Abstract
The hypothalamic–pituitary–adrenal axis (HPA axis) is a pathway through which childhood trauma may increase risk for negative health outcomes. The HPA axis is sensitive to stress throughout development; however, few studies have examined whether timing of exposure to childhood trauma is related to differences in later HPA axis functioning. Therefore, we examined the association between age of first trauma and HPA axis functioning among adolescents, and whether these associations varied by sex. Parents of 97 youth (aged 9–16 years) completed the Early Trauma Inventory (ETI), and youth completed the Socially-Evaluated Cold-Pressor Task (SECPT). We measured salivary cortisol response to the SECPT, the cortisol awakening response, and diurnal regulation at home across 2 consecutive weekdays. Exposure to trauma during infancy related to delayed cortisol recovery from peak responses to acute stress, d = 0.23 to 0.42. Timing of trauma exposure related to diverging patterns of diurnal cortisol regulation for males, d = 0.55, and females, d = 0.57. Therefore, the HPA axis may be susceptible to developing acute stress dysregulation when exposed to trauma during infancy, whereas the consequences within circadian cortisol regulation may occur in the context of later trauma exposure and vary by sex. Further investigations are warranted to characterize HPA axis sensitivity to exposure to childhood trauma across child development.
Childhood trauma may lead to health impairments by altering the functioning of the hypothalamic–pituitary–adrenal (HPA) axis (De Bellis & Zisk, 2014; Tarullo & Gunnar, 2006), a critical network that modulates cognitive, immune, and behavioral responses to stress (Lupien, McEwen, Gunnar, & Heim, 2009). There are sensitive periods of HPA axis development, such as infancy, during which exposure to stress can result in long-term perturbations in the regulation of HPA axis functioning, including its sensitivity to future stressors (Levine, 2005). In fact, biological sensitivity to stressful contexts partly explains how some youth are vulnerable to mental illness (Boyce & Ellis, 2005). There is limited research, however, examining whether a child’s age at the time of trauma exposure can lead to different long-term patterns of HPA axis functioning.
By 1 year of age, the HPA axis has become socially regulated and hyporesponsive, which serves to protect the developing infant from the deleterious effects of excessive exposure to glucocorticoids (Tarullo & Gunnar, 2006). In rodents, exposure to maternal separation during infancy (postnatal days 2–14) can lead to dysregulation of the receptors in charge of regulating HPA axis activation, resulting in a hypersensitive stress response and predisposition to internalizing behaviors (Gutman & Nemeroff, 2002). Thus, in humans, exposure to traumatic events during infancy may interfere with the development of a hyporesponsive axis and result in exposure to excess cortisol that may have long-term developmental consequences. This may be one pathway for the development of biological sensitivity to stress (Boyce & Ellis, 2005), which suggests that physiological hyperresponsiveness to stress is deleterious to health for children living in stressful environments. For example, exposure to maternal depression predicts greater responses to stress during preschool (Essex, Klein, Cho, & Kalin, 2002). Further, interpersonal trauma has been linked to impaired downregulation of cortisol in the evening (Weems & Carrion, 2009) and corresponding decreases in brain volume (Carrion, Weems, Richert, Hoffman, & Reiss, 2010). Less is known, however, about timing of a child’s first trauma and the specific aspect of the HPA axis that may be affected.
Cortisol, the final output of the HPA axis, can be noninvasively measured in three ways reflecting different underlying processes: the cortisol awakening response (CAR), diurnal patterns, and response to laboratory stressors. The CAR refers to a rise in cortisol that occurs immediately after awakening (Fries, Dettenborn, & Kirschbaum, 2009), and is influenced by multiple factors including sleep (Vargas & Lopez-Duran, 2014), and daily stress (Chida & Steptoe, 2009). Diurnal cortisol declines from waking to evening in response to circadian modulation from the suprachiasmatic nucleus (SCN) of the hypothalamus (Kalsbeek et al., 2012), thus a declining slope is an indicator of a healthy, intact system (Tsigos & Chrousos, 2002). Finally, reactivity to an acute stressor may indicate speed or intensity of hypothalamic initiation of HPA axis, whereas recovery from acute stress may be an indicator of efficiency of negative feedback by which cortisol downregulates the axis (Ladd, Huot, Thrivikraman, Nemeroff, & Plotsky, 2004).
To our knowledge, no study has examined how age of trauma may affect different HPA axis indices in the same sample. Evidence from separate studies, however, suggests that age of trauma may influence what aspect of the HPA axis is affected by trauma. For example, exposure to maternal depression during infancy prospectively predicted a sensitized HPA axis to stress during preschool (Essex et al., 2002), whereas the combination of maternal depression and marital conflict before age 5 years prospectively predicted at age 15 higher cortisol in the morning and flattened diurnal slope (Essex et al., 2011). Prenatal and early postnatal adversity (Bosch et al., 2012) and early childhood parent separation (Pesonen et al., 2010) may sensitize the HPA axis to greater reactivity to acute stress during adolescence and adulthood. In contrast, exposure to stressors during middle childhood may contribute to upregulation of tonic HPA axis stress hormones (Heim, Newport, Mletzko, Miller, & Nemeroff, 2008), thus contributing to greater cortisol exposure following stressors in adolescence (Bosch et al., 2012). Taken together, timing of stress exposure may matter for long-term HPA axis function, but such evidence comes from studies that examined a single HPA axis index, limiting the conclusions we can draw across studies.
Another well-documented source of variability in HPA axis development is sex (Gunnar & Vazquez, 2006). For example, excess testosterone in the brain during infancy may lead to greater vulnerability to stress in some males (Schwarz, Sholar, & Bilbo, 2012), suggesting that gonadal hormones demonstrate early organizing effects on HPA axis functioning. Animal models of the effect of physical neglect on males and females, however, have returned inconsistent results (Gunnar & Vazquez, 2006). Male and female children may have different periods of sensitivity to traumatic experiences that result in anomalous HPA axis functioning later in life. For example, adult men who lost a parent during childhood demonstrated greater adrenal sensitivity to the dexamethasone/corticotropin-releasing hormone test compared to females with parental loss (Tyrka et al., 2008). Likewise, HPA axis anomalies in youth depression, which has been extensively linked to early stress, have primarily been observed in males but not females (Hartman, Hermanns, de Jong, & Ormel, 2013; Owens et al., 2014). Therefore, in this study we also examined sex as a moderator of the association between age of first trauma and adolescent HPA axis functioning.
The purpose of this study was to examine the relationship between age of first trauma and adolescent HPA axis functioning, including CAR, diurnal cortisol, and acute stress response, as well as examining sex effects. Given the paucity of research on this association and the methodological variation between existing studies, our hypotheses were somewhat exploratory. We hypothesized that earlier exposure to childhood trauma would be associated with greater stress reactivity whereas later trauma would be associated with more elevated diurnal cortisol. Further, we hypothesized that males would demonstrate greater vulnerability to early trauma exposure.
Method
Participants and Procedure
Participants were a subgroup of 97 youth from a larger study of 138 subjects examining the neuroendocrine correlates of youth anxiety and depression (Kuhlman, Geiss, Vargas, & Lopez-Duran, 2015). Inclusion criteria for the larger study was any youth between ages 9–16 years; youth were excluded if they were currently taking asthma medication, had a major medical condition such as cancer, had a history of mania or psychosis, or had a developmental disorder such as autism. Youth were only included in the present study if their parents reported at least one traumatic event; ages were 9–16 years (M = 12.91, SD = 2.25; 51.6% male). This trauma-exposed group was 78.4% Caucasian, 5.9% African American, 2.0% Asian, 9.8% biracial, 2.9% Latino, and 1.0% other. Youth in this subgroup lived predominantly with both biological parents (65.4%) and in well-educated families (75.3% of participants’ mothers had earned a bachelor’s degree or higher). These youth did not differ from the larger sample in age, sex, race, maternal education, or current psychopathology, all p > .200. All eligible participants and their parents provided signed assent and consent to participate and youth were compensated for their time. This research was approved by the University of Michigan Health Sciences and Behavioral Sciences Institutional Review Board.
On two consecutive weekdays, participants provided passive drool into Sarstedt on four occasions: waking, 45-min postwaking, dinner, and bedtime. Participants were instructed to refrain from eating or drinking for 1 hour before each sample, record each sampling time, sleep and wake times, and any stressors, then store samples in the freezer.
All visits were conducted after 1 p.m. The stress task consisted of a 30-min baseline phase, a 5-min stress task, and a 60-min recovery period. The stress task used in this study was the Socially-Evaluated Cold Pressor Task (Schwabe, Haddad, & Schachinger, 2008). The participant placed their hand in ice water (33–39° F) for up to 3 min while a research assistant “watched [their] facial expressions” and recorded them with a video camera. The participant then watched a 60-min National Geographic documentary. HPA axis reactivity was estimated from cortisol extracted from seven saliva samples: one upon arrival, just before the stress task, and 25, 35, 45, 55, and 65 min after stress task initiation. Each child provided passive drool directly into a salivette tube. No agents (e.g., chewing gum) were used to facilitate saliva production. All salivettes were stored at −20° Celsius. Samples were assayed in duplicate using a commercial enzyme immunoassay kit (Salimetrics, LLC, Carlsbad, CA; assay sensitivity = 0.01 l g/dl) at the university’s core assay facility within 6 months. All samples from the same child were assayed together.
Measures
Parents (n = 79 mothers) completed the Early Trauma Inventory about their child (Bremner, Vermetten, & Mazure, 2000). Parents marked yes or no to a series of traumatic events including multiple items constituting physical abuse (hit to the point of bruising), sexual abuse (forced to engage in sexual acts), emotional abuse (persistently insulted by a caregiver), or non-intentional trauma (serious injury, parent separation). The score for each subtype was computed as the total number of events to which each child was exposed multiplied by the number of times any event occurred. These subtypes were then summed to create a total score ranging from 1 to 20 events. Total trauma exposure showed acceptable reliability (α = .79). For items marked yes, parents indicated the age (in years) of the child at the time of the event. Some parents indicated that a potential trauma, such as a family member with a mental illness, was present from birth which was quantified as 0 years, whereas other events were reported in months, quantified as a fraction of a year. Age of first trauma, identified as the youngest age reported for any event, reflects the child’s age at the time of their first exposure to a traumatic event, and was always treated as a continuous variable.
Data Analysis
We conducted models examining CAR, diurnal cortisol, and stress reactivity from total trauma exposure, age of first trauma, and the interaction between age of first trauma and sex using SPSS 22.0. Age was included in all models. Cortisol values were transformed using the Box-Cox transformation (Miller & Plessow, 2013), all other continuous independent variables were log-transformed to address skewness and kurtosis, and centered at the mean. Only participants with complete data (n = 78 for CAR, n = 88 for diurnal functioning, and n = 97 for stress reactivity) on childhood trauma exposure and HPA axis functioning were included in the present analyses. Missing samples are accounted for by failure to return home saliva samples to the laboratory or had missing, noncompliant diurnal samples.
We conducted hierarchical linear regressions accounting for change from waking to 45-min postwaking (CAR); testing the main effects of trauma exposure and age of first trauma, then the interaction between age of first trauma and sex. We then conducted growth curve models using linear mixed modeling to account for waking cortisol (intercept) and slope of diurnal cortisol from trauma exposure, age of first trauma, and the interaction between age of first trauma and sex. We used an unstructured covariance matrix, accounted for the impact of waking cortisol on diurnal slope, and included random effects for the intercept (waking) and the linear slope. For acute stress reactivity, we used growth curve analysis with landmark registration (Lopez-Duran, Mayer, & Abelson, 2014), where slope of cortisol activation to the stress task, peak cortisol (intercept), and the slope of recovery from the stress task were modeled simultaneously. Again, we modeled the main effects of trauma exposure and age of first trauma, then included the interaction between age of first trauma and sex. A significance threshold of p < .009 maintained a < 5% error rate across our six hypotheses.
Results
Parents reported that youth were exposed to between 1 and 20 traumatic events, beginning between birth and 11 years, with 77.7% of first reported traumatic experiences before age 1. Specifically, 85% of our subgroup was exposed to nonintentional trauma that began around age 1.45 years (SD = 3.23), and 76.9% of reported nonintentional traumas began at or before age 1. Physical abuse was reported for 54% beginning around age 4.47 years (SD = 3.24), and 10.2% of first physical abuse experiences occurred at or before age 1. Emotional abuse was reported for 34% beginning around age 6.23 years (SD = 4.87), and 30.0% of emotional abuse experiences occurred at or before age 1. Finally, 6.7% were exposed to sexual abuse beginning around age 10.57 years (SD = 3.31), the earliest beginning at age 5. See Table 1 for descriptive statistics and correlations between all study variables.
Table 1.
Means, Standard Deviations, and Correlations Between Demographic, Trauma Exposure, and HPA Axis Functioning Measures
| Variable | M | SD or % | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age (years) | 12.9 | 2.3 | – | ||||||||||
| 2. Sex (% female) | 49 | −.15 | – | ||||||||||
| 3. Total traumaa | 3.67 | 3.5 | .23* | .048 | – | ||||||||
| 4. Age of first trauma | 0.98 | 2.4 | −.02 | −.012 | .08 | – | |||||||
| 5. CARb,c | 0.08 | 0.24 | .17 | −.030 | .24* | −.08 | – | ||||||
| 6. Waking cortisolb,c | 0.31 | 0.16 | −.07 | .140 | −.02 | .13 | −.33** | – | |||||
| 7. Afternoon cortisolb,c | 0.12 | 0.15 | .09 | −.042 | −.07 | −.07 | .07 | .03 | – | ||||
| 8. Bedtime cortisolb,c | 0.11 | 0.13 | −.09 | −.308** | −.01 | −.00 | −.07 | .04 | .48** | – | |||
| 9. Baseline cortisolb,c | 0.15 | 0.28 | .24* | .087 | .10 | −.07 | −.13 | .05 | .30** | .13 | – | ||
| 10. AUCgb,c | 8.26 | 11.7 | .19 | .063 | .08 | −.05 | −.09 | .07 | .50** | .30** | .13 | – | |
| 11. Peak cortisolb,c | 0.15 | 0.24 | .16 | .045 | .08 | −.06 | −.05 | .04 | .53** | .33** | .74** | .95** | – |
| 12. AUCib,c | 0.90 | 3.9 | −.08 | .007 | −.01 | .03 | −.01 | .10 | .29** | .19 | −.19 | .21* | .40** |
Note. n = 97. CAR = cortisol awakening response; AUCg = area under the curve with respect to ground; AUCi = area under the curve with respect to increase from baseline.
Log transformed for multivariate analyses;
Box transformed for multivariate analyses;
μg/dl.
p < .05.
p < .01.
Amount of childhood trauma was not significantly associated with greater CAR, p = .078, nor was age of first trauma exposure, p = .085, R2 = .26, F(5, 72) = 6.47, p < .001. When the interaction between age of first trauma and sex was added to the model accounting for CAR, there was no significant interaction between age of first trauma and sex, p = .983, R2=.25, F(6, 70) = 5.31, p < .001. See Table 2 for parameter estimates for trauma exposure, age of first trauma, and the interaction between age of first trauma and sex.
Table 2.
Estimates of Associations With Cortisol Awakening Response, Diurnal Slope, and Stress Reactivity Using Sex, Trauma, Age of First Trauma, and Age by Sex Interactions
| Main effects | Interaction | |||
|---|---|---|---|---|
|
| ||||
| Variable | b | t | b | t |
| CAR | ||||
| Sex | −.05 | −0.49 | −.05 | −0.49 |
| Trauma exposure | .16 | 1.79 | .16 | 1.73 |
| Age of first trauma | −.12 | −1.75 | −.12 | −1.46 |
| Age of First Trauma × Sex | – | – | .00 | 0.02 |
| Diurnal cortisol | ||||
| Sex | .13 | 1.54 | .13 | 1.55 |
| Sex × Time | .03 | 1.03 | .02 | 0.83 |
| Sex × Time2 | −.00 | −1.80 | −.00 | −1.61 |
| Trauma exposure | −.04 | −0.73 | −.03 | −0.65 |
| Trauma Exposure × Time | −.01 | −0.45 | −.02 | −1.12 |
| Trauma Exposure × Time | .00 | 0.63 | .00 | 1.38 |
| Age of first trauma | .08 | 1.39 | .09 | 1.28 |
| Age of First Trauma × Time | −.03 | −1.18 | −.07 | −2.56* |
| Age of First Trauma × Time | .00 | 0.86 | .00 | 2.48* |
| Age of First Trauma × Sex | – | – | −.03 | −0.20 |
| Age of First Trauma × Sex × Time | – | – | .11 | 2.56* |
| Age of First Trauma × Sex × Time | – | – | −.01 | −2.86** |
| Stress reactivity | ||||
| Sex | .02 | 0.25 | .03 | 0.26 |
| Sex × Activation Time | −.00 | −0.77 | −.00 | −0.69 |
| Sex × Activation Time | −1.91E-5 | −0.30 | −1.40E-5 | −0.22 |
| Sex × Recovery Time | .00 | 1.23 | .00 | 1.15 |
| Sex × Recovery Time | −.00 | −1.28 | −9.80E-5 | −1.18 |
| Trauma exposure | −.01 | −0.26 | −.01 | −0.25 |
| Trauma Exposure × Activation Time | −.00 | −0.54 | −.00 | −0.28 |
| Trauma Exposure × Activation Time | −2.16E-6 | −0.07 | 5.34E-6 | 0.16 |
| Trauma Exposure × Recovery Time | .00 | 3.02** | .005 | 2.81** |
| Trauma Exposure × Recovery Time | −.00 | −3.17** | −.00 | −3.02** |
| Age of first trauma | −.02 | −0.22 | −.01 | −0.14 |
| Age of First Trauma × Activation Time | .00 | 0.09 | .00 | 0.75 |
| Age of First Trauma × Activation Time | −1.32E-5 | −0.34 | 1.67E-5 | 0.32 |
| Age of First Trauma × Recovery Time | −.00 | −1.99* | −.00 | −2.48* |
| Age of First Trauma × Recovery Time | .00 | 3.77** | .00 | 4.05** |
| Age of First Trauma × Sex | – | – | −.00 | −0.03 |
| Age of First Trauma × Sex × Activation Time | – | – | −.00 | −1.01 |
| Age of First Trauma × Sex × Activation Time | – | – | −6.70E-5 | −0.82 |
| Age of First Trauma × Sex × Recovery Time | – | – | .01 | 1.51 |
| Age of First Trauma × Sex × Recovery Time | – | – | −.00 | −1.79 |
Note. n = 97.
p < .05.
p < .01.
We then examined unconditional linear and quadratic growth models of diurnal cortisol using waking cortisol as the intercept. The quadratic model was the best fit to the data (linear Akaike information criterion [AIC] = 238.9 vs. quadratic AIC = 233.9). From waking, Intercept b = −1.07, t(86.2) = −28.3, p < .001, cortisol values declined, Time b = ≤.09, t(93.3) = −6.08, p < .001, and this decline decelerated during the evening, Time2 b = .002, t(80.7) = 2.69, p = .009. Age and sex were not related to differences in waking cortisol, p > .192, or the diurnal decline in cortisol, p > .074.
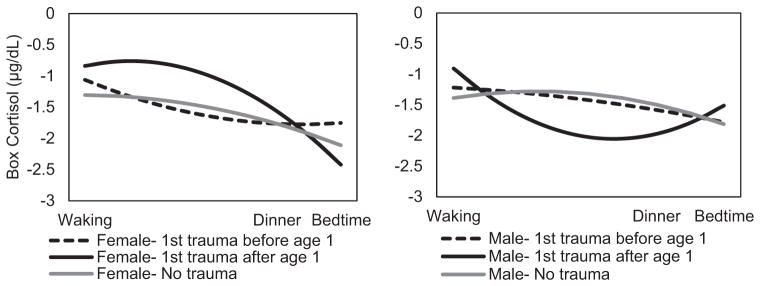
Total trauma exposure and age of first trauma were not associated with waking cortisol or the diurnal cortisol throughout the day. When the interaction between age of first trauma and sex was added, model fit improved (no interactions AIC = 221.6 vs. interactions AIC = 219.4), and sex significantly moderated the association between age of first trauma and diurnal cortisol. See Figure 1 for diurnal cortisol by age of first trauma and sex. In males, earlier age of first trauma related to a flat slope of cortisol decline throughout the day, Age of First Trauma × Time, b = .066, t(80.0) = −2.56, p = .013, but greater decline between dinner and bedtime, Age of First Trauma × Time2, b = .004, t(68.3) = 2.48, p = .016. In contrast, later exposure to a first traumatic experience related to a flatter slope of cortisol decline throughout the day for females, Age of First Trauma × Time (female), b = 113, t(79.9) = 2.56, p = .012, and acceleration of this decline between dinner and bedtime, Age of First Trauma × Time (female), b = −.008, t(68.1) = −2.86, p = .006.
Figure 1.
n = 88. Estimated diurnal cortisol by age of first trauma exposure by sex among trauma-exposed youth. For purposes of comparison only, individuals from the larger sample with no trauma exposure are shown; however, these were not included in any models due to the lack of trauma exposure. For visual purposes, ± 1 SD age of trauma exposure is plotted here; it was a continuous variable in all models.
We then modeled the unadjusted cortisol trajectories in response to acute stress including the slope of cortisol activation, peak (intercept), and slope of cortisol recovery from peak. A model with quadratic slopes for both activation and recovery was the best fit to the data (linear AIC = −229.3 vs. quadratic AIC = −273.8). Cortisol increased toward the peak, Activation Time, b = .007, t(329.9) = 5.22, p < .001, and this increase accelerated over time, Activation Time2, b = .0002, t(333.6) = 6.06, p < .001. Cortisol decreased linearly away from the peak, Recovery Time, b = −.013, t(390.0) = −8.58, p < .001, and decelerated over time, Recovery Time2, b = .0002, t(373.9) = 4.92, p < .001. Age was not related to peak (intercept), or the recovery from this peak. Sex was not related to slope of cortisol reactivity, peak cortisol, or recovery from peak, p > .098.
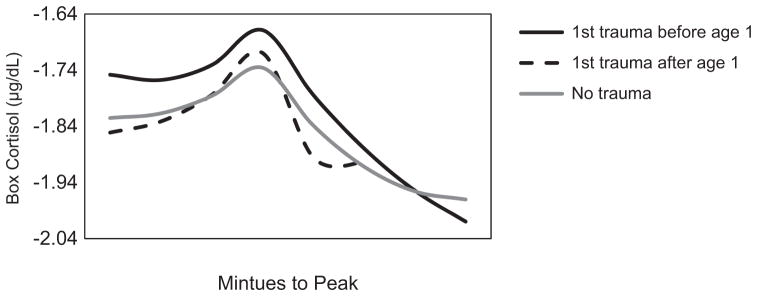
We then conducted conditional, unadjusted models for total trauma exposure, age of onset, and the interaction between age of first trauma and sex. More traumatic experiences were not associated with differences in activation to peak, but related to less steep cortisol recovery from stress, Trauma × Recovery Time, b = .005, t(339.1) = 3.02, p = .003, and Trauma × Recovery Time2, b = −.0002, t(310.5) = −3.17, p = .002. Later exposure to a first traumatic experience related to steeper cortisol recovery from peak, Age of First Trauma × Recovery Time, b = −.005, t(340.3) = −1.99, p = .040, and Age of First Trauma × Recovery Time2, b = .0002, t(313.9) = 3.77, p < .001. When the interaction between age of first trauma and sex were entered into this model, model fit did not improve (no interactions AIC = −237.1 vs. interactions AIC = −231.1), and sex did not moderate the association between age of first trauma and HPA axis reactivity or recovery from the laboratory stressor. See Figure 2 for HPA axis reactivity to acute stress by age of first trauma.
Figure 2.
n = 97. Estimated acute stress response by age of first trauma (± 1 SD). For purposes of comparison only, individuals from the larger sample with no trauma exposure are shown; however, these were not included in any models due to the lack of trauma exposure. For visual purposes, ± 1 SD age of trauma exposure is plotted here for visual purposes; it was a continuous variable in all models.
Differences in HPA axis profiles by trauma subtype were previously observed in this subgroup (Kuhlman et al., 2015) with systematic differences in timing across trauma subtype observed here. Therefore, we conducted a post hoc model predicting HPA axis functioning from count of trauma exposure by subtype and age of first trauma. Age of first trauma was an additional, independent correlate of HPA axis reactivity in this model, exposure to their first trauma increasingly before age 1 (M) correlated with more slowly declining cortisol after the peak response, Age of First Trauma × Recovery, b = .004, p = .019 and Age of First Trauma × Recovery2, b = .0002, p = .001. Thus, it was safe to deduce that both type of childhood trauma exposure and timing of those experiences were unique contributors to later HPA axis functioning in this sample.
Discussion
We examined whether age of first trauma exposure was related to different indices of HPA axis functioning during adolescence, and whether these associations varied by sex. Using a parent-reported measure of childhood trauma, average age of first trauma exposure was 1 year. When accounting for total trauma, trauma exposure after age 1 related to diverging diurnal cortisol slopes for males and females, and exposure before age 1 related to delayed recovery from peak responses to acute physiological stress for both males and females. Given that infancy overlaps with the transition to socially regulated, HPA axis hyporesponsiveness, trauma exposure during this period may disrupt the development of effective regulation of the HPA axis. These findings underscored the potential neurobiological benefit of an infancy that is unremarkable for traumatic experiences.
In this subgroup of trauma-exposed youth, no significant associations were observed between CAR and total reported trauma exposure or age of first trauma. Greater CAR has been most consistently associated with ongoing life stress and anticipation of daily challenges (Chida & Steptoe, 2009). Thus, our findings provide further evidence that CAR does not reflect the physiological consequences of distal stressors and may not be altered by experiences during early development. Alternatively, most studies examining CAR collect a sample at 30-min postwaking, thus our findings may more accurately reflect a lack of association between age of first trauma and ability to downregulate cortisol following CAR. A follow-up study investigating these associations with more densely sampled CAR would address this possibility.
Timing of a first traumatic event was associated with diverging patterns of diurnal cortisol for males and females. Males exposed to their first trauma longer after age 1 exhibited increasingly high cortisol upon waking, lower cortisol throughout the day, and elevated cortisol in the evening compared with infancy exposure, whereas later childhood exposure to a first traumatic event in females related to high circulating cortisol throughout the day compared with early exposure. Diurnal HPA axis regulation is driven by the “central clock” in the SCN of the hypothalamus that responds to changes in light (Nader, Chrousos, & Kino, 2010). Previous research suggested gonadal hormones during infancy, such as testosterone, contribute to the organization of circadian HPA axis regulation and the SCN in the hypothalamus when exposed to isolated stress (Schwarz et al., 2012). This finding suggests that long-term diurnal regulation of the HPA axis may not be programmed during infancy in humans as previously suggested.
Exposure to a first traumatic event during infancy related to slower downregulation of cortisol following acute stress compared to exposure later in childhood. Specifically, exposure to a first traumatic event during infancy may impair negative feedback of the HPA axis, such as reduced density or sensitivity of glucocorticoid receptors in the hippocampus, potentially contributing to biological sensitivity to context later in life. Of note, more total trauma exposure was also associated with slower recovery from acute stress, and early exposure was a significant independent correlate of this pattern of slower recovery. Few studies to our knowledge have examined age of trauma as a predictor of later HPA axis functioning (Bosch et al., 2012; Heim et al., 2008; Pesonen et al., 2010). These studies primarily characterized differences in reactivity of the HPA axis to challenge, whereas our use of a novel analytic approach allowed for disentangling reactivity from recovery to acute challenge.
This study was cross-sectional and no causality or prediction may be inferred. These findings were limited to a sample of youth exposed to at least one traumatic event. Thus future studies, especially when considering sex differences, should include a nontrauma-exposed control group. Furthermore, 77% of the sample experienced nonintentional trauma first, and 98% had not been exposed to a potentially traumatic event in the past 2 years, suggesting that our findings are unlikely to be driven by recent traumatic events. Youth in this sample likely varied in pubertal status, which accounts for divergence in diurnal regulation of the HPA axis between males and females (Netherton, Goodyer, Tamplin, & Herbert, 2004). Information on pubertal status was not collected for this study; however, both age and sex have been accounted for in all of our models, and inclusion of a nonlinear term for age also resulted in no changes to our findings. Childhood trauma exposure was provided retrospectively by parents of our participants and reflects number of exposures rather than duration. Parent report is a common method of assessment of early childhood experiences; however, it is possible that rates of abuse and neglect were underreported. Rates of trauma exposure in this sample are comparable to those reported in nationally representative studies (Flaherty et al., 2009). Further, the prevalence of reported trauma during infancy suggested an unanticipated benefit of parent report and a potential additional limitation of retrospective, self-report of trauma exposure, given that participants may be less likely to report events that occurred during their own infancy. The use of retrospective parent report, however, introduces the possibility that parents are not accurately reporting the age of first trauma for specific events. There were no significant associations between race or mother’s education as correlates of our HPA axis outcomes, nor did our model fit improve with their inclusion. Participants were representative of the local community, which tended to be from highly educated and predominantly Caucasian families.
Age of trauma exposure is important to consider when characterizing physiological dysregulation in trauma-exposed youth, beyond the contribution of trauma exposure quantity. In particular, components of the HPA axis negative-feedback loop may be more plastic during infancy than any other point in childhood, and exposure to trauma during this time may result in poor HPA axis recovery from acute stress that extends at least until adolescence. In contrast, circadian regulation of the HPA axis may be differentially vulnerable to exposure during infancy and other points in childhood based upon sex. Given that infants develop socially regulated, hyporesponsive stress physiology by the end of infancy (Tarullo & Gunnar, 2006), it will be important to understand whether our results indicate the consequences of trauma exposure before this hyporesponsiveness has developed or whether trauma exposure impairs the development of hyporesponsiveness. These findings converge with sensitive periods in animal models by showing that exposure to stress during infancy resulted in poor regulation of glucocorticoids via reduced glucocorticoid receptor sensitivity (Meaney et al., 1996). Our findings highlight the importance of examining the potential for the differential impact of age of trauma exposure on physiology in children to better translate experimental animal models. Several studies have linked age of trauma to patterns of symptom presentation (e.g., Dunn, McLaughlin, Slopen, Rosand, & Smoller, 2013; Kuhlman, Maercker, Bachem, Simmen, & Burri, 2013), while studies are emerging demonstrating that different neurobiological systems are sensitive to stress exposure at different periods of development (Andersen & Teicher, 2008). These findings underscore the need for more investigations of childhood trauma exposure timing as a contributor to later functioning of key neurobiological systems.
Acknowledgments
This research would not be possible if not for the support of several individuals and organizations. Among them are the team at MichiganPAL for collecting this data 7 days a week for 2 years, the families who generously gave their time to improve our understanding of the underpinnings of anxiety and depression, the faculty and fellows of the International Max Planck Research School on the Life Course who have provided valuable insight on the development of this project, and the following organizations for their financial support of this research: Blue Cross Blue Shield of Michigan Foundation, Barbara A. Oleshansky Memorial Award, American Psychological Foundation Elizabeth Munsterberg-Koppitz Award, and Rackham Graduate School at University of Michigan.
References
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Bosch NM, Riese H, Reijneveld SA, Bakker MP, Verhulst FC, Ormel J, Oldehinkel AJ. Timing matters: Long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response. The TRAILS study. Psychoneuroendocrinology. 2012;37:1439–1447. doi: 10.1016/j.psyneuen.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis B. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/S0954579405050145. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: The Early Trauma Inventory. Depression and Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Richert K, Hoffman BC, Reiss AL. Decreased prefrontal cortical volume associated with increased bedtime cortisol in traumatized youth. Biological Psychiatry. 2010;68:491–493. doi: 10.1016/j.biopsych.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Cortisol awakening response and psychosocial factors: A systematic review and meta-analysis. Biological Psychology. 2009;80:265–278. doi: 10.1016/j.biopsycho.2008.10.004. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Zisk A. The biological effects of childhood trauma. Child and Adolescent Psychiatric Clinics of North America. 2014;23:185–222. doi: 10.1016/j.chc.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn EC, McLaughlin KA, Slopen N, Rosand J, Smoller JW. Developmental timing of child maltreatment and symptoms of depression and suicidal ideation in young adulthood: Results from the National Longitudinal Study of Adolescent Health. Depression and Anxiety. 2013;30:955–964. doi: 10.1002/da.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/S0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, … Armstrong JM. Influence of early life stress on later hypothalamic–pituitary–adrenal axis functioning and its covariation with mental health symptoms: A study of the allostatic process from childhood into adolescence. Development and Psychopathology. 2011;23:1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaherty EG, Thompson R, Litrownik AJ, Zolotor AJ, Dubowitz H, Runyan DK, … Everson MD. Adverse childhood exposures and reported child health at age 12. Academic Pediatrics. 2009;9:150–156. doi: 10.1016/j.acap.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol 2: Developmental neuroscience. 2. Hoboken, NJ: Wiley; 2006. pp. 533–577. [Google Scholar]
- Gutman DA, Nemeroff CB. Neurobiology of early life stress: Rodent studies. Seminars in Clinical Neuropsychiatry. 2002;7:89–95. doi: 10.1053/scnp.2002.31781. [DOI] [PubMed] [Google Scholar]
- Hartman CA, Hermanns VW, de Jong PJ, Ormel J. Self-or parent report of (co-occurring) internalizing and externalizing problems, and basal or reactivity measures of HPA-axis functioning: A systematic evaluation of the internalizing-hyperresponsivity versus externalizing-hyporesponsivity HPA-axis hypothesis. Biological Psychology. 2013;94:175–184. doi: 10.1016/j.biopsycho.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo–pituitary–adrenal (HPA) axis. Molecular and Cellular Endocrinology. 2012;349:20–29. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Kuhlman KR, Geiss EG, Vargas I, Lopez-Duran NL. Differential associations between childhood trauma subtypes and adolescent HPA-axis functioning. Psychoneuroendocrinology. 2015;54:103–114. doi: 10.1016/j.psyneuen.2015.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman KR, Maercker A, Bachem R, Simmen K, Burri A. Developmental and contextual factors in the role of severe childhood trauma in geriatric depression: The sample case of former indentured child laborers. Child Abuse & Neglect. 2013;37:969–978. doi: 10.1016/j.chiabu.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Plotsky PM. Long-term adaptations in glucocorticoid receptor and mineralocorticoid receptor mrna and negative feedback on the hypothalamo–pituitary–adrenal axis following neonatal maternal separation. Biological Psychiatry. 2004;55:367–375. doi: 10.1016/j.biopsych.2003.10.007. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Lopez-Duran NL, Mayer SE, Abelson JL. Modeling neuroendocrine stress reactivity in salivary cortisol: Adjusting for peak latency variability. Stress. 2014;17:285–295. doi: 10.3109/10253890.2014.915517. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, … Plotsky PM. Early environmental regulation of fore-brain glucocorticoid receptor gene expression: Implications for adreno-cortical responses to stress. Developmental Neuroscience. 1996;18:61–72. doi: 10.1159/000111396. [DOI] [PubMed] [Google Scholar]
- Miller R, Plessow F. Transformation techniques for cross-sectional and longitudinal endocrine data: Application to salivary cortisol concentrations. Psychoneuroendocrinology. 2013;38:941–946. doi: 10.1016/j.psyneuen.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends in Endocrinology & Metabolism. 2010;21:277–286. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherton C, Goodyer I, Tamplin A, Herbert J. Salivary cortisol and dehydroepiandrosterone in relation to puberty and gender. Psychoneuroendocrinology. 2004;29:125–140. doi: 10.1016/S0306-4530(02)00150-6. [DOI] [PubMed] [Google Scholar]
- Owens M, Herbert J, Jones PB, Sahakian BJ, Wilkinson PO, Dunn VJ, … Goodyer IM. Elevated morning cortisol is a stratified population-level biomarker for major depression in boys only with high depressive symptoms. Proceedings of the National Academy of Sciences. 2014;111:3638–3643. doi: 10.1073/pnas.1318786111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen AK, Raikkonen K, Feldt K, Heinonen K, Osmond C, Phillips DIW, … Kajantie E. Childhood separation experience predicts HPA axis hormonal responses in late adulthood: A natural experiment of World War II. Psychoneuroendocrinology. 2010;35:758–767. doi: 10.1016/j.psyneuen.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33:890–895. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. Journal of Neurochemistry. 2012;120:948–963. doi: 10.1111/j.1471-4159.2011.07630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Hormones and Behavior. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Chrousos GP. Hypothalamic–pituitary–adrenal axis, neuroendocrine factors and stress. Journal of Psychosomatic Research. 2002;53:865–871. doi: 10.1016/S0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL. Childhood parental loss and adult hypothalamic–pituitary–adrenal function. Biological Psychiatry. 2008;63:1147–1154. doi: 10.1016/j.biopsych.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas I, Lopez-Duran N. Dissecting the impact of sleep and stress on the cortisol awakening response in young adults. Psychoneuroendocrinology. 2014;40:10–16. doi: 10.1016/j.psyneuen.2013.10.009. [DOI] [PubMed] [Google Scholar]
- Weems CF, Carrion VG. Brief report: Diurnal salivary cortisol in youth—Clarifying the nature of posttraumatic stress dysregulation. Journal of Pediatric Psychology. 2009;34:389–395. doi: 10.1093/jpepsy/jsn087. [DOI] [PMC free article] [PubMed] [Google Scholar]