Abstract
Various in vitro and in vivo studies have linked mesenchymal stem cells (MSCs) with cancer, but little is known about the effect of MSCs on tumor progression. The present study aimed to analyze the role of the MSCs from different tissues, consisting of human bone marrow, adipose and the umbilical cord tissues, and the heterogeneity of tumors in tumor progression. By collecting the culture supernatants of MSCs as MSC-conditioned media (CMs), the present study found that MSC-CM produces no significant effect on the proliferation of MDA-MB-231 and A549 tumor cells. The migration of MDA-MB-231 cells was enhanced upon incubation with MSC-CM, while that of A549 cells was inhibited. Furthermore, the phosphorylation of insulin receptors (IRs) was upregulated in MSC-CM-treated MDA-MB-231 cells, while in MSC-CM-treated A549 cells, the phosphorylation of human epidermal growth factor receptor 3 (Her3) was downregulated. Taken together, the findings suggest that the phosphorylation of IR and Her3 may contribute to the discrepant effects of MSC-CM on the migration of the 2 cell lines.
Keywords: mesenchymal stem cell, tumor migration, conditioned medium, insulin receptor, human epidermal growth factor receptor 3
Introduction
Globally, cancer is a significant public health problem, with ~14.1 million new patients and 8.2 million cancer-associated mortalities reported worldwide in 2012 (1). Currently, radiotherapy, chemotherapy and surgery are common tumor treatments. However, the therapeutic effects are unsatisfactory due to serious side effects, inability of the treatments to accurately distinguish between normal and tumor cells, resistance to chemoradiotherapy and the loss of effect against later metastasis (2,3). Therefore, the development of specific and highly efficient therapies is important. Mesenchymal stem cells (MSCs) are multipotent progenitor cells with the capacity to self-renew and differentiate becoming osteoplastic, adipogenic or chondrogenic. MSCs are not only derived primarily from the bone marrow (BM-MSCs) but also have been successfully derived from numerous tissues, including adipose tissue (AD-MSCs) and the umbilical cord (UC-MSCs) (4). These MSCs share useful characteristics, such as immune modulation, cytokine secretion and differentiation, which make them popular candidates for use in the development of new tumor therapies.
The stroma of solid cancers contains multiple cell types and non-cellular components, creating a complex signaling network to maintain tumor development (5). Decades of research has proven that MSCs can be recruited to the stroma of solid tumors, via direct contact or paracrine signaling, to effect the cell growth, apoptosis and metastasis of the surrounding tumor cells (6). Additionally, MSCs have been suggested to act as a cellular delivery system, owing to their targeted recruiting ability, which derives from the innate tropism of MSCs to tumors. MSCs can deliver interferon (IFN)-β to inhibit the proliferation of malignant tumor cells in in vitro co-culture systems (7). Combination treatment with AD-MSC-IFN-β and cisplatin synergistically reduces tumor volume. Exogenous tumor necrosis factor-α (TNF-α) primed human MSCs express high levels of membrane-bound TNF-related apoptosis-inducing ligand (TRAIL) and induce apoptosis in MDA cells (8). Our previous research has indicated that the administration of MSCs inhibits tumor migration in mice with Lewis lung cancer (9). However, experimental data has also revealed the capability of MSCs to promote tumor progression and metastasis. MSCs integrate into the prostate tumor stroma, are converted into cancer-associated fibroblasts (CAFs), and secrete chemokine (C-X-C motif) ligand 12 (CXCL12), which ultimately leads to an epithelial-to-mesenchymal transition and distant metastasis of the tumor (10). Subsequent to being preactivated by the inflammatory cytokines TNF-α and IFN-γ, BM-MSCs accelerate colon cancer growth in vivo to a greater degree, by expressing higher levels of vascular endothelial growth factor to promote angiogenesis (11). Tsai et al reported that immunodeficient mice injected with MSCs and human colorectal cancer prominin-1 (CD133)−/cluster of differentiation 166−/epithelial cell adhesion molecule− cells exhibited enhanced tumor formation and the expression of CD133 through interleukin-6/signal transducer and activator of transcription 3; furthermore, MSC-derived CAFs participated in this process (12).
Taken together, these findings suggest the discrepant effects of MSCs on tumor progression; however, the factors to which these effects are attributable remain unclear. The heterogeneity of MSCs from different tissues may underlie the discrepant effects. In addition, differences in surface receptors and the different signal cascades they activate in different tumor cells may induce different biological behaviors (13). The aim of the present study was to determine the role of MSC sources and tumor cell receptors in the interaction between MSCs and tumor progression.
Materials and methods
Human MSC preparation and cell culture
UC-, AD- and BM-MSCs were isolated from human umbilical cord, adipose tissues and bone marrow, respectively. The samples were obtained with consent from donors at the Chinese People's Liberation Army Hospital (Beijing, China) between March, 2013 and November, 2013. The cells were maintained in α-modified minimum essential medium (α-MEM) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a humidified 5% CO2 atmosphere. Human lung adenocarcinoma A549 cells and human breast adenocarcinoma MDA-MB-231 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS at 37°C in a humidified 5% CO2 atmosphere. The study was approved by the Ethical Committee of the Academy of Military Medical Sciences (Beijing, China). All samples were collected with the informed consent of patients.
In vitro adipogenic or osteogenic differentiation analysis
UC-, AD- and BM-MSCs were seeded in 24-well plates at a density of 20,000 cells/well with adipogenic-differentiation medium (Cyagen Biosciences, Santa Clara, CA, USA) or 5,000 cells/well with osteogenic-differentiation medium (Cyagen Biosciences), and cultured with α-MEM supplemented with adipogenic- or osteogenic-differentiation medium. Subsequent to 3 weeks of culturing, the cells were fixed with 4% paraformaldehyde at room temperature and stained with oil red O or alkaline phosphatase (Cyagen Biosciences) to detect adipogenic or osteogenic differentiation.
Immunophenotyping analysis
For detection of surface antigens, UC-, AD- and BM-MSCs (1×106 cells for each sample) were trypsinized with the use of 0.05% trypsin, centrifuged (112 × g for 5 min at room temperature), and incubated for 30 min at 4°C with the following monoclonal antibodies with 2% FBS: Phycoerythrin-conjugated anti-human CD-45 (catalogue no., 560975; 5 µg per 106 cells), CD-73 (catalogue no., 550257; 5 µg per 106 cells), CD-90 (catalogue no., 561970; 5 µg per 106 cells), and CD-105 (catalogue no., 562380; 5 µg per 106 cells), and fluoroisothiocyanate (FITC)-conjugated anti-human CD-19 (catalogue no., 560994; 5 µg per 106 cells) and CD-34 (catalogue no., 555821; 5 µg per 106 cells) (Biolegend, Inc., San Diego, CA, USA) at room temperature for 30 min. The cells were then harvested using a FACScan flow cytometer and analyzed by FlowJo vX.0.7 (FC500; Beckman Coulter, Inc., Brea, CA, USA).
Collection of conditioned medium
UC-, AD- and BM-MSCs were cultured in basic α-MEM medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS, until they reached 70% confluence. The culture medium was then replaced with serum-free DMEM (Thermo Fisher Scientific, Inc.), and the cells were incubated for an additional 24 h. The complete supernatant was collected (112 × g for 5 min at room temperature), filtered through 0.45 µm filters, and designated as MSC-conditioned medium (MSC-CM) (6).
Cell proliferation analysis
A549 and MDA-MB-231 cells were seeded at a density of 2×103 cells/well into 96-well plates and incubated with MSC-CM (200 µl/well) at 37°C for 48 h. Tumor cells cultured in serum-free DMEM served as the normal control. At the experimental endpoint, 10 µl Cell Counting Kit-8 (CCK-8) solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added to each well, and the cells were incubated at 37°C for an additional 2 h. The optical density (OD) value was determined at 450 nm on a microplate reader (Model 680; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Cell migration analysis
The present study loaded 24-well polycarbonate inserts (8 µm; Corning Incorporated, Corning, NY, USA) in 24-well plates for Transwell assays. UC-, AD- and BM-MSCs at 5×104 cells/well were incubated at 37°C in DMEM with 10% FBS and added to the lower chamber (600 µl/well) until they adhered to the well. Tumor cells at 4×104 cells/well were incubated in 200 µl/well serum-free medium and plated into the upper chamber. Subsequent to being cultured at 37°C for 7 h, the cells remaining on the upper surface of the membrane were wiped away with a cotton tip applicator. The cells on the lower surface were fixed with 4% paraformaldehyde and stained with 1% crystal violet. Images in 5 random fields were captured for quantification using microscopy (Eclipse TS100; Nikon, Tokyo, Japan).
Western blot analysis
A549 and MDA-MB-231 cells were seeded at a density of 5×105 cells/well into 25 cm2 flasks and grown to 70% confluence. Subsequent to being starved in serum-free DMEM for 24 h, the tumor cells were incubated with MSC-CM for 30 min at room temperature. Cell membrane proteins were used for Human phospho-receptor-associated tyrosine kinase (RTK) array (RayBiotech, Norcross, GA, USA), according to the manufacturer's protocol. Membrane protein concentration was determined using a BCA protein assay kit (Beijing Yuanpinghao Biological Technology Co., Ltd., Beijing, China). In total, 20 µg proteins were separated using 10% SDS-PAGE and were transferred to a nitrocellulose membrane by electroblotting. The membrane was blocked with 5% non-fat milk in 0.1%, v/v Tris-buffered saline/Tween-20 and incubated overnight at 4°C with the appropriate primary antibodies. The antibodies used were as follows: P-IR (dilution, 1:1,000; catalogue no., 3023) and P-Her3 (dilution, 1:1,000; catalogue no., 2842; Cell Signaling Technology, Inc., Boston, MA, USA); and rabbit monoclonal antibody against GAPDH (dilution, 1:3,000; catalogue no., 21018; Santa Cruz Biotechnology, Inc., Santa Cruz Biotechnology, Inc., CA, USA). The membrane was washed and incubated with a secondary anti-rabbit IgG antibody conjugated with horseradish peroxidase (dilution, 1:5,000; catalogue no., ab6734; Abcam, Cambridge, UK) at room temperature for 2 h, and proteins were detected using ECL Plus Western Blotting Detection Reagents (EMD Millipore, Billerica, MA, USA). Bands were compared against GAPDH and data was presented as relative density ratios (Quantity One software; Bio-Rad Laboratories, Inc.).
Statistical analysis
Statistical analysis was performed using Student's t-test or one-way analysis of variance and GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
MSCs from disparate sources share the same characteristics
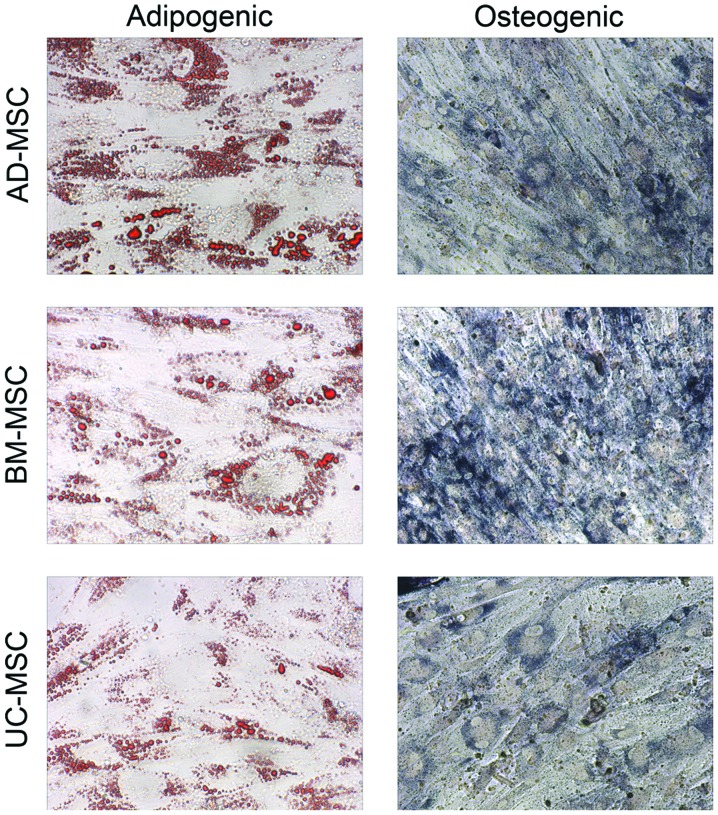
MSCs were harvested, and the immunophenotypes of the cells from the 3 sources was analyzed using flow cytometry. As shown in Table I, the surface markers of MSCs were not changed. Cells from all 3 sources expressed CD29, CD44, CD73 and human leukocyte antigen (HLA)-A, B, C-FITC and did not express CD14, CD31, CD34, CD45 and HLA-DR-FITC. The present study next considered the differentiation of the MSCs into osteogenic cells and adipogenic cells. It was found that MSCs were successfully induced to undergo chondrogenic and adipogenic differentiation, revealing they had the same differentiation ability (Fig. 1).
Table I.
Flow cytometry assay of the immunophenotype of MSCs.
| Variables | UC-MSC | AD-MSC | BM-MSC |
|---|---|---|---|
| CD14-PE | − | − | − |
| CD29-PE | +++ | +++ | +++ |
| CD31-PE | − | − | − |
| CD34-PE | − | − | − |
| CD44-PE | +++ | +++ | +++ |
| CD45-PE | − | − | − |
| CD73-PE | +++ | +++ | +++ |
| HLA-A,B,C-FITC | +++ | +++ | +++ |
| HLA-DR-FITC | − | − | − |
+++, >90%; -, <5%; MSC, mesenchymal stem cells; UC-MSC, umbilical cord-MSC; AD-MSC, adipose tissue-MSC; BM-MSC, bone marrow-MSC; PE, phycoerythrin; FITC, fluoroisothiocyanate; HLA, human leukocyte antigen.
Figure 1.
Osteogenic and adipogenic differentiation assay of MSCs showed that MSCs from disparate sources share the same ability to be induced to chondrogenic and adipogenic differentiation (magnification, ×100). MSC, mesenchymal stem cells; UC-MSC, umbilical cord-MSC; BM-MSC, bone marrow-MSC; AD-MSC, adipose tissue-MSC.
UC-MSC-CM has no effect on tumor cell proliferation
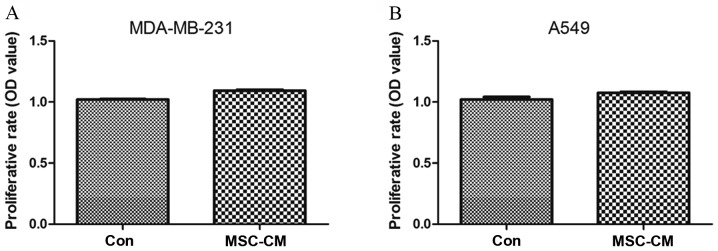
Breast cancer MDA-MB-231 cells and lung cancer A549 cells were seeded at a density of 3,000 cells/well into 96-well plates for 12 h and then replaced with UC-MSC-CM for 48 h. Cell viability was determined using a CCK-8 assay, and the results were expressed as the OD value to represent the relative proliferation ability of the cells. The results showed that UC-MSC-CM had no significant effect on the proliferation of MDA-MB-231 (P=0.1770) and A549 cells (P=0.0766) (Fig. 2).
Figure 2.
Effect of MSC-CM on tumor cell proliferation. (A) Proliferative rate of MDA-MB-231 cells. (B) Proliferative rate of A549 cells. UC-MSC-CM had no significant effect on the proliferation of MDA-MB-231 and A549 cells. MSC-CM, mesenchymal stem cell-conditioned medium; Con, serum-free DMEM acted as the normal control.
MSC-CM inhibits the migration of A549 cells but promotes the migration of MDA-MB-231 cells
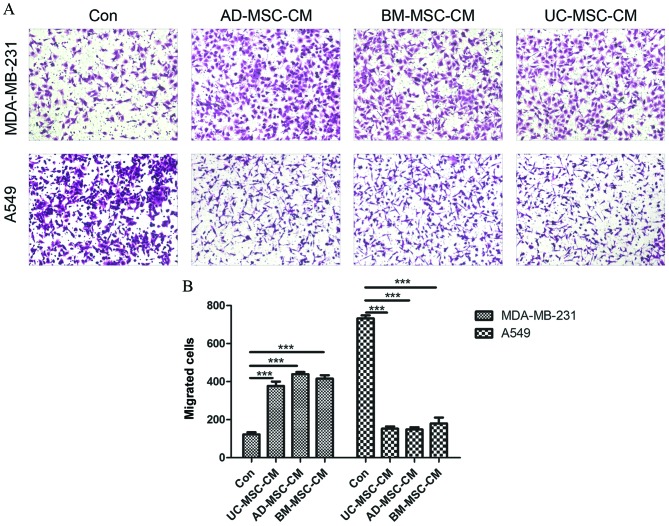
The present study investigated the effect of MSC-CM on the migration of A549 and MDA-MB-231 cells by using 24-well polycarbonate Transwell inserts. As shown in Fig. 3A, compared with basic α-MEM medium, MSC-CM significantly attenuated the migration of A549 cells (UC-MSC, P<0.0001; AD-MSC, P<0.0001; BM-MSC, P<0.0001) but enhanced the migration of MDA-MB-231 cells (UC-MSC, P<0.0001; AD-MSC, P<0.0001; BM-MSC, P<0.0001). Differences in the sources of MSCs had no effect on the cell migration ability. The quantitative determination of cell migration is shown in Fig. 3B.
Figure 3.
Effect of MSC-CM on tumor cell migration. (A) Images of transferred cells stained by crystal violet at ×100 magnification. (B) The quantitative determination of transferred cells. MSC-CM inhibits the migration of A549 cells but promotes the migration of MDA-MB-231 cells. Differences in the sources of MSCs had no effect on the cell migration ability. ***P<0.005.
Levels of phosphorylated insulin receptor and human epidermal growth factor receptor 3 differed between the 2 cell lines
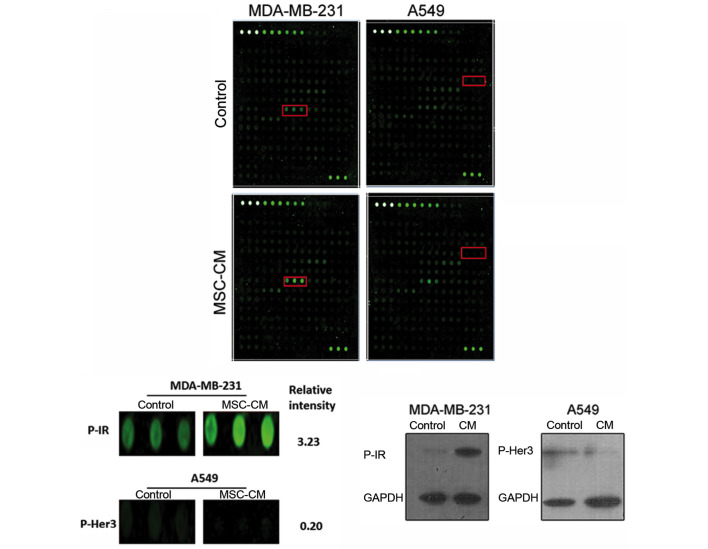
To investigate the cause of the difference in the motility of A549 and MDA-MB-231 cells under the effect of MSC-CM, a phospho-RTK array analysis of the membrane protein extracts of the 2 cell lines was performed. As shown in Fig. 4, the phosphorylation of insulin receptors (IRs) in MDA-MB-231 cells was enhanced due to the MSC-CM treatment, whereas in A549 cells, human epidermal growth factor receptor 3 (Her3) phosphorylation was attenuated. Western blot analysis was also performed, and consistent results were acquired, confirming the findings of the phosphor-RTK array analysis (Fig. 4C).
Figure 4.
Phosphorylated analysis in tumor cells. (A) Phospho-receptor-associated tyrosine kinase array analysis of tumor cell membrane proteins. (B) Red box in MDA-MB-231 represents phospho-IR; red box in A549 represents phospho-Her3. (C) Western blot analysis of IR and HER3 phosphorylation in tumor cells. The phosphorylation of IRs in MDA-MB-231 cells was enhanced due to the MSC-CM treatment, whereas in A549 cells, Her3 phosphorylation was attenuated. IR, insulin receptor; Her3, human epidermal growth factor receptor 3; MSC, mesenchymal stem cells; CM, conditioned medium.
Discussion
In the present study, initial evidence has been provided that MSC-CM has opposite effects on the motility of the lung cancer A549 cell line and the breast cancer MDA-MB-231 cell line. The present results showed a significant promotion of MDA-MB-231 cell migration and a significant inhibition of A549 cell migration. However, MSC-CM appeared to have no significant impact on tumor cell proliferation in the present study. The data also indicated that MSCs derived from different tissues, namely, the umbilical cord, adipose tissue and bone marrow, did not have discrepant effects on tumor cell migration. Phospho-RTK array analysis indicated an upregulation of IR phosphorylation in MSC-CM-treated MDA-MB-231 cells and a downregulation of Her3 phosphorylation in MSC-CM-treated A549 cells, suggesting that the modulation of IR and Her3 phosphorylation may a major role in the promotion and inhibition, respectively, of tumor cell migration by MSC-CM.
By binding to IRs, insulin enhances the proliferation and migration of MCF-7 via extracellular signal-regulated kinase (ERK) signaling in vivo and in vitro (14,15). Consistent with this, the present results showed that the level of phosphorylated IRs was increased in MSC-pretreated human breast cancer MDA-MB-231 cells, without significant alteration in the level of phosphorylated insulin-like growth factor 1 receptors (IGF-1Rs) and the subsequent c-Jun N-terminal kinase (JNK) activation, thus enhancing cell proliferative and migratory abilities. It was therefore deduced that MSC-CM mainly stimulates IRs rather than IGF-1Rs to act as a pro-tumor effector in a JNK-independent manner. However, this conclusion should be explored in additional experiments.
Her3 is a transmembrane receptor and a member of the erythroblastosis oncogene B family, which is associated with tumor cell proliferation, motility and survival by the ligand-driven activation of the RTK domains, stimulating downstream signaling cascades (16). Previously, accumulating evidence has indicated that Her3 is a potential therapeutic target in cancers (17,18). Her3 was shown to be highly expressed in lung adenocarcinomas and associated with decreased survival in patients (19). Sheng et al reported that the loss of Her3 function slows ovarian tumor progression in vitro and prolongs survival in mouse xenograft models of ovarian cancer (17). Notably, in the present experiments, inactivation and decreased phosphorylation of Her3 in A549 cells was observed; these changes inhibited cell migration, indicating an anti-tumor effect of MSC-CM on lung cancer.
In oncology, receptor patterns in patients have been the basis of promising, individualized, biological anti-tumor therapies, such as the HER2-targeting monoclonal antibody trastuzumab for breast cancer and lung cancer (20). In addition, MSC-based therapy is becoming a new strategy, due to the abundant secreted cytokines, which bind to their corresponding receptors and stimulate anti-tumor signals (21). Zhu et al (22) reported that conditioned medium derived from BM-MSCs enhance human gastric cancer cell growth via the activation of Ras homolog gene family, member A-guanosine-5′-triphosphate and ERK1/2 signaling in vivo and in vitro. Similarly, BM-MSCs promote the invasion and tumorigenesis of colorectal cancer cells by secreting soluble neuregulin1 to bind Her3 and activate the PI3K/AKT signaling cascade in cancer cells (23). In the present study, MSCs were not found to be suitable for all types of tumors. In breast cancer cells induced by MSCs, IRs were phosphorylated, enhancing the malignant traits of the cells; opposite effects were observed in lung cancer cells, in which the phosphorylation of Her3 was decreased. Additionally, more precise and specific therapeutic strategies should be formulated based on the different receptors expressed in tumors, particularly for MSC-based therapy.
In conclusion, the present research demonstrated that MSC-CM promotes MDA-MB-231 cell migration and inhibits A549 cell migration by modulating the phosphorylation of IR and Her3, respectively. Differences in the source of MSCs have no impact on tumor progression. The present results suggest that the 2 cancer cell lines behaved differentially owing to the effect of MSC-CM. A better understanding of the conditions in which MSCs enhance tumor progression is crucial to safely develop MSCs as a therapeutic tool and to prevent tumor progression.
Acknowledgements
This study was supported by the National Science Foundation of China (grant nos. 81270894 and 81201760).
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Baskar R, Yap SP, Chua KL, Itahana K. The diverse and complex roles of radiation on cancer treatment: Therapeutic target and genome maintenance. Am J Cancer Res. 2012;2:372–382. [PMC free article] [PubMed] [Google Scholar]
- 3.Collins KK, Liu Y, Schootman M, Aft R, Yan Y, Dean G, Eilers M, Jeffe DB. Effects of breast cancer surgery and surgical side effects on body image over time. Breast Cancer Res Treat. 2011;126:167–176. doi: 10.1007/s10549-010-1077-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rastegar F, Shenaq D, Huang J, Zhang W, Zhang BQ, He BC, Chen L, Zuo GW, Luo Q, Shi Q, et al. Mesenchymal stem cells: Molecular characteristics and clinical applications. World J Stem Cells. 2010;2:67–80. doi: 10.4252/wjsc.v2.i4.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Egeblad M, Nakasone ES, Werb Z. Tumors as organs: Complex tissues that interface with the entire organism. Dev Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kucerova L, Skolekova S, Matuskova M, Bohac M, Kozovska Z. Altered features and increased chemosensitivity of human breast cancer cells mediated by adipose tissue-derived mesenchymal stromal cells. BMC Cancer. 2013;13:535. doi: 10.1186/1471-2407-13-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn J, Lee HW, Seo KW, Kang SK, Ra JC, Youn HY. Anti-tumor effect of adipose tissue derived-mesenchymal stem cells expressing interferon-β and treatment with cisplatin in a xenograft mouse model for canine melanoma. PLoS One. 2013;8:e74897. doi: 10.1371/journal.pone.0074897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee RH, Yoon N, Reneau JC, Prockop DJ. Preactivation of human MSCs with TNF-α enhances tumor-suppressive activity. Cell stem cell. 2012;11:825–835. doi: 10.1016/j.stem.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Di GH, Jiang S, Li FQ, Sun JZ, Wu CT, Hu X, Duan HF. Human umbilical cord mesenchymal stromal cells mitigate chemotherapy-associated tissue injury in a pre-clinical mouse model. Cytotherapy. 2012;14:412–422. doi: 10.3109/14653249.2011.646044. [DOI] [PubMed] [Google Scholar]
- 10.Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. doi: 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Han ZP, Zhang SS, Jing YY, Bu XX, Wang CY, Sun K, Jiang GC, Zhao X, Li R, et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem. 2011;286:25007–25015. doi: 10.1074/jbc.M110.213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai KS, Yang SH, Lei YP, Tsai CC, Chen HW, Hsu CY, Chen LL, Wang HW, Miller SA, Chiou SH, et al. Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology. 2011;141:1046–1056. doi: 10.1053/j.gastro.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 13.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., III Concise review: Dissecting a discrepancy in the literature: Do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan F, Hong LQ. Insulin promotes proliferation and migration of breast cancer cells through the extracellular regulated kinase pathway. Asian Pac J Cancer Prev. 2014;15:6349–6352. doi: 10.7314/APJCP.2014.15.15.6349. [DOI] [PubMed] [Google Scholar]
- 15.Gallagher EJ, Alikhani N, Tobin-Hess A, Blank J, Buffin NJ, Zelenko Z, Tennagels N, Werner U, LeRoith D. Insulin receptor phosphorylation by endogenous insulin or the insulin analog AspB10 promotes mammary tumor growth independent of the IGF-I receptor. Diabetes. 2013;62:3553–3560. doi: 10.2337/db13-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison MM, Hutchinson K, Williams MM, Stanford JC, Balko JM, Young C, Kuba MG, Sánchez V, Williams AJ, Hicks DJ, et al. ErbB3 downregulation enhances luminal breast tumor response to antiestrogens. J Clin Invest. 2013;123:4329–4343. doi: 10.1172/JCI66764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheng Q, Liu X, Fleming E, Yuan K, Piao H, Chen J, Moustafa Z, Thomas RK, Greulich H, Schinzel A, et al. An activated ErbB3/NRG1 autocrine loop supports in vivo proliferation in ovarian cancer cells. Cancer Cell. 2010;17:298–310. doi: 10.1016/j.ccr.2009.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gala K, Chandarlapaty S. Molecular pathways: HER3 targeted therapy. Clin Cancer Res. 2014;20:1410–1416. doi: 10.1158/1078-0432.CCR-13-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sithanandam G, Smith GT, Masuda A, Takahashi T, Anderson LM, Fornwald LW. Cell cycle activation in lung adenocarcinoma cells by the ErbB3/phosphatidylinositol 3-kinase/Akt pathway. Carcinogenesis. 2003;24:1581–1592. doi: 10.1093/carcin/bgg125. [DOI] [PubMed] [Google Scholar]
- 20.Ma J, Lyu H, Huang J, Liu B. Targeting of erbB3 receptor to overcome resistance in cancer treatment. Mol Cancer. 2014;13:105. doi: 10.1186/1476-4598-13-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greco SJ, Rameshwar P. Mesenchymal stem cells in drug/gene delivery: Implications for cell therapy. Ther Deliv. 2012;3:997–1004. doi: 10.4155/tde.12.69. [DOI] [PubMed] [Google Scholar]
- 22.Zhu W, Huang L, Li Y, Qian H, Shan X, Yan Y, Mao F, Wu X, Xu WR. Mesenchymal stem cell-secreted soluble signaling molecules potentiate tumor growth. Cell Cycle. 2011;10:3198–3207. doi: 10.4161/cc.10.18.17638. [DOI] [PubMed] [Google Scholar]
- 23.De Boeck A, Pauwels P, Hensen K, Rummens JL, Westbroek W, Hendrix A, Maynard D, Denys H, Lambein K, Braems G, et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression through paracrine neuregulin 1/HER3 signalling. Gut. 2013;62:550–560. doi: 10.1136/gutjnl-2011-301393. [DOI] [PubMed] [Google Scholar]