endogenous circadian rhythmicity of ANS independent of behavioral or sleep–wake cycles (see Hilton et al., 2000). It should also be noted that studies of biological effects of bright light on CB and the ANS suggest that physical variables of light such as intensity, temperature, wavelength, etc. exert differential effects on the ANS as measured by HRV (Ishibashi et al., 2007). Other considerations necessarily include retinal sensitivity of different photoreceptive elements and circadian phase and phase shifting.
In general, periods of sleep are associated with increased parasympathetic activity during all sleep phases – during REM and non-REM sleep – although sympathetic tone and the sympathovagal ratio exhibit phase-dependent variations (Tsunoda et al., 2001). During REM sleep, sympathetic tone is elevated and may even be elevated above levels found during wakefulness; however, the sympathovagal ratio is decreased selectively during slow wave sleep (Tsunoda et al., 2001). Similarly, delta waves on EEG are inversely correlated with low-frequency and LF/HF HRV (each an oblique measure sympathetic tone) providing further evidence that deeper levels of sleep are accompanied by decrements in sympathetic activity (Ako et al., 2003).
BLT: EFFECTS ON CB AND THE ANS IN NS-MDD
BLT: effects on CB in NS-MDD
Research on the effects of BLT in non-seasonally depressed individuals’ CB is limited – generally being extrapolated from data in SAD. For example, as cited above, DV of mood positively predicts response to BLT, and one study has found an association between degree of phase advance and depression response to BLT (Terman et al., 2001). Interest, though, has been growing in clock gene machinery in depression, particularly related to the rapid antidepressant effects of wake therapy and other chronotherapeutic modalities (Bunney & Potkin, 2008). Wulff et al. (2010; see Supplement 2) and Etain et al. (2011) have reviewed data on clock gene machinery implicated in affective disorders including both seasonal and non-seasonal depression; however, data remain limited on the role that such genetic associations play in modulating response to BLT or other chronobiological interventions. In view of the work of Roecklein et al. (2009) who demonstrated an association between a missense variant of the melanopsin (OPN4) gene and a subset of patients with winter seasonal depression, one wonders whether OPN4 gene variants would be associated with response to BLT as melanopsin is integrally responsible for the chronobiological effects of light.
BLT: effects on the ANS in NS-MDD
A systematic search of Medline crossing three groups of related terms – those related to depression, BLT and the ANS4 – yielded a select few relevant articles despite the clear effects of bright light on the ANS and the established antidepressant properties of BLT.
As described above (see Neurobiology of BLT: the ANS), a trial by Rechlin et al. (1995b) compared the effects of BLT on HRV in non-seasonally depressed inpatients (n = 30) versus non-medicated, euthymic controls (n = 18). Patients whose depression improved by day 4 were found to have greater HF-HRV than those without improvement suggesting a correlation between study-defined response and greater parasympathetic tone. A host of limitations, though, constrain any conclusions that can be drawn. More salient limitations include the following: (1) defining response by VAS or CGI (rather than HDRS) and that on day four of treatment, which is expected to be premature, (2) using concurrent anticholinergic medication that would be expected to confound measurements of HRV, (3) implementing non-standard light intensity and duration by current recommendations, and (4) limiting evaluations of ANS tone to two 5-minute epochs immediately post-exposure. A host of ancillary evaluations or assessments could provide meaningful clinical information such as measures of circadian rhythm shifts or chronobiological correlates (e.g. dim-light melatonin onset, Tcore, etc.), EEG or polysomnography.
Two other studies indirectly address the question of BLT’s effect on the ANS in depression. In the first of these, Choi et al. (2011; also cited under Neurobiology of BLT: the ANS above) demonstrated wavelength-specific effects, which were further modified by state anxiety or depression. In a separate study, 19 patients with non-psychotic bipolar depression were evaluated by single proton magnetic resonance spectroscopy before and after repeated wake therapy combined with BLT (Benedetti et al., 2009). A decrease in (glutamate + glutamine)/creatine ratio accompanied improvements in both objective and subjective measure in depressive symptoms, although the implementation of wake therapy in addition to BLT confounds any conclusions that can be drawn regarding BLT-specific autonomic effects.
BLT: effects on circadian rhythm of the ANS in NS-MDD
The literature search as defined above (see footnote 4 under BLT: effects on the ANS in NS-MDD) yielded no clear evidence that bright light’s effects on the chronobiological aspects of the ANS in non-seasonally depressed patients has been investigated, although we expect that this area of research may hold great potential in view of the data catalogued above. In summary, we have proposed that the chronobiological effects of BLT on the ANS may be integral to its antidepressant properties, emphasizing the role of BLT in non-seasonal depression. We have marshaled a corpus of evidence in support of our hypothesis, which we imagine could have both treatment and prognostic implications (e.g. could limited HRV predict positive response to BLT?). We further reiterate that BLT and chronotherapeutics have the potential for application in investigating the link between depression and coronary events. This review seeks to raise important questions for future investigation by providing the rationale in support of a body of testable hypotheses.
TABLE 2.
Potential limitations of BLT versus antidepressant medication.
| 1. | Greater up-front cost relative to medications (i.e. the cost of the light-delivery device). |
| 2. | Requirement of at least half an hour investment each day for use based on current recommendations (American Psychiatric Association, 2010). |
| 3. | Recommendation that it be used in the morning as evening BLT has generally been shown to be less efficacious (Wirz-Justice, 1998). |
| 4. | Evening BLT may cause initial insomnia. |
| 5. | As with the relative rapidity of clinical effect (within a week), the loss of efficacy can be seen within days of discontinuation. |
| 6. | Unclear application in patients with retinal/ocular pathology or on photosensitizing medications. |
| 7. | Very limited potential UV radiation exposure. |
| 8. | Menstrual irregularities have been reported. |
| 9. | Light-delivery devices do not qualify as “durable medical equipment” by Medicare; hence, they:a a. Are not covered by Medicare although coverage varies among private insurance companies b. Are not regulated by the U.S. FDA making it difficult to discern the accuracy of manufacturers’ claims c. Do not carry legally-prescribed indications per the FDA d. Are not currently under patent for use as BLT (see Studwell, 2004). |
| 10. | Absence of true “dose-finding” (phase I) studies akin to those required for medications. |
| 11. | In terms of defining standard of care, BLT (and other chronotherapeutic techniques) is largely excluded from treatment algorithms and guidelines on the management of non-seasonal depression (see Casher et al., 2012). |
The implications here are myriad. That BLT does not require a prescription by a licensed medical clinician (i.e. physician or nurse practitioner) may be interpreted as a limitation (i.e. we maintain that BLT should be exercised in concert with a clinician with knowledge of major depression, the various treatment options, and who has clinical experience with BLT) or a benefit (i.e. clinical psychologists may develop proficiency in the use of BLT for their patients). Furthermore, manufacturers of light-delivery devices have limited financial incentive to pursue efficacy trials, which constrains the literature on BLT. Such heterogeneity in the literature regarding BLT “may have created the unsubstantiated impression that the treatment itself has limitations in terms of its efficacy” (Golden et al., 2005).
Footnotes
We use the term NS-MDD to identify all presentations of MDD exclusive of seasonal affective disorder (SAD). Per the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition (DSM 5), this includes all instances of MDD Single Episode and MDD Recurrent that do not meet criteria for the With Seasonal Pattern specifier.
This author acknowledges the linguistic and practical inaccuracy of the term “prescribing” related to chronotherapeutics, particularly as no prescription is required. However, the words of Dilsaver (1989) remain timely over two decades later: “Light can be regarded both as a Zeitgeber and as a drug.” Furthermore, we are currently conducting such a survey to evaluate the attitudes of U.S. psychiatrists toward BLT.
In this proposal, we appeal to the pioneer of the randomized clinical trial, A. B. Hill, who articulated criteria by which to assess causality. He cites “biological gradient,” “plausibility” and “coherence” as integral to establishing causation, each of which speaks to a biological premise upon which to base clinical judgment (Hill, 1965).
Included search terms related to depression (depress*, emotion, mood and affective), BLT (bright light, light therapy, chronotherap*, phototherap*, color* and illuminat*) and ANS (autonomic, sympathetic, adren*, parasympathetic, sympathovagal, vagus, vagal, ganglion*, *cholin* and muscarinic).
DECLARATION OF INTEREST: Neither author has any financial conflicts of interest to disclose.
References
- Ako M, Kawara T, Uchida S, et al. Correlation between electroencephalography and heart rate variability during sleep. Psychiatry Clin Neurosci. 2003;57:59–65. doi: 10.1046/j.1440-1819.2003.01080.x. [DOI] [PubMed] [Google Scholar]
- Altimus C, Güler A, Villa K. Rods-cones and melanopsin detect light and dark to modulate sleep independent of image formation. Proc Natl Acad Sci USA. 2008;105:19998–20003. doi: 10.1073/pnas.0808312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association, Work Group on Major Depressive Disorder. Practice guideline for the treatment of patients with major depressive disorder. (3rd) 2010 Accessed at http://psychiatryonline.org/data/Books/prac/PG_Depression3rdEd.pdf on 12 August 2012.
- Huang An M, Shimomura J, Katsuura YT. Time-of-day-dependent effects of monochromatic light exposure on human autonomic nervous system. J Hum Environ Syst. 2009;12:55–61. [Google Scholar]
- Anderson J, Vasile R, Mooney J, et al. Changes in norepinephrine output following light therapy for fall/winter seasonal depression. Biol Psychiatry. 1992;32:700–4. doi: 10.1016/0006-3223(92)90299-f. [DOI] [PubMed] [Google Scholar]
- Andruškevičius S. Parameters of the spectral analysis of the heart rate variability in treating depression. Medicina (Kaunas) 2009;45:214–20. [PubMed] [Google Scholar]
- Avissar S, Schreiber G, Nechamkin Y, et al. The effects of seasons and light therapy on G protein levels in mononuclear leukocytes of patients with seasonal affective disorder. Arch Gen Psychiatry. 1999;56:178–83. doi: 10.1001/archpsyc.56.2.178. [DOI] [PubMed] [Google Scholar]
- Bär KJ, Greiner W, Jochum T, et al. The influence of major depression and its treatment on heart rate variability and pupillary light reflex parameters. J Affect Disord. 2004;82:245–52. doi: 10.1016/j.jad.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colombo C, Pontiggia A, et al. Morning light treatment hastens the antidepressant effect of citalopram: A placebo-controlled trial. J Clin Psychiatry. 2003a;64:648–58. doi: 10.4088/jcp.v64n0605. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Colombo C, Serretti A, et al. Antidepressant effects of light therapy combined with sleep deprivation are influenced by a functional polymorphism within the promoter of the serotonin transporter gene. Biol Psychiatry. 2003b;54:687–92. doi: 10.1016/s0006-3223(02)01894-2. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Calabrese G, Bernasconi A, et al. Spectroscopic correlates of antidepressant response to sleep deprivation and light therapy: A v3.0 Tesla study of bipolar depression. Psychiatry Res. 2009;173:238–42. doi: 10.1016/j.pscychresns.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Berger S, Kliem A, Yeragani V, Bär K. Cardio-respiratory coupling in untreated patients with major depression. J Affect Disorders. 2012;139:166–71. doi: 10.1016/j.jad.2012.01.035. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Picq C, Sinniger V, et al. Vagus nerve stimulation: From epilepsy to the cholinergic anti-inflammatory pathway. Neurogastroenterol Motil. 2013;25:208–21. doi: 10.1111/nmo.12076. [DOI] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Brown R, James C, Henderson L, Macefield V. Autonomic markers of emotional processing: Skin sympathetic nerve activity in humans during exposure to emotionally charged images. Front Physiol. 2012;3:1–6. doi: 10.3389/fphys.2012.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney J, Potkin S. Circadian abnormalities, molecular clock genes and chronobiological treatments in depression. Br Med Bulletin. 2008;86:23–32. doi: 10.1093/bmb/ldn019. [DOI] [PubMed] [Google Scholar]
- Bunney B, Bunney W. Rapid-acting antidepressant strategies: Mechanisms of action. Int J Neuropsychopharmacol. 2012;15:695–713. doi: 10.1017/S1461145711000927. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Zeitzer J, Czeisler C, Dijk D. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Münch M, Kobialka S, et al. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–16. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- Cajochen C. Alerting effects of light. Sleep Med Rev. 2007;11:453–64. doi: 10.1016/j.smrv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Campbell P. A decade for psychiatric disorders. Nature. 2010;463:9. doi: 10.1038/463009a. [DOI] [PubMed] [Google Scholar]
- Carney R, Freedland K. Depression and heart rate variability in patients with coronary heart disease. Cleve Clin J Med. 2009;76:S13–17. doi: 10.3949/ccjm.76.s2.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney R, Freeland K, Stein P, et al. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med. 2000;62:639–47. doi: 10.1097/00006842-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Casher M, Schuldt S, Haq A, Burkhead-Weiner D. Chronotherapy in treatment-resistant depression. Psychiatric Annals. 2012;42:166–9. [Google Scholar]
- Chambers A, Allen J. Vagal tone as indicator of treatment response in major depression. Psychophysiology. 2002;39:861–4. doi: 10.1111/1469-8986.3960861. [DOI] [PubMed] [Google Scholar]
- Chang H, Chang C, Chen C, et al. Major depression is associated with cardiac autonomic dysregulation. Acta Neuropsychiatrica. 2012;24:318–27. doi: 10.1111/j.1601-5215.2011.00647.x. [DOI] [PubMed] [Google Scholar]
- Chellappa S, Viola A, Schmidt C, et al. Human melatonin and alerting response to blue-enriched light depend on a polymorphism in the clock gene PER3. J Clin Endocrinol Metab. 2012;97:E433–7. doi: 10.1210/jc.2011-2391. [DOI] [PubMed] [Google Scholar]
- Choi C, Kim K, Kim C, et al. Reactivity of heart rate variability after exposure to colored lights in healthy adults with symptoms of anxiety and depression. Int J Psychophysiol. 2011;79:83–8. doi: 10.1016/j.ijpsycho.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Czeisler C, Allan J, Stragatz S, et al. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–71. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- Dahl K, Avery D, Lewy A, et al. Dim light melatonin onset and circadian temperature during a constant routine in hypersomnic winter depression. Acta Psychiatr Scand. 1993;88:60–6. doi: 10.1111/j.1600-0447.1993.tb03414.x. [DOI] [PubMed] [Google Scholar]
- Dai J, Swaab D, Van der Vliet J, Buijs R. Postmortem tracing reveals the organization of hypothalamic projections of the suprachiasmatic nucleus in the human brain. J Comp Neurol. 1998;400:87–102. doi: 10.1002/(sici)1096-9861(19981012)400:1<87::aid-cne6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Danilenko K, Putilov A, Russkikh G, et al. Diurnal and seasonal variations of melatonin and serotonin in women with seasonal affective disorder. Arct Med Res. 1994;53:137–45. [PubMed] [Google Scholar]
- Dilsaver S. Neurobiologic effects of bright artificial light. Brain Res Brain Res Rev. 1989;14:311–33. doi: 10.1016/0165-0173(89)90016-7. [DOI] [PubMed] [Google Scholar]
- Duman R, Aghajanian G. Synaptic dysfunction in depression: Potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emens J, Lewy A, Kinzie J, et al. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168:259–61. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Enezi J, Revell V, Brown T, et al. A “melanopic” spectral efficiency function predicts the sensitivity of melanopsin photoreceptors to polychromatic lights. J Biol Rhythms. 2011;26:314–23. doi: 10.1177/0748730411409719. [DOI] [PubMed] [Google Scholar]
- Etain B, Milhiet V, Bellivier F, Leboyer M. Genetics of circadian rhythms and mood spectrum disorders. Eur Neuropsychopharmacology. 2011;21:S676–82. doi: 10.1016/j.euroneuro.2011.07.007. [DOI] [PubMed] [Google Scholar]
- Fabbian F, Smolensky M, Tiseo R, et al. Dipper and non-dipper blood pressure 24-hour patterns: Circadian rhythm-dependence physiological and pathophysiologic mechanisms. Chronobiol Int. 2013;30:17–30. doi: 10.3109/07420528.2012.715872. [DOI] [PubMed] [Google Scholar]
- Fischer R, Kasper S, Pjrek E, Winkler D. On the application of light therapy in German-speaking countries. Eur Arch Psychiatry Clin Neurosci. 2012;262:501–5. doi: 10.1007/s00406-011-0286-3. [DOI] [PubMed] [Google Scholar]
- Germain A, Kupfer D. Circadian rhythm disturbances in depression. Hum Psychopharmacol. 2008;23:571–85. doi: 10.1002/hup.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetzel R, Pei X, Tabrizi M, et al. Ten modifiable health risk factors are linked to more than one-fifth of employer-employee health care spending. Health Aff (Millwood) 2012;31:2474–84. doi: 10.1377/hlthaff.2011.0819. [DOI] [PubMed] [Google Scholar]
- Golden R, Gaynes B, Ekstrom R, et al. The efficacy of light therapy in the treatment of mood disorders: A review and meta-analysis of the evidence. Am J Psychiatry. 2005;162:656–62. doi: 10.1176/appi.ajp.162.4.656. [DOI] [PubMed] [Google Scholar]
- Gooley J, Lu J, Fischer D, Saper C. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Stein P. Circadian rhythm in the cardiovascular system: Considerations in non-invasive electrophysiology. Card Electrophysiol Rev. 2002;6:267–72. doi: 10.1023/a:1016337210738. [DOI] [PubMed] [Google Scholar]
- Hatanaka M, Tanida M, Shintani N, et al. Lack of light-induced elevation of renal sympathetic nerve activity and plasma corticosterone levels in PACAP-deficient mice. Neurosci Lett. 2008;444:153–6. doi: 10.1016/j.neulet.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–49. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. The environment and disease: Association or causation? Proc R Soc Med. 1965;58:295–300. [PMC free article] [PubMed] [Google Scholar]
- Hilton M, Umali M, Czeisler C, et al. Endogenous circadian control of the human autonomic nervous system. Comput Cardiol. 2000;27:197–200. [PubMed] [Google Scholar]
- Huang J, An M, Shimomura Y, Katsuura T. Non-visual biological effect of monochromatic light on human circadian rhythm of physiological and psychological parameters. Proc Int Assoc Soc Design Res. 2009:3887–90. [Google Scholar]
- Insel T. Disruptive insights in psychiatry: Transforming a clinical discipline. J Clin Invest. 2009;199:700–5. doi: 10.1172/JCI38832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi K, Kitamura S, Kozaki T, Yasukouchi A. Inhibition of heart rate variability during sleep in humans by 6700 K pre-sleep light exposure. J Physiol Anthropol. 2007;26:39–43. doi: 10.2114/jpa2.26.39. [DOI] [PubMed] [Google Scholar]
- Ishida S, Nakagawa M, Fujino T, et al. Circadian variation of QT interval dispersion: Correlation with heart rate variability. J Electrocardiol. 1997;30:205–10. doi: 10.1016/s0022-0736(97)80005-2. [DOI] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, et al. Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metab. 2005;2:297–307. doi: 10.1016/j.cmet.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Jung C, Khalsa S, Scheer F, et al. Acute effects of bright light exposure on cortisol levels. J Biol Rhythms. 2010;25:208–16. doi: 10.1177/0748730410368413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper S, Ruhrmann S, Neumann S, Möller H. Use of light therapy in German psychiatric hospitals. Eur Psychiatry. 1994;9:288–92. [Google Scholar]
- Kendler K. The dappled nature of causes of psychiatric illness: Replacing the organic–functional/hardware–software dichotomy with empirically based pluralism. Mol Psychiatry. 2012;17:377–88. doi: 10.1038/mp.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Chiu W, Demler O, Walters E. Prevalence, severity, and comorbidity of twelve-month DSM-IV disorders in the National Comorbidity Survey Replication (NCS-R) Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa S, Jewett M, Cajochen C, Czeisler C. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–52. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohsaka M, Kohsaka S, Fukuda N, et al. Effects of bright light exposure on heart rate variability during sleep in young women. Psychiatry Clin Neurosci. 2001;55:283–4. doi: 10.1046/j.1440-1819.2001.00861.x. [DOI] [PubMed] [Google Scholar]
- Kraft M, Martin R. Chronobiology and chronotherapy in medicine. Dis Mon. 1995;41:506–75. doi: 10.1016/s0011-5029(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Lam R, Bowering T, Tam E, et al. Effects of rapid tryptophan depletion in patients with seasonal affective disorder in natural summer remission. Psychol Med. 2000;30:79–87. doi: 10.1017/s003329179900152x. [DOI] [PubMed] [Google Scholar]
- Lam R, Levitt A, Levitan R, et al. The Can-SAD study: A randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. Am J Psychiatry. 2006;163:805–12. doi: 10.1176/ajp.2006.163.5.805. [DOI] [PubMed] [Google Scholar]
- Lanfumey L, Mongeau R, Hamon M. Biological rhythms and melatonin in mood disorders and their treatments. Pharmacol Ther. 2013;138:176–84. doi: 10.1016/j.pharmthera.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Laufer L, Láng E, Izsó L, Németh E. Psychophysiological effects of coloured lighting in older adults. Lighting Res Technol. 2009;41:371–8. [Google Scholar]
- Leucht S, Hierl S, Kissling W, et al. Putting the efficacy of psychiatry and general medicine medication into perspective: Review of meta-analyses. Br J Psychiatry. 2012;200:97–106. doi: 10.1192/bjp.bp.111.096594. [DOI] [PubMed] [Google Scholar]
- Lewy A, Sack R, Miller L, Hoban T. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235:352–4. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- Lewy A, Bauer V, Cutler N, et al. Morning vs. evening light treatment of patients with winter depression. Arch Gen Psychiatry. 1998;55:890–6. doi: 10.1001/archpsyc.55.10.890. [DOI] [PubMed] [Google Scholar]
- Licht C, Penninx B, de Geus E. Effects of antidepressants, but not psychopathology, on cardiac sympathetic control: A longitudinal study. Neuropsychopharmacology. 2012;37:2487–95. doi: 10.1038/npp.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht C, de Geus E, Zitman F, et al. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Arch Gen Psychiatry. 2008;65:1358–67. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- Lieberman H, Garfield G, Waldhauser F, et al. Possible behavioral consequences of light-induced changes in melatonin availability. Ann N Y Acad Sci. 1985;453:242–52. doi: 10.1111/j.1749-6632.1985.tb11814.x. [DOI] [PubMed] [Google Scholar]
- Lindsey K, Rohan K, Roecklein K, Mahon J. Surface facial electromyography, skin conductance, and self-reported emotional responses to light- and season-relevant stimuli in seasonal affective disorder. J Affect Disord. 2011;133:311–19. doi: 10.1016/j.jad.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockley S, Gooley J. Circadian photoreception: Spotlight on the brain. Curr Biol. 2006;16:R795–7. doi: 10.1016/j.cub.2006.08.039. [DOI] [PubMed] [Google Scholar]
- Martiny K. Adjunctive bright light in non-seasonal major depression. Acta Psychiatr Scand. 2004;425:7–28. doi: 10.1111/j.1600-0447.2004.00460_2.x. [DOI] [PubMed] [Google Scholar]
- McCarthy M Welsh D. Cellular circadian clocks in mood disorders. J Biol Rhythms. 2012;27:339–52. doi: 10.1177/0748730412456367. [DOI] [PubMed] [Google Scholar]
- McEwen B. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Mehta R, Zhu R. Blue or red? Exploring the effect of color on cognitive task performances. Science. 2009;323:1226–9. doi: 10.1126/science.1169144. [DOI] [PubMed] [Google Scholar]
- Myers B, Badia P. Immediate effects of different light intensities on body temperature and alertness. Physiol Behav. 1993;54:199–202. doi: 10.1016/0031-9384(93)90067-p. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Praschak-Rieder P, Hesselmann B, et al. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;554:133–8. doi: 10.1001/archpsyc.1997.01830140043008. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Turner E, Matthews J, et al. Effects of tryptophan depletion vs catecholamine depletion in patients with seasonal affective disorder in remission with light therapy. Arch Gen Psychiatry. 1998;55:524–30. doi: 10.1001/archpsyc.55.6.524. [DOI] [PubMed] [Google Scholar]
- Okamoto-Mizuno K, Mizuno K. Effects of thermal environment on sleep and circadian rhythm. J Physiol Anthropol. 2012;31:1–9. doi: 10.1186/1880-6805-31-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono H, Taguchi T, Kido Y, et al. The usefulness of bright light therapy for patients after oesophagectomy. Intensive Crit Care Nurs. 2011;27:158–66. doi: 10.1016/j.iccn.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Packard G, Sollars P. Intrinsically photosensitive retinal ganglion cells. Rev Physiol Biochem Pharmacol. 2012;162:59–90. doi: 10.1007/112_2011_4. [DOI] [PubMed] [Google Scholar]
- Pail G, Huf W, Pjrek E, et al. Bright-light therapy in the treatment of mood disorders. Neuropsychobiology. 2011;64:152–62. doi: 10.1159/000328950. [DOI] [PubMed] [Google Scholar]
- Parry B, Maurer E. Light treatment of mood disorders. Dialogues Clin Neurosci. 2003;5:353–65. doi: 10.31887/DCNS.2003.5.4/bparry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges S. The polyvagal theory: Phylogenetic substrates of a social nervous system. Int J Psychophysiol. 2001;42:123–46. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Hermida R. Circadian rhythms in cardiac arrhythmias and opportunities for their chronotherapy. Adv Drug Deliv Rev. 2007;59:940–51. doi: 10.1016/j.addr.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Rajae-Joordens R. The effects of colored light on valence and arousal: Investigating responses through subjective evaluations and psycho-physiological measurements. In: Westerink J, Krans M, Ouwerkerk M, editors. Sensing emotions. Vol. 12. Dordrecht, Netherlands: Philips Research Book Series; 2011. pp. 65–84. [Google Scholar]
- Rao M, Müller-Oerlinghausen B, Mackert A, et al. The influence of phototherapy on serotonin and melatonin in nonseasonal depression. Pharmacopsychiatry. 1990;23:155–8. doi: 10.1055/s-2007-1014499. [DOI] [PubMed] [Google Scholar]
- Rechlin T, Weis M, Kaschka W. Is diurnal variation of mood associated with parasympathetic activity? J Affect Disord. 1995a;34:249–55. doi: 10.1016/0165-0327(95)00011-b. [DOI] [PubMed] [Google Scholar]
- Rechlin T, Weis M, Schneider K, et al. Does bright-light therapy influence autonomic heart-rate parameters? J Affect Disord. 1995b;34:131–7. doi: 10.1016/0165-0327(95)00010-k. [DOI] [PubMed] [Google Scholar]
- Robillard R, Naismith S, Rogers N, et al. Delayed sleep phase in young people with unipolar or bipolar affective disorders. J Affect Disord. 2013;145:260–3. doi: 10.1016/j.jad.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Roecklein K, Rohan K, Duncan W, et al. A missense variant (P10L) of the melanopsin (OPN4) gene in seasonal affective disorder. J Affect Disord. 2009;114:279–85. doi: 10.1016/j.jad.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N, Levendosky A, Skwerer R, et al. Effects of light treatment on core body temperature in seasonal affective disorder. Biol Psychiatry. 1990;27:39–50. doi: 10.1016/0006-3223(90)90018-w. [DOI] [PubMed] [Google Scholar]
- Rosenthal N, Wehr T. Towards understanding the mechanisms of action of light in seasonal affective disorder. Pharmacopsychiatry. 1992;25:56–60. doi: 10.1055/s-2007-1014389. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Chambers A, Allen J, Manber R. Cardiac vagal control on the severity and course of depression: The importance of symptomatic heterogeneity. J Affect Disord. 2007;103:173–9. doi: 10.1016/j.jad.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudorfer M, Skwerer R, Rosenthal N. Biogenic amines in seasonal affective disorder: Effects of light therapy. Psychiatry Res. 1993;46:19–28. doi: 10.1016/0165-1781(93)90004-z. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Kasper S, Hawellek B, et al. Effects of fluoxetine versus bright light in the treatment of seasonal affective disorder. Psychol Med. 1998;28:923–33. doi: 10.1017/s0033291798006813. [DOI] [PubMed] [Google Scholar]
- Rüger M, Gordijn M, Beersma D, et al. Time-of-day-dependent effects of bright light exposure on human psychophysiology: Comparison of daytime and nighttime exposure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1413–20. doi: 10.1152/ajpregu.00121.2005. [DOI] [PubMed] [Google Scholar]
- Rush A, Siefert S. Clinical issues in considering vagus nerve stimulation for treatment-resistant depression. Exp Neurol. 2009;219:36–43. doi: 10.1016/j.expneurol.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Saito Y, Shimizu T, Takahashi Y, et al. Effect of bright light exposure on muscle sympathetic nerve activity in human. Neurosci Lett. 1996;219:135–7. doi: 10.1016/s0304-3940(96)13171-2. [DOI] [PubMed] [Google Scholar]
- Sakakibara S, Honma H, Kohsaka M, et al. Autonomic nervous function after evening bright light therapy: Spectral analysis of heart rate variability. Psychiatry Clin Neurosci. 2000;54:363–4. doi: 10.1046/j.1440-1819.2000.00716.x. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalco A, Rondon M, Trombetta I, et al. Muscle sympathetic nervous activity in depressed patients before and after treatment with sertraline. J Hypertens. 2009;27:2429–36. doi: 10.1097/HJH.0b013e3283310ece. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Kratky K. The effect of colored illumination on heart rate variability. Forsch Komplementmed. 2006;13:167–73. doi: 10.1159/000092644. [DOI] [PubMed] [Google Scholar]
- Scheer F, Ter Horst G, van der Vliet J, Buijs R. Physiological and anatomic evidence for regulation of the heart by suprachiasmatic nucleus in rats. Am J Physiol Heart Circ Physiol. 2001;280:H1391–9. doi: 10.1152/ajpheart.2001.280.3.H1391. [DOI] [PubMed] [Google Scholar]
- Scheer F, Kalsbeek A, Buijs R. Cardiovascular control by the suprachiasmatic nucleus: Neural and neuroendocrine mechanism in human and rat. Biol Chem. 2003;384:697–709. doi: 10.1515/BC.2003.078. [DOI] [PubMed] [Google Scholar]
- Scheer F, Van Doornen L, Buijs R. Light and diurnal cycle affect autonomic cardiac balance in human; possible role for the biological clock. Auton Neurosci. 2004;110:44–8. doi: 10.1016/j.autneu.2003.03.001. [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger A, Rosenkaimer A. Depressive symptoms and attenuated physiological reactivity to laboratory stressors. Biol Psychiatry. 2011;87:430–8. doi: 10.1016/j.biopsycho.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Selvi Y, Aydin A, Boysan M, et al. Associations between chronotype, sleep quality, suicidality, and depressive symptoms in patients with major depression and healthy controls. Chronobiol Int. 2010;27:1813–28. doi: 10.3109/07420528.2010.516380. [DOI] [PubMed] [Google Scholar]
- Shin K, Minamitani H, Onishi S, et al. Assessment of training-induced autonomic adaptations in athletes with spectral analysis of cardiovascular variability signals. Jpn J Physiol. 1995;45:1053–69. doi: 10.2170/jjphysiol.45.1053. [DOI] [PubMed] [Google Scholar]
- Simmoneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: A review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55:325–95. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- Sletten T, Vincenzi S, Redman J, et al. Timing of sleep and its relationship with the endogenous melatonin rhythm. Front Neurol. 2010;1:1–8. doi: 10.3389/fneur.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedh K, Spigset O, Allard P, et al. Platelet [3H]paroxetine and [3H]lysergic acid diethylamide binding in seasonal affective disorder and the effect of bright light therapy. Biol Psychiatry. 1999;45:464–70. doi: 10.1016/s0006-3223(98)00069-9. [DOI] [PubMed] [Google Scholar]
- Stinson D, Thompson C. Clinical experience with phototherapy. J Affect Disord. 1990;18:129–35. doi: 10.1016/0165-0327(90)90069-k. [DOI] [PubMed] [Google Scholar]
- Streeter C, Gerbarg P, Saper R, et al. Effects of yoga on the autonomic nervous system, gamma-amino-butyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med Hypotheses. 2012;78:571–9. doi: 10.1016/j.mehy.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Studwell J. All sleepless and light. Financial Times. 2004 Accessed at http://www.cet.org/docs/press/chrono/Financial_Times_2004.pdf on April 9, 2013.
- Szádóczky E, Falus A, Németh A, et al. Effect of phototherapy on 3H-imipramine binding sites in patients with SAD, non-SAD and in healthy controls. J Affect Disord. 1991;22:179–84. doi: 10.1016/0165-0327(91)90063-x. [DOI] [PubMed] [Google Scholar]
- Székely M. The vagus nerve in thermoregulation and energy metabolism. Auton Neurosci. 2000;85:26–38. doi: 10.1016/S1566-0702(00)00217-4. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- Terman J, Terman M, Lo E, Cooper T. Circadian time of morning light administration and therapeutic response in winter depression. Arch Gen Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- Terman M, Terman J. Light therapy for seasonal and nonseasonal depression: Efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–63. doi: 10.1017/s1092852900019611. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene D. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J, Lane R. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev. 2009;33:81–8. doi: 10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Thayer J, Brosschot J. Psychosomatics and psychopathology: Looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–8. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Trestman R, Yehuda R, Coccaro E, et al. Diurnal neuroendocrine and autonomic function in acute and remitted depressed male patients. Biol Psychiatry. 1995;37:448–56. doi: 10.1016/0006-3223(94)00184-5. [DOI] [PubMed] [Google Scholar]
- Trevizani G, Benchimol-Barbosa P, Nadal J. Effects of age and aerobic fitness on heart rate recovery in adult men. Arq Bras Cardiol. 2012;99:802–10. doi: 10.1590/s0066-782x2012005000069. [DOI] [PubMed] [Google Scholar]
- Tsunoda M, Endo T, Hashimoto S, et al. Effects of light and sleep stages on heart rate variability in humans. Psychiatry Clin Neurosci. 2001;55:285–6. doi: 10.1046/j.1440-1819.2001.00862.x. [DOI] [PubMed] [Google Scholar]
- Udupa K, Sathyaprabha T, Thirthalli J, et al. Alteration of cardiac autonomic functions in patients with major depression: A study using heart rate variability measures. J Affect Disord. 2007;100:137–41. doi: 10.1016/j.jad.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Viola A, James L, Archer S, Dijk D. PER3 polymorphism and cardiac autonomic control: Effects of sleep debt and circadian phase. Am J Physiol Heart Circ Physiol. 2008;295:H2156–63. doi: 10.1152/ajpheart.00662.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Flaxman A, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010. A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warthen D, Provencio I. The role of intrinsically photosensitive retinal ganglion cells in nonimage-forming responses to light. Eye Brain. 2012;4:43–8. doi: 10.2147/EB.S27839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A. Beginning to see the light. Arch Gen Psychiatry. 1998;55:861–2. doi: 10.1001/archpsyc.55.10.861. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Berger M, et al. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol Med. 2005;35:939–44. doi: 10.1017/s003329170500437x. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A. Diurnal variation of depressive symptoms. Dialogues Clin Neurosci. 2008;10:337–43. doi: 10.31887/DCNS.2008.10.3/awjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirz-Justice A, Benedetti F, Terman M. Chronotherapeutics for affective disorders: A clinician’s manual for light and wake therapy. Basel: Karger; 2009. [DOI] [PubMed] [Google Scholar]
- Wirz-Justice A, Bader A, Frisch U, et al. A randomized, double-blind, placebo-controlled study of light therapy for antepartum depression. J Clin Psychiatry. 2011;72:986–93. doi: 10.4088/JCP.10m06188blu. [DOI] [PubMed] [Google Scholar]
- Ursinus Wu YH, Zhou JJ, et al. Alterations of melatonin receptors MT1 and MT2 in the hypothalamic suprachiasmatic nucleus during depression. J Affect Disord. 2013;148:357–67. doi: 10.1016/j.jad.2012.12.025. [DOI] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein J, Foster R. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nat Rev Neurosci. 2010;11:1–12. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- Xhyheri B, Manfrini O, Mazzolini M, et al. Heart rate variability today. Prog Cardiovasc Dis. 2012;55:321–31. doi: 10.1016/j.pcad.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Zhou L, Zhu D. Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical applications. Nitric Oxide. 2009;20:223–30. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]


