Abstract
Tumor protein 53 (TP53), a tumor suppressor gene, is a vital cellular cancer suppressor in multicellular organisms. Murine double minute-2 (MDM2) is an oncoprotein that inhibits TP53 activity. A number of studies have examined the association of TP53 and MDM2 polymorphisms with the risk of common forms of cancer, but the findings remain inconclusive. The present study aimed to evaluate the impact of the 40-bp insertion/deletion (I/D) polymorphism (rs3730485) in the MDM2 promoter region and the 16-bp I/D polymorphism (rs17878362) in TP53 on the susceptibility of prostate cancer (PCa) in a sample of the Iranian population. This case-control study included 103 patients with pathologically confirmed PCa and 142 patients with benign prostatic hyperplasia. The MDM2 40-bp I/D and TP53 16-bp I/D polymorphism was determined using polymerase chain reaction analysis. The results demonstrated that the MDM2 40-bp I/D polymorphism increased the risk of PCa in a co-dominant inheritance model [odds ratio (OR)=1.88; 95% confidence interval (CI)=1.11–3.19; P=0.023, D/D vs. I/I], while this variant marginally increased the risk of PCa in a dominant model (OR=1.69; 95% CI=1.00–2.83; P=0.051, I/D+D/D vs. I/I). No significant association was observed between the TP53 16-bp I/D polymorphism and PCa. In conclusion, the present study demonstrated that the 40-bp I/D polymorphism in the MDM2 promoter increased the risk of PCa in an Iranian population. Further investigations with diverse ethnicities and larger sample sizes are required to verify these results.
Keywords: prostate cancer, tumor protein 53, murine double minute-2, insertion/deletion, polymorphism
Introduction
Prostate cancer (PCa) is the most prevalent form of cancer among males in the United States (1). In Iran, the incidence of PCa is ~9.6 cases per 100,000 individuals, with a range of 3.2–16.0 per 100,000 in various geographical settings (2,3). This is comparable with the Asia-Pacific region (9.9 per 100,000), but significantly lower than in the rest of the world (32.8 per 100,000) (4). The median age at diagnosis is ~66 years and the 5-year survival rate of patients with PCa has been estimated to be 98.9% (5).
The molecular mechanisms underlying the progression and carcinogenesis of PCa have not yet been clarified. Additional molecular markers that may be employed to detect PCa and to individualize patient therapy and prognosis are of great clinical importance. A number of large cohort and case-control studies with various populations suggest that family history is a primary risk factor for PCa (6–10).
In humans, the tumor protein 53 (TP53) gene is located on the short arm of chromosome 17 (17p13.1) (11). TP53, an important tumor suppressor gene, is a crucial regulator of apoptosis and the cell cycle (12–15). When it is mutated, this regulation may be lost, leading to uncontrolled cell proliferation and potentially tumorigenesis (16).
The human MDM2 gene is mapped on chromosome 12q14.3-15 (17). MDM2 functions as a key negative regulator of TP53 (18), inhibiting the transcriptional activity of TP53 and enhancing proteolytic P53 degradation (19).
The impact of a 16-bp duplication [insertion (I)/deletion (D)] polymorphism (rs17878362) within intron 3 of TP53 and a 40-bp I/D variant of MDM2 on cancer susceptibility has been examined, but studies have reported conflicting results (20–28). Therefore, the current study aimed to determine the possible association of the 16-bp I/D polymorphism within intron 3 of TP53 and the 40-bp I/D polymorphism in the promoter region of MDM2 with PCa in a sample of an Iranian population.
Materials and methods
Patients
The present case-control study enrolled 103 histopathologically-confirmed patients with PCa and 142 age-matched men with benign prostatic hyperplasia (BPH), who had been referred to the Department of Urology, Shahid Labbafinejad Medical Center, Shahid Beheshti University of Medical Sciences (Tehran, Iran). The enrollment process and study design were performed as previously described (29). Ethical approval for the study was obtained from the Ethics Committee of Zahedan University of Medical Sciences, and written informed consent was obtained from all participating patients. Blood samples were collected in EDTA-containing tubes from patients and controls, and DNA was extracted using the salting out method as previously described (30).
Genotyping
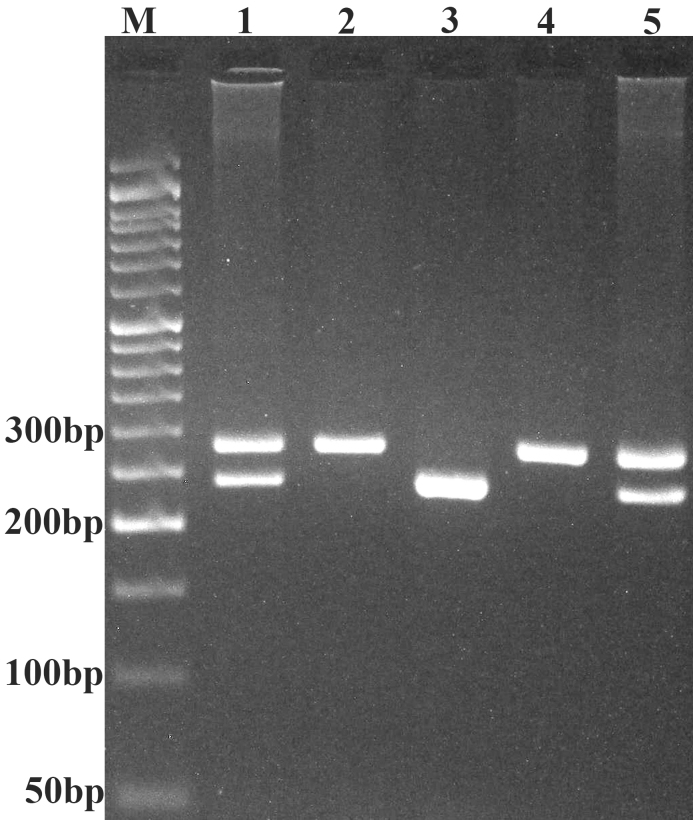
MDM2 40-bp I/D polymorphism genotyping was performed using forward (5′-GACCACTATGTTTAAGGAAG-3′) and reverse (5′-TGACTCACCTACTTTCCCAC-3′) primers, as described previously (28). Polymerase chain reaction (PCR) was performed using commercially available Prime Taq Premix (Genet Bio, Daejeon, South Korea), according to the manufacturer's recommended protocol. The PCR cycling conditions were as follows: An initial denaturation at 95°C for 5 min, followed by 30 cycles at 95°C for 30 sec, 59°C for 25 sec, 72°C for 30 sec and a final extension step at 72°C for 10 min. The product sizes for the I and D alleles were 287 and 247 bp, respectively. The PCR products were verified using 2.5% agarose gels containing 0.5 µg/ml ethidium bromide, and observed under UV light (Fig. 1).
Figure 1.
Electrophoresis pattern of the polymerase chain reaction product of the 40-bp I/D polymorphism of murine double minute-2 resolved by 2.5% agarose gel electrophoresis. M, 50 bp DNA marker; lanes 1 and 5, I/D; lanes 2 and 4, I/I; lane 3, D/D. I, insertion; D, deletion.
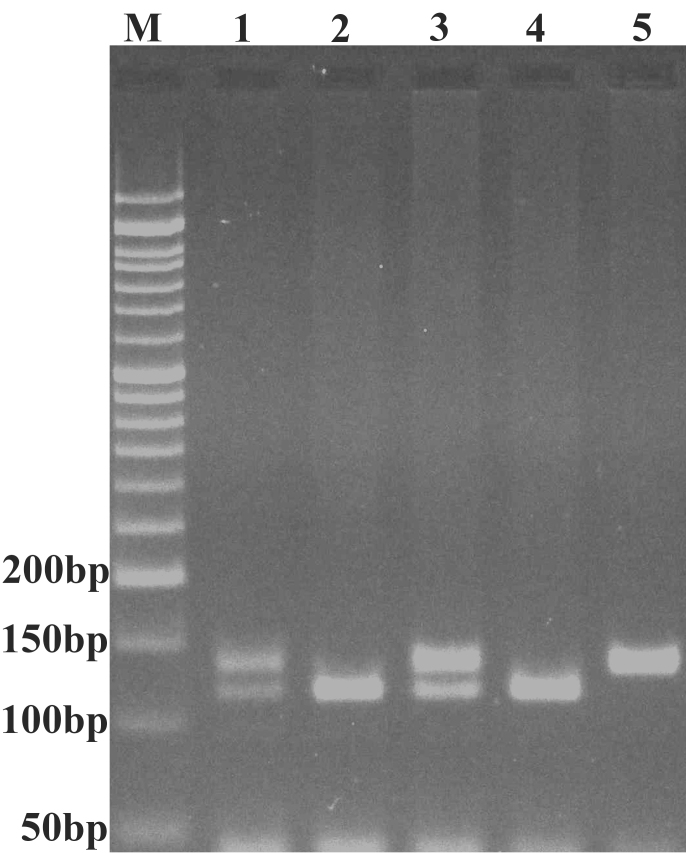
Genotyping of the 16-bp duplication polymorphism in TP53 was performed by PCR using forward (5′-CTGAAAACAACGTTCTGGTA-3′) and reverse (5′-AAGGGGGACTGTAGATGGGTG-3′) primers (31) as previously described (32). The PCR conditions were as follows: An initial denaturation step at 95°C for 5 min, followed by 30 cycles at 95°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec, with a final extension step at 72°C for 10 min. The TP53 wild type allele, the D allele (no duplication), resulted in a 119-bp fragment and the variant alleles, the I allele (with 16-bp duplication), resulted in a 135-bp fragment (Fig. 2). Approximately 15% of the samples were randomly selected for confirmation and the results were all consistent.
Figure 2.
Representative polymerase chain reaction products resolved by agarose gel electrophoresis to detect the presence or absence of the 16 bp I/D polymorphism of the tumor protein 53 gene. M, 50 bp DNA marker; lanes 1 and 3, I/D; lanes 2 and 4, D/D; lane 5, I/I. I, insertion; D, deletion.
Statistical analysis
Statistical analysis was performed using SPSS v18 (SPSS, Inc., Chicago, IL, USA). Continuous and categorical data were analyzed using the independent sample t-test and the χ2 test, respectively. Associations of the MDM2 40-bp I/D and the TP53 16-bp I/D polymorphism with PCa were calculated by computing the odds ratio (OR) and 95% confidence intervals (95% CIs) from logistic regression analyses. The genotype distribution of variants was tested for Hardy-Weinberg equilibrium (HWE) separately for cases and controls. P<0.05 was considered to indicate a statistically significant difference.
Results
Patient characteristics
In total, 103 patients with PCa were enrolled with a mean age of 61.03±6.03 years, and 142 patients with BPH (control group) were enrolled with a mean age of 62.57±7.85 years. No significant difference in age was observed between the groups (P=0.097). The clinicopathological characteristics of the patients with PCa are summarized in Table I.
Table I.
Clinicopathological characteristics of patients with prostate cancer.
| Characteristics | Number of patients |
|---|---|
| Mean age, years (range) | 61.0 (43–72) |
| PSA level at diagnosis (ng/ml), mean ± SD | 14.1±13.3 |
| Gleason Score, n (%) | |
| ≤6 | 36 (35) |
| 7 | 46 (44.7) |
| >7 | 21 (20.4) |
| Stage, n (%) | |
| pT1 | 8 (7.8) |
| pT2a | 20 (19.4) |
| pT2b | 7 (6.8) |
| pT2c | 39 (37.9) |
| pT3a | 8 (7.8) |
| pT3b | 21 (20.4) |
| Perineural invasion, n (%) | 60 (58.3) |
| Impotency, n (%) | 18 (17.5) |
| Loss of libido, n (%) | 16 (15.5) |
| Post-void residual, mean ± SD (ml) | 26.7±27.6 |
| Addiction, n (%) | 4 (3.9) |
| Hypertension, n (%) | 8 (7.8) |
| Diabetes mellitus, n (%) | 7 (6.8) |
| History of smoking, n (%) | 12 (11.7) |
| Alcohol consumption, n (%) | 1 (0.97) |
PSA, prostate specific antigen; SD, standard deviation. pT1, pT2a, pT2b, pT2c, pT3a and pT3b are clinical stages of prostate cancer.
TP53 16-bp I/D and MDM2 40-bp I/D polymorphism variant and PCa risk
The TP53 16-bp I/D variant was not associated with PCa in any tested inheritance models (co-dominant, dominant and recessive; Table II). The genotype and allele frequencies of the MDM2 40-bp I/D polymorphism in patients with PCa and the controls are presented in Table III. A significant difference was observed between patients with PCa and controls regarding the MDM2 40-bp I/D polymorphism (χ2=7.06; P=0.029). The MDM2 40-bp I/D polymorphism increased the risk of PCa in a co-dominant inheritance model (OR=1.88; 95% CI=1.11–3.19; P=0.023, I/D vs. I/I), while the variant marginally increased the risk of PCa in a dominant model (OR=1.69; 95% CI=1.00–2.83; P=0.051, I/D+D/D vs. I/I). The D allele was not associated with PCa (OR=1.23; 95% CI=0.84–1.82; P=0.320), compared with the I allele.
Table II.
Genotypic and allelic frequencies of the TP53 16-bp I/D polymorphism in patients with PCa, and in controls.
| TP53 16-bp I/D | PCa, n (%) | Control, n (%) | OR (95% CI) | P-value |
|---|---|---|---|---|
| Codominant | ||||
| D/D | 36 (35.0) | 57 (40.1) | 1.00 | – |
| D/I | 50 (48.5) | 69 (48.6) | 1.15 (0.66–1.99) | 0.673 |
| I/I | 17 (16.5) | 16 (11.3) | 1.68 (0.76–3.76) | 0.223 |
| Dominant | ||||
| D/D | 36 (35.0) | 57 (40.1) | 1.00 | – |
| D/I+I/I | 67 (65.0) | 84 (59.9) | 1.26 (0.75–2.14) | 0.425 |
| Recessive | ||||
| DD+D/I | 86 (83.5) | 126 (88.7) | 1.00 | – |
| I/I | 17 (16.5) | 16 (11.3) | 1.56 (0.75–3.25) | 0.259 |
| Allele | ||||
| D | 122 (59.2) | 183 (64.4) | – | |
| I | 84 (40.8) | 101 (35.6) | 1.25 (0.86–1.81) | 0.258 |
TP53, tumor protein 53; I, Insertion; D, deletion; PCa, prostate cancer; OR, odds ratio; CI, confidence interval.
Table III.
Genotypic and allelic frequencies of the MDM2 40-bp I/D polymorphism in patients with PCa and controls.
| MDM2 40-bp I/D | PCa, n (%) | Control, n (%) | OR (95% CI) | P-value |
|---|---|---|---|---|
| Codominant | ||||
| I/I | 39 (37.9) | 72 (50.7) | 1.00 | – |
| I/D | 60 (58.2) | 59 (41.6) | 1.88 (1.11–3.19) | 0.023 |
| D/D | 4 (3.9) | 11 (7.7) | 0.67 (0.20–2.25) | 0.578 |
| Dominant | ||||
| I/I | 39 (37.9) | 72 (50.7) | 1.00 | – |
| I/D+D/D | 64 (62.1) | 70 (49.3) | 1.69 (1.00–2.83) | 0.051 |
| Recessive | ||||
| I/I+I/D | 99 (96.1) | 131 (92.3) | 1.00 | – |
| D/D | 4 (3.9) | 11 (7.7) | 0.45 (0.15–1.56) | 0.284 |
| Alleles | ||||
| I | 138 (67.0) | 203 (71.5) | 1.00 | – |
| D | 68 (33.0) | 81 (28.5) | 1.23 (0.84–1.82) | 0.320 |
MDM2, murine double minute-2; I, insertion; D, deletion; PCa, prostate cancer; OR, odds ratio; CI, confidence interval.
The MDM2 I/D and TP53 16-bp variant genotype frequencies were individually tested for patients and controls using the HWE. The TP53 16-bp I/D polymorphism in patients and controls were observed to be in equilibrium (χ2=0.002, P=0.958; χ2=0.52, P=0.473, respectively). For the MDM2 40-bp I/D, the genotype was in equilibrium in controls (χ2=0.51; P=0.820), but not in patients (χ2=10.36, P=0.001).
Association of MDM2 I/D and TP53 I/D with clinicopathological characteristics
As presented in Table IV, the MDM2 I/D variant was not associated with clinicopathological characteristics, including age, stage, prostate specific antigen level, grade (Gleason score), perineural invasion or surgical margin. However, there was an association between the TP53 I/D polymorphism and surgical margin (P=0.035).
Table IV.
Association between MDM2 40-bp I/D and TP53 16-bp I/D polymorphisms with clinicopathological parameters in patients with prostate cancer.
| MDM2 40-bp I/D | TP53 16-bp I/D | |||||||
|---|---|---|---|---|---|---|---|---|
| Factors | II | ID | DD | P-value | DD | DI | II | P-value |
| Age at diagnosis (years), n | 0.976 | 0.529 | ||||||
| ≤65 | 30 | 45 | 3 | 25 | 40 | 13 | ||
| >65 | 9 | 15 | 1 | 11 | 10 | 4 | ||
| Stage | 0.152 | 0.241 | ||||||
| pT1 | 3 | 5 | 0 | 4 | 3 | 1 | ||
| pT2a | 5 | 12 | 3 | 7 | 6 | 7 | ||
| pT2b | 3 | 4 | 0 | 2 | 4 | 1 | ||
| pT2c | 18 | 21 | 0 | 16 | 18 | 5 | ||
| pT3a | 1 | 6 | 1 | 2 | 4 | 2 | ||
| pT3b | 9 | 12 | 0 | 5 | 15 | 1 | ||
| PSA level at diagnosis (ng/ml), n | 0.647 | 0.538 | ||||||
| ≤4 | 1 | 0 | 0 | 1 | 0 | 0 | ||
| 4–10 | 18 | 29 | 1 | 19 | 21 | 8 | ||
| >10 | 20 | 31 | 3 | 16 | 29 | 9 | ||
| Gleason score, n | 0.475 | 0.674 | ||||||
| ≤7 | 32 | 46 | 4 | 30 | 38 | 14 | ||
| >7 | 7 | 14 | 0 | 6 | 12 | 3 | ||
| Perineural invasion, n | 0.886 | 0.878 | ||||||
| Positive | 22 | 36 | 2 | 21 | 30 | 9 | ||
| Negative | 17 | 24 | 2 | 15 | 20 | 8 | ||
| Surgical margin, n | 0.925 | 0.035 | ||||||
| Positive | 16 | 24 | 2 | 13 | 26 | 3 | ||
| Negative | 23 | 36 | 2 | 23 | 24 | 14 | ||
MDM2, murine double minute-2; TP53, tumor protein 53; I, insertion; D, deletion; PSA, prostate specific antigen. pT1, pT2a, pT2b, pT2c, pT3a and pT3b are clinical stages of prostate cancer.
Discussion
It has been suggested that genetic and environmental factors each contribute to the pathogenesis of human PCa (33–35). The present study investigated the impact of the 16-bp I/D polymorphism within intron 3 of TP53 and the 40-bp I/D polymorphism in the promoter region of MDM2 on the risk of PCa in a sample of the Iranian population. The results demonstrated that the TP53 16-bp I/D polymorphism was not associated with the risk of PCa. However, a significant association was observed between the MDM2 I/D variant and the risk of PCa, and the I/D genotype increased the risk of PCa, compared with I/I.
The TP53 gene is a key tumor suppressor that encodes a 53-kDa protein, which is essential in DNA repair, cell cycle arrest and apoptosis in response to DNA damage (12–15,26,36). TP53 mutations are the most frequent gene mutations in the majority of types of human cancer, and they permit cells to proliferate and survive (37). Rapid phosphorylation of TP53 by ataxia telangiectasia mutated (ATM) following DNA damage leads to increased TP53 stability and activity (38,39). TP53-induced gene expression leads to cell cycle arrest and apoptosis (40). When TP53 is mutated, this effect may be lost, resulting in uncontrolled cell proliferation that may result in tumorigenesis (16). It has been proposed that genetic polymorphisms in TP53 may affect a number of its functions (41).
MDM2, a key negative regulator of TP53, is able to bind directly to TP53 and inhibit its transcriptional activity (18). MDM2 also promotes the ubiquitination and subsequent degradation of TP53 (19,42). Promoter polymorphisms in the MDM2 gene may affect MDM2 cellular protein levels (43), and MDM2 overexpression has been reported in several forms of human cancer (44–46).
In agreement with the results of the present study, Mittal et al (47) reported no significant association between the TP53 16-bp I/D polymorphism and PCa in an Indian population. Certain studies have demonstrated that the 16-bp I/D variant increased the risk of various types of cancer, including breast (20,21,48–50), colorectal (22), lung (23,24,51), esophageal and gastric (52) cancer. Conversely, a number of studies observed no association between the TP53 16-bp I/D variant and certain forms of cancer, including breast (53) and pancreatic (54) cancer. The 16-bp I/D polymorphism in TP53 has been demonstrated to be associated with lymph node metastasis in breast cancer (55).
The intron sequences in TP53 are involved in regulating gene expression and in DNA-protein interactions (56,57). It has been proposed that the TP53 intron 3 16-bp I variant is associated with lower levels of TP53 transcripts, which suggests that this duplication polymorphism causes an alteration in mRNA processing and may be a risk factor for developing cancer (57). In addition, it has been suggested that the TP53 codon 72 variant may be a low-penetrant risk factor for developing PCa in Caucasians, but not in Asians (58), and variants within the TP53 binding sites may be valuable biomarkers for the prognosis of patients with PCa (59).
The current study observed that the 40-bp I/D polymorphism in the MDM2 promoter increased the risk of PCa. A recent study reported that the MDM2 40-bp I/D variant increased the risk of breast cancer in an Iranian population (28). Furthermore, a significant association between MDM2 I/D and lung cancer was detected in the Chinese population (26). In addition, the MDM2 40-bp I/D variant has been demonstrated to be a risk factor for hepatocellular carcinoma in the Chinese population (25). By contrast, no association was identified between the MDM2 40-bp I/D polymorphism and breast cancer in the Chinese population (27).
The limitations of the present study are as follows: Firstly, relatively small sample sizes were used, therefore repetition with larger samples is required; secondly, gene-environment interactions were not determined. It has been proposed that environmental and genetic factors each serve a role in PCa development. In conclusion, the results of the present study indicate that the 40-bp I/D polymorphism in the MDM2 gene promoter increases the risk of PCa in a sample of the Iranian population. Larger sample sizes with differing ethnicities are required to further investigate these findings.
Acknowledgements
The current study was supported by Zahedan University of Medical Sciences, Zahedan, Iran (grant no. 6728), a University of Manitoba Start-Up grant (grant no. 315992) and a Manitoba Medical Services Foundation grant (grant no. 8-2015-11). The authors would like to thank all individuals who willingly participated in the study. In addition, all authors acknowledge Dr Judi Smith (Research Facilitator, Health Sciences, University of Manitoba, Canada) for editing the original manuscript.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Farahmand M, Khademolhosseini F, Mehrabani D. Trend of prostate cancer in Fars Province, Southern Iran, 2001–2007. J Res Med Sci. 2010;15:295–297. [PMC free article] [PubMed] [Google Scholar]
- 3.Talaiezadeh A, Tabesh H, Sattari A, Ebrahimi S. Cancer incidence in southwest of iran: First report from khuzestan population-based cancer registry, 2002–2009. Asian Pac J Cancer Prev. 2013;14:7517–7522. doi: 10.7314/APJCP.2013.14.12.7517. [DOI] [PubMed] [Google Scholar]
- 4.Baade PD, Youlden DR, Cramb SM, Dunn J, Gardiner RA. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int. 2013;1:47–58. doi: 10.12954/PI.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.SEER stat fact sheets: Prostate cancer. http://seer.cancer.gov/statfacts/html/prost.html. Surveillance e and end results program. 2014 Sep 2; [Google Scholar]
- 6.Carter BS, Beaty TH, Steinberg GD, Childs B, Walsh PC. Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci USA. 1992;89:3367–3371. doi: 10.1073/pnas.89.8.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gronberg H, Damber L, Damber JE. Familial prostate cancer in Sweden. A nationwide register cohort study. Cancer. 1996;77:138–143. doi: 10.1002/(SICI)1097-0142(19960101)77:1<138::AID-CNCR23>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Johnson AM, Zuhlke KA, Plotts C, McDonnell SK, Middha S, Riska SM, Schaid DJ, Thibodeau SN, Douglas JA, Cooney KA. Mutational landscape of candidate genes in familial prostate cancer. Prostate. 2014;74:1371–1378. doi: 10.1002/pros.22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen LS, Fann JC, Chiu SY, Yen AM, Wahlfors T, Tammela TL, Chen HH, Auvinen A, Schleutker J. Assessing interactions of two loci (rs4242382 and rs10486567) in familial prostate cancer: Statistical evaluation of epistasis. PLoS One. 2014;9:e89508. doi: 10.1371/journal.pone.0089508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teerlink CC, Thibodeau SN, McDonnell SK, Schaid DJ, Rinckleb A, Maier C, Vogel W, Cancel-Tassin G, Egrot C, Cussenot O, et al. Association analysis of 9,560 prostate cancer cases from the International Consortium of Prostate Cancer Genetics confirms the role of reported prostate cancer associated SNPs for familial disease. Hum Genet. 2014;133:347–356. doi: 10.1007/s00439-013-1384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McBride OW, Merry D, Givol D. The gene for human p53 cellular tumor antigen is located on chromosome 17 short arm (17p13) Proc Natl Acad Sci USA. 1986;83:130–134. doi: 10.1073/pnas.83.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tong WM, Hande MP, Lansdorp PM, Wang ZQ. DNA strand break-sensing molecule poly(ADP-Ribose) polymerase cooperates with p53 in telomere function, chromosome stability, and tumor suppression. Mol Cell Biol. 2001;21:4046–4054. doi: 10.1128/MCB.21.12.4046-4054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong H, Takahashi K, Ichisaka T, Aoi T, Kanagawa O, Nakagawa M, Okita K, Yamanaka S. Suppression of induced pluripotent stem cell generation by the p53-p21 pathway. Nature. 2009;460:1132–1135. doi: 10.1038/nature08235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marte B. Cancer: Super p53. Nature. 2002;420:279. doi: 10.1038/420279a. [DOI] [PubMed] [Google Scholar]
- 15.Levine AJ, Oren M. The first 30 years of p53: Growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhuo W, Zhang Y, Xiang Z, Cai L, Chen Z. Polymorphisms of TP53 codon 72 with breast carcinoma risk: Evidence from 12226 cases and 10782 controls. J Exp Clin Cancer Res. 2009;28:115. doi: 10.1186/1756-9966-28-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Momand J, Zambetti GP. Mdm-2: ‘Big brother’ of p53. J Cell Biochem. 1997;64:343–352. doi: 10.1002/(SICI)1097-4644(19970301)64:3<343::AID-JCB1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 18.Bond GL, Hu W, Levine AJ. MDM2 is a central node in the p53 pathway: 12 years and counting. Current Cancer Drug Targets. 2005;5:3–8. doi: 10.2174/1568009053332627. [DOI] [PubMed] [Google Scholar]
- 19.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 20.Wu D, Zhang Z, Chu H, Xu M, Xue Y, Zhu H, Zhang Z. Intron 3 sixteen base pairs duplication polymorphism of p53 contributes to breast cancer susceptibility: Evidence from meta-analysis. PloS One. 2013;8:e61662. doi: 10.1371/journal.pone.0061662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bisof V, Salihović MP, Narancić NS, Skarić-Jurić T, Jakić-Razumović J, Janićijević B, Turek S, Rudan P. TP53 gene polymorphisms and breast cancer in Croatian women: A pilot study. Eur J Gynaecol Oncol. 2010;31:539–544. [PubMed] [Google Scholar]
- 22.Mammano E, Belluco C, Bonafé M, Olivieri F, Mugianesi E, Barbi C, Mishto M, Cosci M, Franceschi C, Lise M, Nitti D. Association of p53 polymorphisms and colorectal cancer: Modulation of risk and progression. Eur J Surg Oncol. 2009;35:415–419. doi: 10.1016/j.ejso.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 23.Xu B, Xu Z, Cheng G, Min ZC, Mi Y, Zhang ZZ, Tao J, Li PC, Wang ML, Tang JL, et al. Association between polymorphisms of TP53 and MDM2 and prostate cancer risk in southern Chinese. Cancer Genet Cytogenet. 2010;202:76–81. doi: 10.1016/j.cancergencyto.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Mahasneh AA, Abdel-Hafiz SS. Polymorphism of p53 gene in Jordanian population and possible associations with breast cancer and lung adenocarcinoma. Saudi Med J. 2004;25:1568–1573. [PubMed] [Google Scholar]
- 25.Dong D, Gao X, Zhu Z, Yu Q, Bian S, Gao Y. A 40-bp insertion/deletion polymorphism in the constitutive promoter of MDM2 confers risk for hepatocellular carcinoma in a Chinese population. Gene. 2012;497:66–70. doi: 10.1016/j.gene.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Hu Z, Ma H, Lu D, Qian J, Zhou J, Chen Y, Xu L, Wang X, Wei Q, Shen H. Genetic variants in the MDM2 promoter and lung cancer risk in a Chinese population. Int J Cancer. 2006;118:1275–1278. doi: 10.1002/ijc.21463. [DOI] [PubMed] [Google Scholar]
- 27.Ma H, Hu Z, Zhai X, Wang S, Wang X, Qin J, Jin G, Liu J, Wang X, Wei Q, Shen H. Polymorphisms in the MDM2 promoter and risk of breast cancer: A case-control analysis in a Chinese population. Cancer Lett. 2006;240:261–267. doi: 10.1016/j.canlet.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 28.Hashemi M, Omrani M, Eskandari-Nasab E, Hasani SS, Mashhadi MA, Taheri M. A 40-bp insertion/deletion polymorphism of Murine Double Minute2 (MDM2) increased the risk of breast cancer in Zahedan, Southeast Iran. Iran Biomed J. 2014;18:245–249. doi: 10.6091/ibj.13332.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashemi M, Shahkar G, Simforoosh N, Basiri A, Ziaee SA, Narouie B, Taheri M. Association of polymorphisms in PRKCI gene and risk of prostate cancer in a sample of Iranian Population. Cell Mol Biol (Noisy-le-grand) 2015;61:16–21. [PubMed] [Google Scholar]
- 30.Hashemi M, Bojd H Hanafi, Nasab E Eskandari, Bahari A, Hashemzehi NA, Shafieipour S, Narouie B, Taheri M, Ghavami S. Association of adiponectin rs1501299 and rs266729 gene polymorphisms with nonalcoholic fatty liver disease. Hepat Mon. 2013;13:e9527. doi: 10.5812/hepatmon.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang-Gohrke S, Rebbeck TR, Besenfelder W, Kreienberg R, Runnebaum IB. p53 germline polymorphisms are associated with an increased risk for breast cancer in German women. Anticancer Res. 1998;18:2095–2099. [PubMed] [Google Scholar]
- 32.Eskandari-Nasab E, Hashemi M, Amininia S, Ebrahimi M, Rezaei M, Hashemi SM. Effect of TP53 16-bp and beta-TrCP 9-bp INS/DEL polymorphisms in relation to risk of breast cancer. Gene. 2015;568:181–185. doi: 10.1016/j.gene.2015.05.048. [DOI] [PubMed] [Google Scholar]
- 33.Pourmand G, Ziaee AA, Abedi AR, Mehrsai A, Alavi HA, Ahmadi A, Saadati HR. Role of PTEN gene in progression of prostate cancer. Urol J. 2007;4:95–100. [PubMed] [Google Scholar]
- 34.Shaik AP, Jamil K, Das P. CYP1A1 polymorphisms and risk of prostate cancer: A meta-analysis. Urol J. 2009;6:78–86. [PubMed] [Google Scholar]
- 35.Samzadeh M, Hasanzad M, Jamaldini SH, Haghdoost AA, Afshari M, Ziaee SA. Association of G/A polymorphism, rs266882, in AREI region of the prostate-specific antigen gene with prostate cancer risk and clinicopathological features. Urol J. 2012;9:691–699. [PubMed] [Google Scholar]
- 36.Harris SL, Levine AJ. The p53 pathway: Positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 37.Bennett WP, Hussain SP, Vahakangas KH, Khan MA, Shields PG, Harris CC. Molecular epidemiology of human cancer risk: Gene-environment interactions and p53 mutation spectrum in human lung cancer. J Pathol. 1999;187:8–18. doi: 10.1002/(SICI)1096-9896(199901)187:1<8::AID-PATH232>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 38.Kastan MB, Lim DS. The many substrates and functions of ATM. Nat Rev Mol Cell Biol. 2000;1:179–186. doi: 10.1038/35043058. [DOI] [PubMed] [Google Scholar]
- 39.Tichy A, Vavrova J, Pejchal J, Rezácová M. Ataxia-telangiectasia mutated kinase (ATM) as a central regulator of radiation-induced DNA damage response. Acta Medica (Hradec Kralove) 2010;53:13–17. doi: 10.14712/18059694.2016.57. [DOI] [PubMed] [Google Scholar]
- 40.Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13:83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proestling K, Hebar A, Pruckner N, Marton E, Vinatzer U, Schreiber M. The Pro allele of the p53 codon 72 polymorphism is associated with decreased intratumoral expression of BAX and p21 and increased breast cancer risk. PLoS One. 2012;7:e47325. doi: 10.1371/journal.pone.0047325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 43.Bond GL, Hu W, Levine A. A single nucleotide polymorphism in the MDM2 gene: From a molecular and cellular explanation to clinical effect. Cancer Res. 2005;65:5481–5484. doi: 10.1158/0008-5472.CAN-05-0825. [DOI] [PubMed] [Google Scholar]
- 44.Meddeb M, Valent A, Danglot G, Nguyen VC, Duverger A, Fouquet F, Terrier-Lacombe MJ, Oberlin O, Bernheim A. MDM2 amplification in a primary alveolar rhabdomyosarcoma displaying a t(2;13)(q35;q14) Cytogenet Cell Genet. 1996;73:325–330. doi: 10.1159/000134368. [DOI] [PubMed] [Google Scholar]
- 45.Bueso-Ramos CE, Yang Y, deLeon E, McCown P, Stass SA, Albitar M. The human MDM-2 oncogene is overexpressed in leukemias. Blood. 1993;82:2617–2623. [PubMed] [Google Scholar]
- 46.Watanabe T, Hotta T, Ichikawa A, Kinoshita T, Nagai H, Uchida T, Murate T, Saito H. The MDM2 oncogene overexpression in chronic lymphocytic leukemia and low-grade lymphoma of B-cell origin. Blood. 1994;84:3158–3165. [PubMed] [Google Scholar]
- 47.Mittal RD, George GP, Mishra J, Mittal T, Kapoor R. Role of functional polymorphisms of P53 and P73 genes with the risk of prostate cancer in a case-control study from Northern India. Arch Med Res. 2011;42:122–127. doi: 10.1016/j.arcmed.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Faghani M, Ghasemi FM, Nikhbakht M, Salehi M. TP53 PIN3 polymorphism associated with breast cancer risk in Iranian women. Indian J Cancer. 2011;48:298–302. doi: 10.4103/0019-509X.84925. [DOI] [PubMed] [Google Scholar]
- 49.Lajin B, Sakur A Alhaj, Alachkar A. Association between polymorphisms in apoptotic genes and susceptibility for developing breast cancer in Syrian women. Breast Cancer Res Treat. 2013;138:611–619. doi: 10.1007/s10549-013-2467-4. [DOI] [PubMed] [Google Scholar]
- 50.Hu Z, Li X, Yuan R, Ring BZ, Su L. Three common TP53 polymorphisms in susceptibility to breast cancer, evidence from meta-analysis. Breast Cancer Res Treat. 2010;120:705–714. doi: 10.1007/s10549-009-0488-9. [DOI] [PubMed] [Google Scholar]
- 51.Wu X, Zhao H, Amos CI, Shete S, Makan N, Hong WK, Kadlubar FF, Spitz MR. p53 genotypes and haplotypes associated with lung cancer susceptibility and ethnicity. J Natl Cancer Inst. 2002;94:681–690. doi: 10.1093/jnci/94.9.681. [DOI] [PubMed] [Google Scholar]
- 52.Malik MA, Sharma K, Goel S, Zargar SA, Mittal B. Association of TP53 intron 3, 16 bp duplication polymorphism with esophageal and gastric cancer susceptibility in Kashmir Valley. Oncol Res. 2011;19:165–169. doi: 10.3727/096504011X12935427587920. [DOI] [PubMed] [Google Scholar]
- 53.Pouladi N, Kouhsari SM, Feizi MH, Dehghan R, Azarfam P, Farajzadeh D. Lack of association of intron 3 16 bp polymorphism of TP53 with breast cancer among Iranian-Azeri patients. Asian Pac J Cancer Prev. 2014;15:2631–2634. doi: 10.7314/APJCP.2014.15.6.2631. [DOI] [PubMed] [Google Scholar]
- 54.Naccarati A, Pardini B, Polakova V, Smerhovsky Z, Vodickova L, Soucek P, Vrana D, Holcatova I, Ryska M, Vodicka P. Genotype and haplotype analysis of TP53 gene and the risk of pancreatic cancer: An association study in the Czech Republic. Carcinogenesis. 2010;31:666–670. doi: 10.1093/carcin/bgq032. [DOI] [PubMed] [Google Scholar]
- 55.Costa S, Pinto D, Pereira D, Rodrigues H, Cameselle-Teijeiro J, Medeiros R, Schmitt F. Importance of TP53 codon 72 and intron 3 duplication 16bp polymorphisms in prediction of susceptibility on breast cancer. BMC Cancer. 2008;8:32. doi: 10.1186/1471-2407-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pietsch EC, Humbey O, Murphy ME. Polymorphisms in the p53 pathway. Oncogene. 2006;25:1602–1611. doi: 10.1038/sj.onc.1209367. [DOI] [PubMed] [Google Scholar]
- 57.Gemignani F, Moreno V, Landi S, Moullan N, Chabrier A, Gutiérrez-Enríquez S, Hall J, Guino E, Peinado MA, Capella G, Canzian F. A TP53 polymorphism is associated with increased risk of colorectal cancer and with reduced levels of TP53 mRNA. Oncogene. 2004;23:1954–1956. doi: 10.1038/sj.onc.1207305. [DOI] [PubMed] [Google Scholar]
- 58.Xu B, Mi YY, Min ZC, Cheng G, Tong N, Tao J, Li PC, Wang ML, Tang JL, Zhang ZD, et al. p53 codon 72 increased biochemical recurrence risk after radical prostatectomy in a southern Chinese population. Urol Int. 2010;85:401–405. doi: 10.1159/000315991. [DOI] [PubMed] [Google Scholar]
- 59.Lin VC, Huang CY, Lee YC, Yu CC, Chang TY, Lu TL, Huang SP, Bao BY. Genetic variations in TP53 binding sites are predictors of clinical outcomes in prostate cancer patients. Arch Toxicol. 2014;88:901–911. doi: 10.1007/s00204-014-1196-8. [DOI] [PubMed] [Google Scholar]