Abstract
Objective
Salivary cortisol is increasingly used as a longitudinal indicator of change in neuroendocrine regulation and as a predictor of health outcomes in youth. The purpose of this study was to describe which indices of HPA-axis functioning are sensitive to changes in parent-child conflict over a three week period and to explore the time course under which these changes can be measured.
Methods
Youth (n = 47; ages 8–13) completed daily diaries of their conflict with parents for 56 days. On days 17–18 and 38–39, youth contributed saliva samples upon waking, 30-minutes post-waking, afternoon, and bedtime. We assessed change in average diurnal HPA-axis functioning between day 17–18 and day 38–39 as a function of the slopes of change in parent-child conflict over 3 weeks.
Results
Increasing parent-child conflict was positively associated with concurrent increases in total cortisol output (AUCg), flattening of the diurnal slope, and increases in cortisol at bedtime, but not with change in the cortisol awakening response (CAR). Further, associations between parent-child conflict and both AUCg and bedtime cortisol were observed with at least 14 days of daily diary reporting, whereas any additional ratings of conflict beyond 3 days of daily diaries did not improve model fit for changes in diurnal slope.
Conclusions
This study demonstrates the within-subject up-regulation of the HPA-axis across three weeks in a healthy sample of youth exposed to natural increases in family conflict. In particular, cortisol at bedtime may be the HPA-axis index that is most sensitive to change over time in parent-child conflict, above and beyond conflict occurring that day. Further, when testing associations between family stressors and diurnal cortisol, the optimal schedule for assessing parent-child conflict varies for different indices of HPA-axis functioning.
Keywords: Family conflict, Diurnal cortisol, HPA-axis, Daily diary
1. Introduction
Chronic conflict within families is associated with increased risk for physical and mental illness in children (Repetti et al., 2002). Exposure to daily stress, such as parent-child conflict, may influence health through alterations to one component of the body’s physiological stress response, the hypothalamic-pituitary-adrenal axis (HPA-axis) (Odgers and Jaffee, 2013; Repetti et al., 2011). Functioning of the HPA-axis has been repeatedly identified as a key mediator in the link between stress and both health and mortality (Adam et al., 2010; Seeman et al., 2001; Sephton et al., 2000; Taylor, 2010). Among healthy individuals, cortisol is highest in the morning and continues to decline across the day (Tsigos and Chrousos, 2002). Youth exposed to conflict at home have exhibited deviations from this pattern (Lippold et al., 2014; Slatcher and Robles, 2012), which may be an indicator of cumulative wear-and-tear on the HPA-axis. A recent review of 19 intervention studies examining children’s cortisol regulation as an outcome of various psychosocial interventions also concluded that HPA-axis regulation likely improves as a function of treatment (Slopen et al., 2014). One unifying element of the interventions that demonstrated change in HPA-axis regulation was a focus on improving the parent-child relationship. Yet, we do not know the time frame over which change in interpersonal conflict influences the regulation of biological systems, which is information that could inform future study design as well as our understanding of how conflict gets under the skin to influence lifelong health.
Allostatic load theory describes how chronic stress contributes to wear-and-tear on the body via physiological alterations over time (McEwen, 1998), and naturalistic studies do suggest that the HPA-axis responds to day-to-day changes in the environment (Adam et al., 2006). Further, psychosocial interventions, which occur over several weeks or months, mitigate stress-related HPA-axis functioning (Slopen et al., 2014). There are several indices of the HPA-axis, including the cortisol awakening response (CAR), diurnal slope, and total cortisol output (AUCg), which are each psychobiologically meaningful. For example, CAR may represent adrenal sensitivity to ACTH (Clow et al., 2010) and is influenced by sleep (Vargas and Lopez-Duran, 2014), diurnal slope is a decline in cortisol from waking to evening that is driven by circadian rhythms via the suprachiasmatic nucleus (SCN) of the hypothalamus (Kalsbeek et al., 2012), and AUCg approximates total cortisol production throughout the day which aggregates indices of circadian functioning with responses to stress across the day. Therefore, identifying the indices of HPA-axis functioning that are sensitive to week-to-week changes in conflict and the time course over which associations emerge is a critical gap in our understanding of how conflict gets under the skin. For example, it is possible that increases in parent-child conflict may be reflected in increased sensitivity to stressors throughout the day as reflected in elevated AUCg. Alternatively, increasing parent-child conflict may also disrupt circadian regulation of the system, more likely to be reflected in indices such as CAR or diurnal slope.
Several studies have demonstrated that family functioning, including parenting behaviors and parent-child conflict are associated with variation in daily cortisol (Gunnar and Quevedo, 2007; Lippold et al., 2014; Pendry and Adam, 2007; Repetti et al., 2011; Slatcher and Robles, 2012). For example, more conflict at home has been linked to lower cortisol at waking, and flatter diurnal slope among preschool children (Slatcher and Robles, 2012). Similarly, higher maternal parenting quality predicted steeper diurnal cortisol slopes in kindergarteners (Pendry and Adam, 2007). Thus trait-like family characteristics, such as the overall frequency of parent-child conflict, are associated with indicators of diurnal regulation of cortisol; those and other indices of HPA-axis functioning may be sensitive to short-term changes in the child’s social environment. For example, among adolescents who live in families with low parent-child negativity, higher bedtime cortisol was observed on days with higher reported parent-child negativity (Lippold et al., 2014). No studies to date have collected daily reports on conflict over extended periods of time that would allow examination of the processes through which cumulative, daily interpersonal challenges in the home translate to changes in child diurnal HPA-axis regulation. For example, Lippold et al. (2014) used daily diary data collected over 8 days. The present study directly extends the existing literature because the time course over which this occurs may be weeks rather than days or years although there is little empirical evidence available to address this possibility. Animal models suggest that repeated daily stress causes systemic up-regulation of basal cortisol after 7–21 days depending on the intensity of the stressor (Pitman et al., 1988); in humans there is emerging evidence that psychosocial interventions can alter HPA-axis functioning within several months (Slopen et al., 2014). Careful characterization of how the time course of week-to-week change in conflict exposure relates to change in HPA-axis function may provide insight into how and when interpersonal conflict during childhood gets under the skin and contributes to disease.
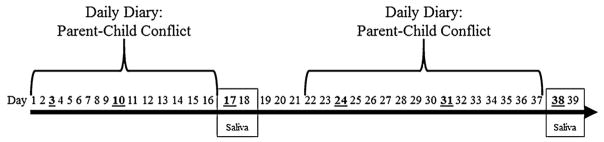
The purpose of this study was to describe how change in daily parent-child conflict relates to change in multiple indices of diurnal HPA-axis regulation among youth, and to identify the optimal number of assessment days to detect these associations. To address the current gaps in the literature, we took advantage of a unique, naturalistic study design that enabled us to examine the association between slope of change in daily reported parent-child conflict and HPA-axis functioning (See Fig. 1). Specifically, we measured the slope of change in child-reported conflict with parents over a 16-day period and tested whether that slope was correlated with diurnal HPA-axis functioning on two consecutive weekdays at the end of the 16-days (Δ Parent-child conflict → Diurnal Cortisol),1 or with change in HPA-axis functioning (Δ Parent-child conflict22–37 → Δ Diurnal Cortisol17–18 to 38–39). Based upon the limited existing literature on healthy youth (Lippold et al., 2014; Slatcher and Robles, 2012), we hypothesized that increases in parent-child conflict would be associated with indicators of an up-regulated HPA-axis: greater cortisol awakening response (CAR), greater total daily cortisol output (AUCg), flatter diurnal slope, and elevated cortisol at bedtime. Further, exploratory analyses sought to identify the number of daily diary assessments of parent-child conflict needed to detect an association between changes in parent-child conflict and changes in HPA-axis functioning.
Fig. 1. Study timeline.
Study timeline indicating days of child-reported parent-child conflict via daily diary and days of salivary cortisol collection included in the present analyses. Study days that fall on a Monday are bold and underlined.
2. Methods
2.1. Participants
Data for the present analyses were collected as part of a larger study of 59 children (47 target children and 12 eligible siblings) that was designed to investigate the association between family interactions and family members’ health and well-being (Reynolds et al., 2015; Robles et al., 2013). Youth were required to be between 8 and 13 years, have at least one parent willing to participate, and be free of major medical conditions that may affect HPA-axis and immune function. Children were recruited from public schools, pediatric clinics, community centers, newspaper advertisements, and direct mailings. This study was approved by the UCLA Institutional Review Board and all parents provided written informed consent and their children provided assent. Data collected for this study occurred across three years between the months of September and May, taking care to not overlap daily diary assessments with major school breaks (e.g., Winter break, Spring break). Due to scheduling conflicts, 12 of the participants did not complete their saliva sampling on the appointed days over the 8-week period. Because the timing of the cortisol sampling days was critical to the design of this study, these children were not included in the present analyses. These children did not differ from the larger sample in sex, age, trait family conflict, slope of parent-child conflict, or any indices of HPA-axis functioning, p > .13. The analyses use data collected from 47 children in 37 families (63.8% female), Mage = 11.13 (SDage = 1.5). Median personal income reported by parents in this study was within a $31,850 to $82,400 bracket, and 57% of mothers (59% of fathers) attained at least a bachelor’s degree or higher. Youth were 46.8% non-Hispanic white, 23.4% Latino/Hispanic, 23.4% African-American, 4.3% Asian, and 2.1% “Other”.
2.2. Procedures
For 8 weeks, participants completed online daily diaries before bedtime, recording their experiences of daily conflict with their parents. During the 3rd and 6th week of the study, participants contributed salivary cortisol samples at home from Saturday through Tuesday. The present analyses (See Fig. 1 for a timeline of study procedures) focus on data collected over 6 weeks, specifically two 18 day periods: parent-child conflict reported on days 1–16, followed by 2 consecutive days of saliva collection (days 17 and 18), and another period of 16 days of daily reported parent-child conflict (days 22–37), followed by 2 more consecutive days of saliva collection (days 38 and 39). Salivary cortisol was limited to the samples taken on weekdays to avoid confounding associations between parent-child conflict and HPA-axis functioning with systematic variation in HPA-axis functioning between weekends and weekdays as observed in adults (Kunz-Ebrecht et al., 2004; Schlotz et al., 2004; Slatcher et al., 2010). We also controlled for parent-child conflict reported on saliva sampling days (17, 18, 38, and 39) to address any same-day effects of conflict on HPA-axis functioning. Further, parent-child conflict on days 19–21 were not included so that each measure of change in parent-child conflict would be based on the same number of days (16 days), beginning and ending on the same days of the week.
2.3. Measures
2.3.1. Daily parent-child conflict
Youth completed daily diaries at bedtime describing their interactions with family members and peers that day using a subset of items from the Youth Everyday Social Interaction and Mood scales (Lehman and Repetti, 2007; Repetti, 1996). Child-reported conflict with each parent was measured through a total of 6-items: “My mom/dad got mad at me today,” “My mom/dad punished me today,” and “I was angry at mom/dad today.” Ratings on a 3-point response scale (1 = not at all, 2 = some, 3 = a lot) were averaged to compute a daily parent-child conflict score. Responses to these conflict items demonstrated acceptable reliability, α= .81. Change in conflict was operationalized as the slope of parent-child conflict over 16 consecutive days (from day 1–16 and from day 22–37). Compliance with online diary procedures was monitored via automated date-time stamping through SurveyMonkey.com. On average, children completed 94% of the expected diaries across the entire study, 98% of which were compliant, defined as having been completed on the evening due or before 9am the following morning. Further, diary measures demonstrated excellent between-subject reliability over time, RKF = .99, and acceptable within-person reliability across days, RC = .74 (See Cranford et al., 2006). For additional information on measurement reactivity and fatigue effects using an 8-week daily diary paradigm in this sample see Reynolds et al. (2015).
2.4. HPA-axis functioning
Diurnal functioning of the HPA-axis was assessed using cortisol collected via saliva on Monday and Tuesday of the 3rd and 6th weeks of the study, Days 17, 18, 38, and 39. Salivary cortisol samples were collected upon waking, 30 min after waking, before dinner, and at bedtime. On sampling days, mean waking time for youth was 6:51 am (SD = 0:45) and mean bedtime was 9:25pm (SD = 0:50). These samples were used to create four indices of diurnal HPA-axis functioning for each of the 4 weekdays: the cortisol awakening response (CAR), total daily cortisol output (AUCg), diurnal slope, and bedtime cortisol. All cortisol data were log transformed to address skew and kurtosis. The CAR was calculated by subtracting the mean waking cortisol concentration from the mean 30 min post-waking cortisol concentration. Negative values for CAR were recoded as 0, but included in the analyses (21.2% of sample days). Area under the curve with respect to ground (AUCg) was calculated using trapezoidal aggregation (Pruessner et al., 2003). Diurnal slope was calculated by computing the difference between cortisol at bedtime and cortisol at waking, divided by the number of hours between waking and bedtime. The post-waking and afternoon samples were not included in calculations of diurnal slope in order to minimize the correlation and overlap in variance between diurnal slope and other indices of HPA-axis functioning tested here, such as CAR and AUCg. Data from Monday and Tuesday were averaged to create a single index for that week. Sample timing data were collected using an electronic time stamp, and MEMS caps were used to record when these saliva samples were completed for a subset of participants (29%) to determine adherence to sampling instructions. Fidelity to the sample timing instructions was defined as <30 min correspondence between the paper recording form and MEMS cap time record. Fidelity for this sample was 97.6%. Saliva samples taken more than 45 min after waking (7.6%) were considered non-compliant and were not included in calculations of CAR. Cortisol samples were excluded from analyses if cortisol concentrations were greater than 60 nmol/l (n = 8) to minimize the influence of extreme values on the results. The day after each saliva sampling period, a research assistant picked up saliva samples from the family’s home and transported them on ice to the lab where they were frozen and stored at −20 °C at the UCLA Health Psychology Laboratory. Saliva samples were later shipped to the Biological Psychology Laboratory at the Technical Institute of Dresden (Dresden, Germany), directed by Clemens Kirschbaum, and assayed with commercial kits (IBL, Hamburg, Germany) for free cortisol using chemiluminescence immunoassay (50 μl saliva required; minimum detection limit <.003 μg/dL, mean intra- and interassay coefficients of variance (CV) below 10%).
2.5. Data analysis
We operationalized change in daily reported parent-child conflict using slopes from day 1–16 and day 22–37. Individual participant slopes of parent-child conflict from day 1–16 and day 22–37 were computed using linear regressions predicting daily parent-child conflict from study day. To test our hypotheses, we conducted marginal models using the mixed procedure in SPSS with time in weeks as the repeated effect to model each measure of HPA-axis functioning and the slope of change in each of those cortisol measures over 21 days. Four separate models predicted CAR, AUCg, diurnal slope, and bedtime cortisol. All models were conducted both unadjusted then adjusted for covariates, and accounted for non-independence of data by nesting individuals within families. Based on previous literature (Kudielka et al., 2004; Kudielka and Kirschbaum, 2005) and our research questions, the covariates considered for the present analyses were child age, sex, average daily parent-child conflict reported on the days of saliva sampling, household income, and waking time or bedtime depending on the HPA-axis index. Household income was not associated with CAR, B = −.167, p = .237, AUCg, B = −.084, p = .349, diurnal slope, B = −.021, p = .664, or cortisol at bedtime, B = −.073, p = .527, and therefore was not including in our models. Each of these models included a within-subjects parameter estimating the fixed effect of change in parent-child conflict on HPA-axis functioning (Δ Parent-child conflict ↔ Diurnal Cortisol), and the parameter of interest, the fixed effect of change in parent-child conflict from day 22–37 on change in HPA-axis functioning from day 17–18 to day 38–39 (Δ Parent-child conflict → Δ Cortisol17–18 to 38–39). We also retested each of our models with time reverse-coded to assure that change in HPA-axis functioning was not accounted for by regression to the mean (Campbell and Kenny, 1999). A significance threshold of p < .013 maintains less than 5% error rate across our 4 hypotheses. Finally, we re-examined our results using the slope of change in parent-child conflict computed from 3, 9, and 14 days prior to each HPA-axis assessment to determine whether fewer diary days could detect associations between conflict and change in HPA-axis functioning in future studies. We chose these time intervals to represent the inclusion of an increasing number of weekend days, given that families spend more time together on weekends than weekdays. We compared these models using Akaike Information Criterion (AIC) as comparative fit indices that provide a measure of relative quality of a model (Akaike, 1974). We then computed the change in variance accounted for by this model compared with the AIC of the unconditional model of change in HPA-axis functioning over time (Nakagawa and Schielzeth, 2013).
3. Results
Youth in our sample reported low daily parent-child conflict; no conflict was reported on 67% of study days. Mean reported conflict on day 1 of the study was 1.20 (SD =.31), and decreased over the 56 days of assessment (BParent–child Conflict1–16 = −.005, p=.026; BParent–child Conflict22–37 = −.001, p = .029). At the same time, 48% of youth demonstrated a positive slope of parent-child conflict from day 1–16 and 60% of youth reported a positive slope from day 22–37.2 Neuroendocrine functioning for the participants was consistent with what would be expected in a healthy sample of children. For example, on average, youth demonstrated an increase in cortisol following waking (CAR) and decline in cortisol from waking to bedtime. See Table 1 for descriptive statistics for child-reported family conflict and cortisol indices by study week and their bivariate correlations.
Table 1.
Means, standard deviations and correlations between all study variables.
| Mean3 (SD) | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. | 12. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | 11.13 (1.50) | 1.0 | |||||||||||
| 2. Δ Parent-child conflict1–16 | −.001 (.016) | .23 | 1.0 | ||||||||||
| 3. Parent-child conflict 17–18 | 6.33 (1.60) | −.09 | .33* | 1.0 | |||||||||
| 4. CAR 17–18 1 | 7.18 (12.4) | −.18 | .05 | −.02 | 1.0 | ||||||||
| 5. AUCg 17–18 1 | 237.57 (232.3) | .08 | −.08 | .08 | .29† | 1.0 | |||||||
| 6. Diurnal Slope 17–18 1 | −1.00 (1.22) | −.17 | .07 | −.16 | −.31* | −.14 | 1.0 | ||||||
| 7. Bedtime Cortisol 17–18 1 | 3.89 (9.7) | .15 | −.11 | −.01 | .31* | .78** | .04 | 1.0 | |||||
| 8. Δ Parent-child conflict 22–37 | .004 (.013) | −.04 | .48** | .38** | .06 | .02 | −.14 | −.01 | 1.0 | ||||
| 9. Parent-child conflict 38–39 | 6.42 (1.83) | −.13 | .59** | .29† | −.05 | .12 | .21 | .10 | .48** | 1.0 | |||
| 10. CAR 38–39 1 | 5.96 (8.92) | .05 | −.02 | −.01 | .15 | .11 | .07 | −.24 | .05 | .19 | 1.0 | ||
| 11. AUCg 38–39 | 217.23 (124.98) | .10 | .12 | .38* | .27 | .62** | −.37* | .56** | .34* | .002 | −.25 | 1.0 | |
| 12. Diurnal Slope 38–39 1 | −.96 (.67) | −.15 | .29 | .12 | −.08 | .27 | .06 | .07 | .37* | .33* | .01 | .38* | 1.0 |
| 13. Bedtime Cortisol 38–39 1 | 4.22 (10.73) | .06 | .20 | .25 | .28 | .36* | −.10 | .50** | .43** | .12 | −.19 | .70** | .47** |
Note:
LN transformed for correlations, nmol/l;
Change calculated by subtracting week 3 from week 6 values, therefore negative values represent an increase over time;
raw means are reported.
p < .01.
p < .05.
p < .10.
Subscripted numbers correspond to the day the variable was measured within the study.
3.1. Parent-child conflict and CAR
In a conditional, unadjusted model of change in CAR, there was no significant main effect of time on CAR over the three weeks of this study, B = −.093, t(46.14) = −.41, p = .69. Slope of parent-child conflict was not associated with CAR, and slope of parent-child conflict22–27 was not associated with change in CAR17–18 to 38–39, p = .81. In an adjusted model (See Table 2), accounting for sex, age, average parent-child conflict on saliva collection days, and waking time on the day of cortisol sampling, slope of parent-child conflict was not associated with CAR, and slope of parent-child conflict22–37 was not associated with change in CAR17–18 to 38–39, p = .76.
Table 2.
Fixed effects of adjusted models of slope in parent-child conflict predicting CAR, diurnal slope, and bedtime cortisol controlling for child age, sex, state family conflict, and sampling variables (e.g., wake time, bedtime).
| HPA-axis Index | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| CAR1 | AUCg | Diurnal Slope1 | Bedtime | Cortisol1 | ||||
| Predictor | B | t | B | t | B | t | B | t |
| Intercept | .83 | 1.46 | 5.86 | 21.48** | 1.70 | 9.24** | 1.71 | 4.55** |
| Week | −.02 | −.06 | .06 | .75 | −1.12 | −14.29** | −.003 | −.03 |
| Age | −.02 | −.20 | .04 | .70 | −.03 | −1.26 | .04 | .54 |
| Wake time | −.09 | −.48 | −.05 | −.68 | −.006 | .06 | – | – |
| Bedtime | – | – | – | – | – | – | .08 | .75 |
| Sex | .30 | 1.21 | −0.25 | −1.61 | −0.20 | −2.45* | −0.26 | −1.27 |
| Mean Parent-child conflict on sampling days | .08 | .98 | −.08 | −2.11* | .03 | 1.01 | −.08 | −1.61 |
| Δ Parent-child conflict → Diurnal Cortisol | −3.16 | −.26 | −5.16 | .24 | −5.77 | −1.60 | −3.39 | −.53 |
| Δ Parent-child conflict → Δ Diurnal Cortisol17–18 to 38–39 | −5.45 | −.31 | 19.37 | 2.94** | 13.13 | 2.29* | 35.07 | 3.63** |
Note:
p < .05,
p < .01;
ln transformed.
Subscripted numbers correspond to the day the variable was measured within the study.
3.2. Parent-child conflict and total cortisol output (AUCg)
In a conditional, unadjusted model of change in AUCg, there was no significant main effect of time over the three weeks of this study, B = .057, t(30.2) = .68, p = .50. Slopes of parent-child conflict were not associated with AUCg, and there was a non-significant trend suggesting that increasing slope of parent-child conflict22–37 was associated with increases in AUCg17–18 to 38–39, p = .066. In the adjusted model (See Table 2), there continued to be no association between slopes of parent-child conflict and AUCg, however when accounting for age, wake time, sex, and parent-child conflict on saliva assessment days, increases in parent-child conflict22–37 was associated with increases in AUCg17–18to38–39, p = .006.
3.3. Parent-child conflict and diurnal slope
Diurnal cortisol slopes became steeper over the three weeks of this study, B = −1.11, t(49.31) = −14.65, p < .001. In addition, slopes of parent-child conflict 22–37 were positively associated with a flattening diurnal slope17–18 to 38–39, B = 13.87, t(70.42) = 2.56, p = .013. In a model adjusted for age, sex, trait family conflict and waking time (See Table 2), sex was a significant predictor of diurnal slope, such that being female predicted a steeper decline in cortisol from waking to bedtime, B = −.20, t(49.74) = −2.45, p = .018. When accounting for this sex difference and our other covariates, increasing slopes of parent-child conflict22–37 continued to predict a flattening diurnal slope over time, B = 13.13, t(68.69) = 2.29, p = .025. Of note, these associations do not exceed the magnitude required for a significance threshold of .013 that corrects for the testing of multiple hypotheses, and therefore should be interpreted with caution.
3.4. Parent-child conflict and bedtime cortisol
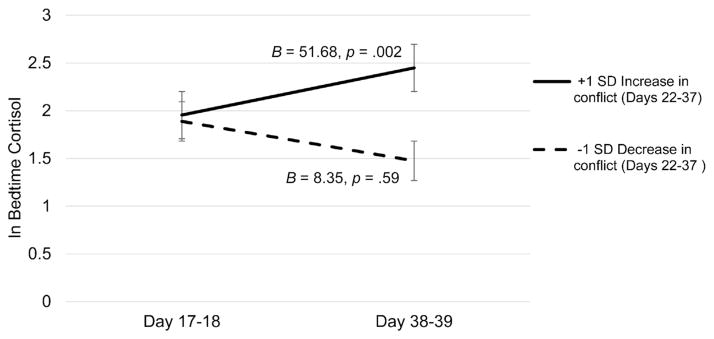
In an unadjusted model of change in bedtime cortisol, there was no significant main effect of time, B = .03, t(44.55) = .33, p = .75. We also found that slopes of parent-child conflict22–37 were positively associated with change in bedtime cortisol17–18 to 38–39, B = 30.28, t(57.97) = 3.23, p = .002. In the adjusted model (See Table 2), increases in parent-child conflict 22–37 continued to predict increases in bedtime cortisol17–18 to 38–39, B = 35.07, t(55.44) = 3.63, p = .001. See Fig. 2.
Fig. 2. Change in bedtime cortisol over three weeks by change in parent-child conflict from Day 22–37.
Estimates of simple slopes are shown. Solid black lines reflect the simple slope for individuals with 1 SD above the mean in slope of parent-child conflict from Day 22–37, dashed lines reflect the simple slope for individuals with 1 SD below the mean in slope of parent-child conflict from Day 22–37.
3.5. Time course of change in parent-child conflict and HPA-axis functioning
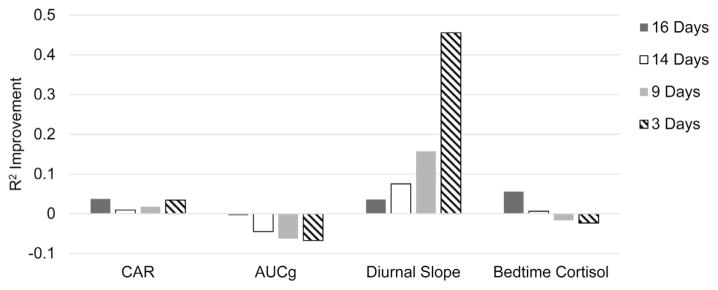
Next we examined each of our adjusted main effect models (shown in Table 2) using slopes of parent-child conflict computed from different numbers of days prior to each HPA-axis assessment. To do this, we calculated slopes of change in daily parent-child conflict based on 3 (days 14–16 and days 35–37), 9 (days 8–16 and days 29–37), and 14 days (days 3–16 and days 24–37) prior to saliva sampling, and retested our models. See Fig. 3 for comparative fit indices for models varying in data collection days.
Fig. 3. Comparative model fit using 16-, 14-, 9-, and 3-days of parent-child conflict reported via daily diary to predict indices of HPA-axis functioning.
Unconditional CAR AIC = 255.17; Unconditional AUCg AIC = 112.63; Unconditional Diurnal Slope AIC = 77.08; Unconditional Bedtime Cortisol AIC = 208.71. Higher values indicate greater improvement in model fit.
With respect to CAR, parent-child conflict slopes calculated from our full (16 day) model was the best fit to the data (AIC = 245.7), followed by a model using conflict computed from the 3 days prior to HPA-axis assessment (AIC = 244.48). In the 3 day model, slopes of parent-child conflict were negatively associated with CAR magnitude, B = −2.13, p = .007, and a steeper slope of increasing parent-child conflict35–37 accounted for increases in CAR17–18 to 38–39, B = 2.81, p = .059. Slopes of parent-child conflict computed from 16 days of daily diaries were also the best fit to our model of change in AUCg over 3 weeks (AIC = 110.53); model fit continued to worsen as fewer days were used to compute slopes of parent-child conflict. Further, the observed association between increasing parent-child conflict and decreases in AUCg17–18 to 38–39 was no longer significant in a model using 14 days of daily diary reports of conflict, B = −8.42, p = .071. In contrast to the other indices of HPA-axis functioning, a model using parent-child conflict computed from 3 days prior to saliva sampling was the best fit to diurnal cortisol slope and change in diurnal slope over three weeks (AIC = 41.96). In the 3 day model, greater increases in parent child conflict during the 3 days prior to HPA-axis assessment were associated with within-subject flattening of diurnal slope17–18 to 38–39, B = −1.40, p = .001. In fact, the model of parent-child conflict reported during the 3 days prior to HPA-axis assessment accounted for an additional 30% of the variance in diurnal slope and its change over the 3 weeks of the study compared to the models using more days of diary assessment. Finally, bedtime cortisol and change in bedtime cortisol over three weeks was best modeled using parent-child conflict computed from 16 days of daily diary data (AIC = 197.07), followed by a model using parent-child conflict slopes computed from 14 days (AIC = 207.46). In the model using slope of conflict from the 14 days prior to saliva sampling, slope of parent-child conflict24–37 was positively associated with increases in cortisol at bedtime17–18 to 38–39, B = 18.08, p = .018, and this effect was not detected in a model using slope of parent-child conflict 9- and 3 days prior to saliva sampling, p > .15. Taken together, a minimum of two weeks of daily diary assessment was necessary to detect associations between slope of change in parent-child conflict and change in AUCg, and bedtime cortisol, while change in diurnal slope was optimally modeled with 3 days of diary reports of change in conflict.
4. Discussion
The purpose of this study was to identify whether and how different indices of HPA-axis functioning, including CAR, diurnal slope, AUCg, and bedtime cortisol, change in response to recent patterns of change in parent-child conflict. Across three weeks, CAR, AUCg, and bedtime cortisol did not change significantly as a function of time, while diurnal slope appeared to flatten. Greater increases in slope of parent-child conflict over 16 days predicted up-regulation of diurnal cortisol across multiple indices, greater AUCg, flatter diurnal slope, and elevated cortisol at bedtime. In contrast, CAR was not responsive to changes in parent-child conflict over 3 weeks. Associations between change in parent-child conflict and change in both AUCg and bedtime cortisol were best modeled with at least 14 days of daily diary data. In contrast, change in diurnal slope and CAR were best modeled with change in parent-child conflict computed from the past 3 days. The time course under which HPA-axis functioning is influenced by changes in the child’s social environment is largely unknown. Thus, our results provide empirical support for future study design, and address a critical gap in our theoretical understanding of allostatic load.
Cumulative increases in parent-child conflict over 16 days predicted increases in cortisol measured by the AUCg, diurnal slope, and cortisol at bedtime. This is consistent with a meta-analysis finding that exposure to uncontrollable stressors is associated with a proximal upregulation of cortisol throughout the day in adults (Miller et al., 2007). Cortisol at bedtime was the most sensitive HPA-axis index to changes in parent-child conflict in this sample, even after controlling for sex, differences in bedtime, and conflict reported on the day of cortisol sampling. Specifically, slopes of parent-child conflict were positively related to increases in bedtime cortisol over 3 weeks. This is consistent with Lippold et al. (2014) who found that among youth with infrequent parent-child conflict, as was the case in our sample, more negative experiences over 8 days predicted elevated cortisol at bedtime. Among adults, daily experiences of tension, anger and stress are also associated with elevated cortisol at bedtime (Adam et al., 2006; Dahlgren et al., 2009). Our finding extends these patterns to a period of several weeks and may suggest that changes in evening regulation of cortisol in response to variations in the social environment drive changes in children’s diurnal slope, the most commonly used index of HPA-axis functioning in intervention studies (Slopen et al., 2014). An inability to down-regulate cortisol around bedtime may have important implications for sleep and circadian regulation (See Buckley and Schatzberg, 2005 for a review), and act as a pathway from interpersonal stress to poorer self-rated health in adulthood (Dahlgren et al., 2009). These results also appear to be consistent with HPA-axis anomalies found in samples exposed to more significant stressors. For example, adolescents who have been exposed to unpredictable traumatic events may have difficulty down-regulating cortisol in the evening (Kuhlman et al., 2015).
In this sample of youth, recent patterns of change in parent-child conflict were not related to changes in CAR over a 3 week period. Previous investigations into the psychobiological significance of CAR have suggested that CAR represents the anticipation of challenges for the coming day (Fries et al., 2009), and has most consistently been linked to recent chronic stress (Chida and Steptoe, 2009). CAR has been characterized as an index of adrenal sensitivity to ACTH exposure and capacity to synthesize cortisol (Clow et al., 2010). The lack of association between slopes of parent-child conflict and change in CAR in this sample of healthy youth exposed to low conflict may simply be an indication that normative fluctuations in parent-child conflict over three weeks are not sufficient to influence the physiological processes that underlie CAR.
We also examined whether the observed associations between parent-child conflict and HPA-axis functioning were detectable using fewer days of daily diary data to inform future study design. For AUCg and bedtime cortisol, slopes of parent-child conflict computed from the maximum available days (16 days) prior to saliva sampling were the best fit to change in cortisol over 3 weeks, and could be similarly modeled with 14 days of daily diary data, but not fewer. In contrast, a model using slopes of parent-child conflict across only 3 days prior to saliva sampling were the best fit to diurnal slope and change in diurnal slope over three weeks, while models of CAR did not benefit in fit from more than 3-days of daily diary report. This is consistent with previous studies of the stability of HPA-axis indices, suggesting that 50% of variability in diurnal indices of HPA-axis functioning are driven by day-to-day changes, and that AUCg was the most stable index among children and adolescents compared with diurnal slope and CAR (Ross et al., 2014). Taken together, our data may suggest that HPA-axis functioning in the morning is particularly sensitive to recent changes in the social environment, while regulation of HPA-axis functioning later in the day is more sensitive to cumulative interpersonal stress across weeks. We encourage other investigators using daily diary methods to examine similar questions of timing in order to inform future study design on features of the social environment that are salient to HPA-axis functioning.
4.1. Limitations
The contribution of this study should be considered in the context of several limitations. First, we aimed to capture parent-child conflict within natural, everyday family settings in a small sample of healthy, two-parent families. Our sample size constrained statistical power to detect subtle associations, as well as the number of variables that could be controlled in the analyses, leaving the investigation of factors such as pubertal status, BMI, sleep, and behavioral symptoms to future studies. Moreover, the characteristics of the participating families may limit the generalizability of results reported here. The diary items do not necessarily reflect potentially harmful family conflict; for instance an endorsement may reflect a child’s perception of the parent’s use of mild discipline that day. Thus, further research is needed to assess how well the patterns we observed apply to families who engage in more serious forms of conflict, such as intimate partner violence and abuse. However, the diary items we used are a sensitive measure of potential conflict between parents and their child, and not necessarily a specific measure of types of conflict to which children can be exposed. It is likely that associations between change in daily parent-child conflict and change in HPA-axis functioning from week-to-week would be stronger in a sample of families whose interactions are more likely to be characterized by hostility, abuse and violence. Related to this point, we designed this study and have interpreted our findings within an allostatic load framework (McEwen, 1998). Thus, our results pertain to children for whom recent patterns of change in parent-child conflict relate to HPA-axis functioning, which may or may not be the case for children exposed to chronically high levels of family conflict. In addition to differences between families, it is likely that there are individual differences in children’s sensitivity to varying intensities and frequencies of parent-child conflict as is postulated in differential susceptibility theory (Belsky and Pluess, 2009; Ellis and Boyce, 2011). With a larger sample, exposed to more family conflict, it would be possible to examine these individual characteristics and their health implications.
A second limitation is the constraint that any non-experimental design places on interpretation of results. Although prospective associations between conflict and HPA-axis functioning were tested, the temporal ordering of variables does not necessarily imply causal priority. The consistency of our findings with both theoretical expectations and, even more so with, intervention trials which do allow causal inference, inspires confidence that further understanding of the pathway from daily conflict exposure to health via functioning of physiological systems warrants further research.
4.2. Conclusion
In this sample of low conflict families, an increase in parent-child conflict over a three week period was associated with a subsequent increase in diurnal cortisol as measured by AUCg, flattening diurnal slope, and cortisol at bedtime but not changes in CAR. These data suggest that change in frequency of conflict over weeks may be more impactful on the HPA-axis, and that the HPA-axis may be resilient to intermittent social stressors. Thus responses to increases in parent-child conflict may be a novel social risk factor for changes to physiological functioning in school aged youth. Bedtime cortisol was the common denominator among the statistically significant findings and may be the aspect of HPA-axis functioning that is most sensitive to changes in parent-child conflict over several weeks. Investigators interested in evaluating improvements in HPA-axis functioning following changes in the family social environment, such as those resulting from psychosocial interventions, should consider assessing bedtime cortisol. To date, cortisol at bedtime has been linked with health outcomes such as sleep (Buckley and Schatzberg, 2005) and mortality in medical populations (Sephton et al., 2000), and thus may be an important indicator of how interpersonal stress can lead to poorer health. The results also speak to the stability of HPA-axis indices, in particular diurnal slope appeared to become steeper as a function of time, and to be sensitive to acute increases in parent-child conflict during the 3 days immediately preceding saliva sampling. Our data clearly suggest that future investigations of the effects of psychosocial interventions on HPA-axis functioning should include control groups to account for changes in HPA-axis functioning that occur over even relatively short periods of time. Finally, associations between parent-child conflict and two of the indices, AUCg and bedtime cortisol, were best modeled with at least 2 weeks of daily diaries, whereas the model fits for diurnal slope and CAR did not benefit from more than 3 days of data on parent-child conflict. Taken together, these findings contribute to converging evidence that the HPA-axis is sensitive to systematic changes in the social environment above and beyond the events on the same day. Additional studies employing dense, repeated sampling of HPA-axis functioning for the assessment of changes that occur from week-to-week would allow further characterization of the time frame in which HPA-axis functioning adapts to changes in the psychosocial environment. This approach could provide insights into short-term adaptations within the neuroendocrine system that may be related to long-term health.
Acknowledgments
Role of the funding source
The submitted research was financially support by the William T. Grant Foundation (Research Grant 9333) and the NIMH (MH015750) who were not involved in study design, the collection, analysis and interpretation of data, writing of the manuscript, or the decision to submit the article for publication.
This research was supported by a Research Grant (9333) from the William T. Grant Foundation. Preparation of this article was supported by a postdoctoral fellowship to Kate R. Kuhlman on MH015750 Biobehavioral Issues in Mental and Physical Health training grant. This research would not have been possible without the parents and children for their participation in this project and dedication to scientific progress, and we would like to thank the graduate students, laboratory staff, and research assistants on the project for their time and effort working with families. We would also like to acknowledge Gayla Margolin and Richard Slatcher for their significant contributions to the completion of this project.
Footnotes
Numbers in subscript following Parent-child conflict and indices of HPA-axis functioning refer to study days. See Fig. 1 for the study days to which these refer.
Among participating children, there was no significant within-person change in child-reported conflict with their parents across all 56 days of the larger study, B = −.001, p > .20. This suggests that the significant, positive slopes of parent-child conflict across these 16 day periods captured short-term and naturalistic increases in parent-child conflict that would not be observed when aggregating over a two-month period.
Conflicts of interest
None.
Contributors
Drs. Repetti, Robles, and Reynolds participated in the acquisition of data. Drs. Kuhlman, Robles, and Repetti contributed to the conception and design of the study, analysis and interpretation of data. Dr. Kuhlman drafted the article and Drs. Repetti, Robles, and Reynolds contributed to revising the manuscript critically for important intellectual content, and all authors provided final approval of the submission.
References
- Adam EK, Hawkley LC, Kudielka BM, Cacioppo JT. Day-to-day dynamics of experience–cortisol associations in a population-based sample of older adults. Proc Natl Acad Sci. 2006;103:17058–17063. doi: 10.1073/pnas.0605053103. http://dx.doi.org/10.1073/pnas.0605053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. http://dx.doi.org/10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19:716–723. http://dx.doi.org/10.1109/TAC.1974.1100705. [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull. 2009;2009(November 135):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm. Exemplary Sleep Disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. http://dx.doi.org/10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- Campbell DT, Kenny DA. A Primer on Regression Artifacts. Publications; Guilford: 1999. [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: a systematic review and meta-analysis. Biol Psychol. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. http://dx.doi.org/10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Clow A, Hucklebridge F, Stalder T, Evans P, Thorn L. The cortisol awakening response: more than a measure of HPA axis function. Neurosci Biobehav Rev. 2010;35:97–103. doi: 10.1016/j.neubiorev.2009.12.011. http://dx.doi.org/10.1016/j.neubiorev.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Cranford JA, Shrout PE, Iida M, Rafaeli E, Yip T, Bolger N. A procedure for evaluating sensitivity to within-person change: can mood measures in diary studies detect change reliably? Pers Soc Psychol Bull. 2006;32:917–929. doi: 10.1177/0146167206287721. http://dx.doi.org/10.1177/0146167206287721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren A, Kecklund G, Theorell T, Åkerstedt T. Day-to-day variation in saliva cortisol—relation with sleep, stress and self-rated health. Biol Psychol. 2009;82:149–155. doi: 10.1016/j.biopsycho.2009.07.001. http://dx.doi.org/10.1016/j.biopsycho.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. Differential susceptibility to the environment: toward an understanding of sensitivity to developmental experiences and context. Dev Psychopathol. 2011;23:1–5. doi: 10.1017/S095457941000060X. http://dx.doi.org/10.1017/S095457941000060X. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. http://dx.doi.org/10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. http://dx.doi.org/10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo–pituitary–adrenal (HPA) axis. Mol Cell Endocrinol. 2012;349:20–29. doi: 10.1016/j.mce.2011.06.042. http://dx.doi.org/10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. http://dx.doi.org/10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. http://dx.doi.org/10.1016/S0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Kuhlman KR, Geiss EG, Vargas I, Lopez-Duran NL. Differential associations between childhood trauma subtypes and adolescent HPA-axis functioning. Psychoneuroendocrinology. 2015;54:103–114. doi: 10.1016/j.psyneuen.2015.01.020. http://dx.doi.org/10.1016/j.psyneuen.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. http://dx.doi.org/10.1016/S0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Lehman BJ, Repetti RL. Bad days dont end when the school bell rings: the lingering effects of negative school events on children’s mood self-esteem, and perceptions of parent–child interaction. Soc Dev. 2007;16:596–618. http://dx.doi.org/10.1111/j.1467-9507.2007.00398.x. [Google Scholar]
- Lippold MA, McHale SM, Davis KD, Almeida DM, King RB. Experiences with parents and youth physical health symptoms and cortisol: a daily diary investigation. J Res Adolesc. 2014 doi: 10.1111/jora.12186. http://dx.doi.org/10.1111/jora.12186 (Epub ahead of print) [DOI] [PMC free article] [PubMed]
- McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann NY Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. http://dx.doi.org/10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychol Bull. 2007;133:25. doi: 10.1037/0033-2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol. 2013;4:133–142. http://dx.doi.org/10.1111/j.2041-210x.2012.00261.x. [Google Scholar]
- Odgers CL, Jaffee SR. Routine versus catastrophic influences on the developing child. Annu Rev Public Health. 2013;34:29–48. doi: 10.1146/annurev-publhealth-031912-114447. http://dx.doi.org/10.1146/annurev-publhealth-031912-114447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendry P, Adam EK. Associations between parents marital functioning, maternal parenting quality, maternal emotion and child cortisol levels. Int J Behav Dev. 2007;31:218–231. http://dx.doi.org/10.1177/0165025407074634. [Google Scholar]
- Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav. 1988;43:47–55. doi: 10.1016/0031-9384(88)90097-2. http://dx.doi.org/10.1016/0031-9384(88)90097-2. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Repetti RL, Taylor SE, Seeman TE. Risky families: family social environments and the mental and physical health of offspring. Psychol Bull. 2002:330–366. [PubMed] [Google Scholar]
- Repetti RL, Robles TF, Reynolds B. Allostatic processes in the family. Dev Psychopathol. 2011;23:921–938. doi: 10.1017/S095457941100040X. http://dx.doi.org/10.1017/S095457941100040X. [DOI] [PubMed] [Google Scholar]
- Repetti RL. The effects of perceived daily social and academic failure experiences on school-age children’s subsequent interactions with parents. Child Dev. 1996;67:1467–1482. http://dx.doi.org/10.2307/1131712. [PubMed] [Google Scholar]
- Reynolds BM, Robles TF, Repetti RL. Measurement reactivity and fatigue effects in daily diary research with families. Dev Psychol. 2015;52:442–456. doi: 10.1037/dev0000081. http://dx.doi.org/10.1037/dev0000081. [DOI] [PubMed] [Google Scholar]
- Robles TF, Reynolds BM, Repetti RL, Chung PJ. Using daily diaries to study family settings, emotions, and health in everyday life. J Soc Pers Relatsh. 2013;30:179–188. http://dx.doi.org/10.1177/0265407512457102. [Google Scholar]
- Ross KM, Murphy ML, Adam EK, Chen E, Miller GE. How stable are diurnal cortisol activity indices in healthy individuals? Evidence from three multi-wave studies. Psychoneuroendocrinology. 2014;39:184–193. doi: 10.1016/j.psyneuen.2013.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlotz W, Hellhammer J, Schulz P, Stone AA. Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom Med. 2004;66:207–214. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. http://dx.doi.org/10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. http://dx.doi.org/10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Slatcher RB, Robles TF. Preschoolers everyday conflict at home and diurnal cortisol patterns. Health Psychol. 2012;31:834–838. doi: 10.1037/a0026774. http://dx.doi.org/10.1037/a0026774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatcher RB, Robles TF, Repetti RL, Fellows MD. Momentary work worries marital disclosure and salivary cortisol among parents of young children. Psychosom Med. 2010;72:887–896. doi: 10.1097/PSY.0b013e3181f60fcc. http://dx.doi.org/10.1097/PSY.0b013e3181f60fcc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slopen N, McLaughlin KA, Shonkoff JP. Interventions to improve cortisol regulation in children: a systematic review. Pediatrics. 2014;133:312–326. doi: 10.1542/peds.2013-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Mechanisms linking early life stress to adult health outcomes. Proc Natl Acad Sci. 2010;107:8507–8512. doi: 10.1073/pnas.1003890107. http://dx.doi.org/10.1073/pnas.1003890107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53:865–871. doi: 10.1016/s0022-3999(02)00429-4. http://dx.doi.org/10.1016/S0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Vargas I, Lopez-Duran N. Dissecting the impact of sleep and stress on the cortisol awakening response in young adults. Psychoneuroendocrinology. 2014;40:10–16. doi: 10.1016/j.psyneuen.2013.10.009. http://dx.doi.org/10.1016/j.psyneuen.2013.10.009. [DOI] [PubMed] [Google Scholar]