Abstract
Timing deficits are observed in patients with schizophrenia. Serotonergic hallucinogens can also alter the subjective experience of time. Characterizing the mechanism through which the serotonergic system regulates timing will increase our understanding of the linkage between serotonin (5-HT) and schizophrenia, and will provide insight into the mechanism of action of hallucinogens. We investigated whether interval timing in mice is altered by hallucinogens and other 5-HT2 receptor ligands. C57BL/6J mice were trained to perform a discrete-trials temporal discrimination task. In the discrete-trials task, mice were presented with two levers after a variable interval. Responding on lever A was reinforced if the interval was < 6.5 s, and responding on lever B was reinforced if the interval was > 6.5 s. A 2-parameter logistic function was fitted to the proportional choice for lever B (%B responding), yielding estimates of the indifference point (T50) and the Weber fraction (a measure of timing precision). The 5-HT2A antagonist M100907 increased T50, whereas the 5-HT2C antagonist SB-242,084 reduced T50. The results indicate that 5-HT2A and 5-HT2C receptors have countervailing effects on the speed of the internal pacemaker. The hallucinogen 2,5-dimethoxy-4-iodoamphetamine (DOI; 3 mg/kg IP), a 5-HT2 agonist, flattened the response curve at long stimulus intervals and shifted it to the right, causing both T50 and the Weber fraction to increase. The effect of DOI was antagonized by M100907 (0.03 mg/kg SC) but was unaffected by SB-242,084 (0.1 mg/kg SC). Similar to DOI, the selective 5-HT2A agonist 25CN-NBOH (6 mg/kg SC) reduced %B responding at long stimulus intervals, and increased T50 and the Weber fraction. These results demonstrate that hallucinogens alter temporal perception in mice, effects that are mediated by the 5-HT2A. It appears that 5-HT regulates temporal perception, suggesting that altered serotonergic signaling may contribute to the timing deficits observed in schizophrenia and other psychiatric disorders.
Keywords: psychedelic, hallucinogen, interval timing, discrete-trials, mice
1. INTRODUCTION
The perception of time is required for the precise organization of behavior as well as the anticipation of outcomes and future events. Temporal perception occurs over multiple timescales, ranging from milliseconds to days, and includes functions such as sensory-motor timing and circadian rhythm (Buhusi and Meck, 2005). Interval typing, another type of temporal perception, is responsible for the discrimination of durations in the seconds to minutes range. Given the crucial importance of timing to the regulation of behavior, impaired temporal processing would have profound consequences. Timing deficits have been observed in patients with a variety of neuropsychiatric disorders, including schizophrenia (Rammsayer, 1990; Tracy et al., 1998; Volz et al., 2001; Davalos et al., 2003, 2011; Elvevag et al., 2003; Carroll et al., 2008, 2009a,b). There is evidence indicating that impaired temporal processing is a core deficit of schizophrenia, contributing to cognitive dysfunction, hallucinations, and inappropriate behavior (Carroll et al., 2008; Ward et al., 2011). Importantly, according to a recent meta-analysis, the timing deficits in schizophrenia patients are independent of impairments of attention, memory, or cognition (Ciullo et al., 2015).
Similar to schizophrenia, serotonergic hallucinogens can markedly alter the subjective experience of time (Kenna and Sedman, 1964; Heimann, 1994). According to anecdotal reports, the serotonergic hallucinogens lysergic acid diethylamide (LSD) and mescaline speed up or slow down the subjective experience of time, or produce feelings of timelessness (Serko,1913; Beringer, 1927; DeShon et al., 1952; Hoch et al., 1952). Recently, psilocybin was found to disrupt timing in human volunteers (Wittmann et al., 2007; Wackerman et al., 2008). Hallucinogens induce their characteristic effects by activating 5-HT2A receptors (Nichols 2004; Halberstadt, 2015), but it remains to be determined whether their effects on temporal perception are mediated by 5-HT2A.
Interval timing is often assessed in rodents using immediate and retrospective timing schedules. Immediate timing schedules require subjects to regulate their behavior based on the passage of time (temporal differentiation), whereas in retrospective schedules subjects are trained to respond differentially depending on the duration of an interval that has already elapsed (temporal discrimination). The discrete-trials task (DTT) developed by Body et al. (2002a) is an example of a retrospective timing schedule. In the DTT, a lamp is illuminated for a variable duration, and then two levers are presented. Responding on lever A is reinforced if the stimulus duration is shorter than a specific value, and responding on lever B is reinforced if the stimulus duration is longer than the value. Timing is measured by the indifference point (T50, the time when animals are equally likely to respond on lever A and lever B), a measure of central pacemaker speed, and by the Weber fraction, a measure of the precision of timing. The Weber fraction is defined as the ratio of the difference limen to T50. When tested in rats performing the DTT, the hallucinogen and 5-HT2A/2C receptor agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) increases the Weber fraction (indicating increased variability of timing) and tends to increase T50 (consistent with a reduction of pacemaker speed) (Asgari et al., 2006; Hampson et al., 2010). DOI also alters temporal differentiation in immediate timing schedules, although it reduces T50 in those tasks (Body et al., 2003, 2006a,b; Cheung et al., 2007). The selective 5-HT2A antagonist M100907 blocks the effects of DOI on interval timing, indicating that its effects are mediated by 5-HT2A receptors (Body et al., 2006a,b; Asgari et al., 2006). Several other 5-HT receptor agonists have effects on interval timing in rodent models, including the 5-HT releasing agent fenfluramine (Body et al., 2004), the 5-HT1A agonist 8-OH-DPAT (Chiang et al., 2000; Body et al., 2002a,b), and the mixed 5-HT2A/3 agonist quipazine (Body et al., 2005; Asgari et al., 2005).
To date, no published studies have assessed whether 5-HT receptor ligands alter interval timing in mice. Given the opportunities provided by genetic mutations to investigate the neural mechanisms for interval timing, these studies are essential. Hence, we developed a version of the DTT that can be used to test whether interval timing is altered by hallucinogens and by 5-HT receptor ligands in mice. The goal of the present investigation was to determine whether DOI alters temporal discrimination in mice. Studies also assessed whether N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenethylamine (25CN-NBOH), a putative 5-HT2A-selective agonist (Hansen et al., 2014), can mimic the effects of DOI on temporal discrimination. We also examined whether timing is altered by blockade of 5-HT2A and 5-HT2C receptors with M100907 and SB-242,084, respectively.
2. MATERIALS AND METHODS
2.1. Subjects
Mice were housed in a vivarium at the University of California San Diego (UCSD), an AAALAC-approved animal facility that meets Federal and State requirements for care and treatment of laboratory animals. Male C57BL/6J mice were obtained from Jackson Labs (Bar Harbor, ME); they were allowed to acclimate for approximately 1 week after arrival. All mice were housed up to 4 per cage in a climate-controlled room with a reversed light cycle (lights on at 1900 hours, off at 0700 hours). All testing occurred between 1000 and 1800 hours; animal testing was conducted in accord with the ‘Principles of laboratory Animal Care’ NIH guidelines and were approved by the UCSD animal care committee.
2.2. Drugs
Drugs used were 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride (DOI; Sigma Chemical Co., St Louis, MO); 6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride (SB-242,084), N-(2-hydroxybenzyl)-2,5-dimethoxy-4-cyanophenethylamine hydrochloride (25CN-NBOH; Tocris Bioscience, Ellisville, MO); and (R)-(+)-α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol (M100907; Hoechst Marion Roussel Inc., Kansas City, MO). The purity of M100907 was confirmed by LC-MS analysis. DOI was dissolved in isotonic saline. M100907 and 25CN-NBOH were dissolved in water containing 5% Tween 80. SB 242,084 was dissolved in water containing 1% Tween 80. DOI was administered intraperitoneally at a volume of 5 mL/kg body weight. 25CN-NBOH, M100907, and SB-242,084 were administered subcutaneously at a volume of 5 mL/kg body weight.
2.3. Assessment of the head-twitch response
The head twitch response (HTR) was assessed using a head-mounted magnet and a magnetometer coil, as described previously (Halberstadt and Geyer, 2013, 2014). Food and water were provided ad libitum, except during behavioral testing. 25CN-NBOH was administered immediately prior to the start of a 30-min assessment. Total HTR counts were analyzed by non-linear regression, as well as by one-way analysis of variance (ANOVA) and post-hoc Tukey’s test. The alpha level was set at 0.05.
2.4. Binding Studies
Radioligands used for 44 binding assays are listed in Table S1. The screening was performed by the NIMH Psychoactive Drug Screening Program (NIMH PDSP). 25CN-NBOH was dissolved in DMSO and primary binding screens were conducted in quadruplicate. Sites exhibiting >50% inhibition at 10,000 nM were subjected to a competitive binding assay to determine Ki values. Detailed experimental details are available from the NIMH PDSP website at http://pdsp.med.unc.edu/UNC-CH%20Protocol%20Book.pdf.
2.5. Discrete-trials timing task
2.5.1. Apparatus
Training and testing in the discrete-trials task occurred in 2-lever operant chambers (21.6×17.8×12.7 cm, Med Associates Inc., St. Albans, VT). One wall in each chamber contains a food-delivery magazine (Lafayette Instruments, Lafayette, IN) and 2 motor-driven retractable levers 2.2 cm above the grid floor and spaced 10.4 cm apart, with an incandescent house-light located near the ceiling. Two white light-emitting diodes (LEDs), mounted horizontally near the top of the magazine, were used to signal reward availability, and a green LED was mounted horizontally above each lever. Liquid reinforcement (Strawberry Nesquik® plus non-fat milk, 20 μL) was delivered by a peristaltic pump (Lafayette Instruments, Lafayette, IN) to a well located in the floor of the magazine. Magazine entries were monitored using an infrared beam mounted horizontally, 5 mm above the floor and recessed 6 mm into the magazine. The chamber was located in a sound-attenuating box, ventilated by a fan that also provided a low level of background noise. The control of stimuli and recording of responses were managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates Inc., St. Albans, VT) using custom programming.
2.5.2. Procedure
Mice were maintained at 85% of their free-feeding weight and trained 5 days a week, at the same time each day, during the dark (awake) phase of their light-dark cycle. Mice were first trained using a session in which reinforcement was dispensed every 15 s into the magazine, which was simultaneously illuminated. Magazine entries resulted in the light being extinguished until the next reinforcement is delivered. This training was repeated daily (Monday–Friday) until there were ≥30 magazine entries in 10 min for 2 consecutive days. In the second session, mice were trained to respond on either lever in order to obtain reinforcement under an FR1 schedule. This session was repeated daily (Monday–Friday) until all mice made >70 lever presses within a 30 min session on 2 consecutive days.
The mice were subsequently trained daily (Monday–Friday) in the discrete-trials task, with each training session lasting 30 min or 120 trials (whichever was completed first). At the beginning of each session, the house light was extinguished and the magazine light and the green LEDs above the levers were illuminated; trials were initiated when the mouse entered and then exited the magazine. At the beginning of each trial, the magazine light was extinguished, and then, following a variable duration (2.5, 5.0, 8.0, or 10.5 s, in pseudorandom order), the levers were presented for 10 s. Responding on lever A was reinforced if the interval was <6.5 s, and responding on lever B was reinforced if the interval was >6.5 s. The position of the two levers (left vs. right) was counterbalanced across subjects. An incorrect response or omission (failure to respond within 10 s) resulted in a time-out “punishment” where the levers were retracted, the house light was illuminated, and no aperture was responsive for 4 s. The next trial was initiated when the mouse entered and then exited the magazine. One-fifth of the trials were forced choice trials where only one lever was extended and was not retracted until a response was made. Training occurred until all mice attained a %correct [correct/(correct+incorrect)*100] score of >85% for the 2.5 and 10.5 s trials. Correct, incorrect, omissions, and reward latencies were also recorded. Test sessions were identical to the training sessions except that more durations (2.5, 3.4, 4.3, 5.2, 6.1, 6.9, 7.8, 8.7, 9.6, and 10.5 s) were used, there were no forced choice trials, and the sessions lasted 45 min or 200 trials.
2.5.3. Experimental Design
There was a minimum of 7 days between experimental test sessions; group assignments were randomized. DOI and 25CN-NBOH were administered 10 min and 5 min prior to the start of the test session, respectively. For dose-response experiments, M100907 and SB-242,084 were administered 10 min prior to the start of the test session. For blockade experiments, M100907 and SB-242,084 were administered 30 min prior to the start of the test session.
2.5.4. Data Analysis
The proportion of responses made on lever B (%B responding) was analyzed by two- or three-way ANOVA with pretreatment and/or treatment as between-subject factors and stimulus duration as a repeated measure. Post-hoc analyses were carried out using Dunnett’s test or Tukey’s test. The alpha level was set at 0.05.
A 2-parameter logistic function was used to fit the data: %B = 1/(1 + exp−ε(t−T50)), where ε is the slope of the function and t is the stimulus duration. The difference limen was calculated as (T75 − T25)/2, and the Weber Fraction was calculated as: (T75 − T25)/(2 × T50). The Weber fraction, difference limen, slope (ε), T50, goodness of fit (r2), and the number of trials completed were analyzed by one- or two-way ANOVA with pretreatment and/or treatment as between-subject factors. Post-hoc analyses were carried out using Dunnett’s test or Tukey’s test. Subjects were excluded from analysis if they failed to complete at least 100 trials during an experiment or if the logistic function failed to fit their %B responding data.
3. RESULTS
3.1. Acquisition and baseline performance of the discrete-trials interval-timing task
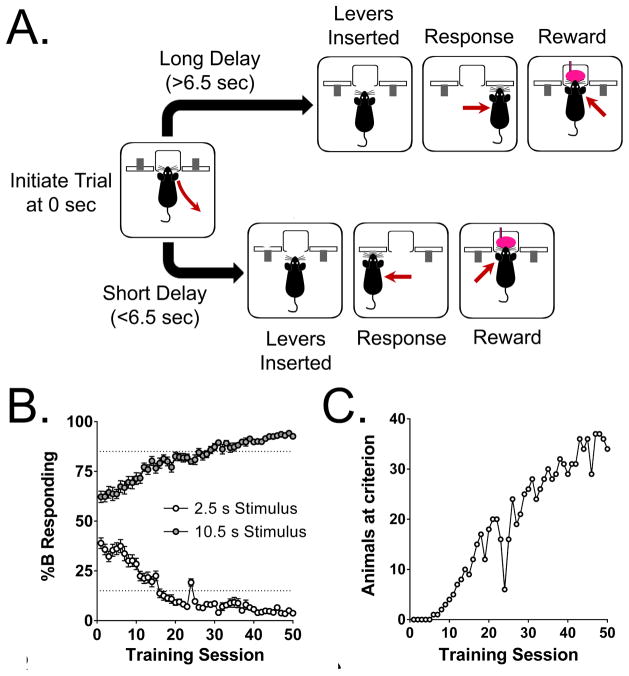
C57BL/6J mice rapidly learned to perform the DTT (Fig. 1A). Figure 1B shows the proportional choice of lever B (%B responding) induced by the two extreme stimulus durations (2.5 and 10.5 s) during the first 50 training sessions, and Fig. 1C shows the number of animals responding at criterion. The mice performed slightly better than chance during the first training session because of the use of forced choice trials, which were included so that a bias did not develop for either lever. The cohort of mice (n=40) began to discriminate the 2.5 s stimulus (≥85% correct responding) on the 16th training session and the 10.5 s stimulus on the 29th training session. For individual mice, the average number of sessions required to reach criterion was 16.6±1.1 (mean±SEM), with a range of 6–42. Figure 2 shows %B responding during a vehicle challenge in 38 mice that had reached criterion and were stably performing the task. As expected, the proportion of responses on lever B increased progressively with the stimulus duration. A two-parameter logistic function was used to analyze %B responding. The value of T50 (6.47±0.12 s) was almost exactly the same as the criterion of the task (6.5 s, the arithmetic mean of the range of durations). The two-parameter logistic function provided a highly accurate fit for the data (r2 = 0.927±0.010). The Weber fraction for the saline challenge was calculated as 0.218±0.017, and the difference limen was 1.36±0.07 s.
Figure 1.
(A) Format of the discrete-trials task. (B) Change in proportional choice of lever B (%B responding) for the two extreme stimulus durations (2.5 and 10.5 s) over the first 50 training sessions. The dotted lines show criterion (≥85% correct responding). Data shown are group means±SEM, (C) Number of animals responding at criterion over the first 50 training sessions.
Figure 2.
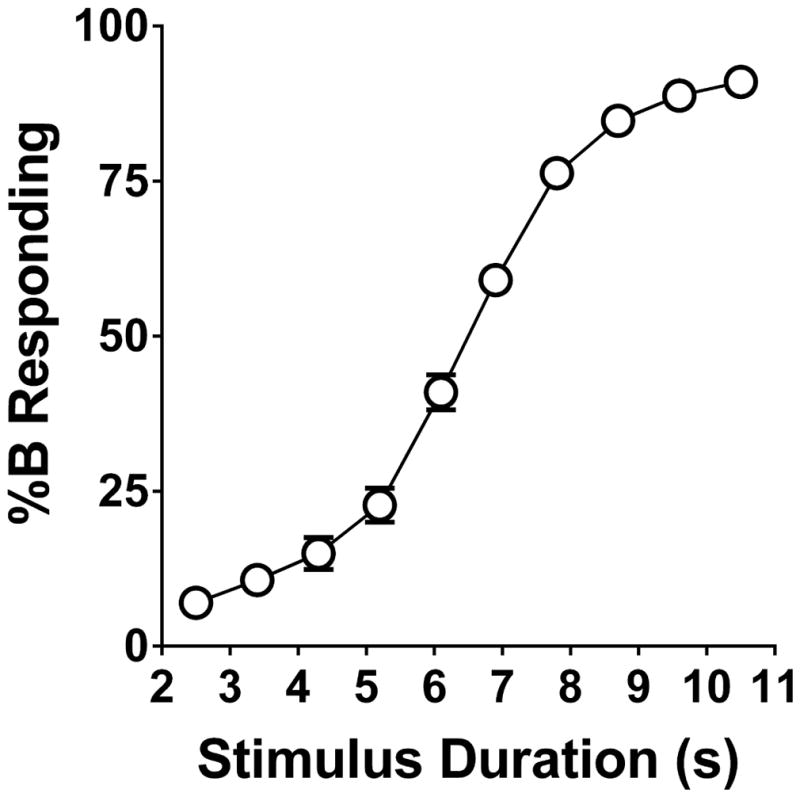
Proportional choice of lever B (%B responding) in 38 mice injected with saline. Data shown are group means±SEM,
3.2. Effect of M100,907
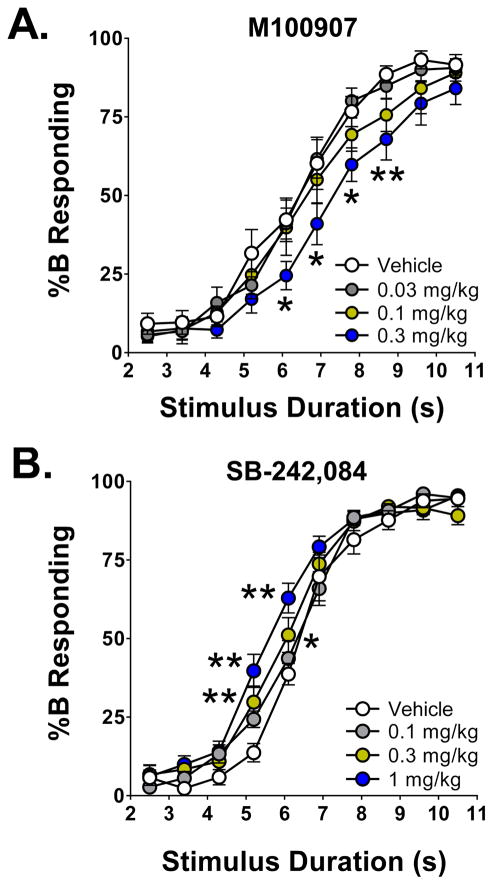
We examined the effect of M100907 on timing in the DTT. M100907 was tested at doses known to be highly effective at blocking 5-HT2A receptors in C57BL/6J mice (Halberstadt and Geyer, 2014). There was a main effect of stimulus duration on %B responding (F(9,297)=258.7, p<0.0001); all subsequent experiments showed a similar effect (data not shown). There was also a main effect of M100907 on %B responding (F(3,33)=2.95, p<0.05). The highest dose of M100907 (0.3 mg/kg) shifted the psychometric response curve to the right (see Fig. 3A), resulting in a significant reduction of %B responding 6.1, 6.9, 7.8, and 8.7 s after trial onset (p<0.01, 0.05, Dunnett’s test), and increasing T50 from 6.35±0.32 s to 7.71±0.45 s (p<0.05, Dunnett’s test). By contrast, as shown in Table 1, M100907 had no effect on the Weber fraction, difference limen, or the slope parameter (ε). The highest dose of M100907 reduced the number of trials completed from 167.2±3.6 to 141.3±8.9 (p<0.05, Dunnett’s test), but the number of trials completed was sufficient to yield an accurate assessment of timing.
Figure 3.
Effects of M100907 (A) and SB-242,084 (B) on temporal discrimination. The data shown (group means±SEM) are the proportional choice of lever B (%B responding) as a function of stimulus duration. *Significant difference from the control group, p<0.05. **Significant difference from the control group, p<0.01.
Table 1.
Effect of M100907 on the discrete-trials task.
| Vehicle | 0.03 mg/kg | 0.1 mg/kg | 0.3 mg/kg | ANOVA | |
|---|---|---|---|---|---|
| (n = 10) | (n = 9) | (n = 8) | (n = 10) | ||
| Weber Fraction | 0.197±0.021 | 0.214±0.051 | 0.220±0.021 | 0.206±0.021 | F3,33=0.10, NS |
| Difference Limen (s) | 1.21±0.10 | 1.31±0.25 | 1.48±0.15 | 1.60±0.20 | F3,33=0.96, NS |
| Slope (ε) | 0.961±0.070 | 0.997±0.114 | 0.791±0.070 | 0.857±0.184 | F3,33=0.55, NS |
| T50 (s) | 6.35±0.32 | 6.42±0.23 | 6.80±0.37 | 7.71±0.45* | F3,33=3.21, p<0.04 |
| r2 | 0.935±0.011 | 0.939±0.019 | 0.895±0.039 | 0.912±0.025 | F3,33=0.68, NS |
| Trials completed | 167.2±3.6 | 163.6±6.5 | 155.4±7.4 | 141.3±8.9* | F3,33=2.98, p<0.05 |
Significantly different from vehicle control, p<0.05 (Dunnett’s test).
3.3. Effect of SB-242,084
There was a main effect of treatment with SB-242,084 on %B responding (F(3,34)=3.99, p<0.02) and an interaction between treatment and stimulus duration (F(27,306)=2.42, p=0.0002). The two highest doses of SB-242,084 (0.3 and 1 mg/kg) shifted the psychometric response curve to the left, resulting in a significant increase in %B responding 5.2 and 6.1 s after trial onset (p<0.05, p<0.01, Tukey’s test; Fig. 3B). Administration of 1 mg/kg SB-242,084 also significantly reduced T50, from 6.51±0.17 s to 5.66±0.17 s (p<0.01, Tukey’s test). SB-242,084 had no effect on the Weber fraction, difference limen, ε, or the number of trials completed (Table 2).
Table 2.
Effect of SB-242,084 on the discrete-trials task.
| Vehicle | 0.1 mg/kg | 0.3 mg/kg | 1 mg/kg | ANOVA | |
|---|---|---|---|---|---|
| (n = 9) | (n = 9) | (n = 10) | (n = 10) | ||
| Weber Fraction | 0.146±0.025 | 0.156±0.016 | 0.173±0.015 | 0.191±0.018 | F3,34=1.09, NS |
| Difference Limen (s) | 0.90±0.12 | 0.98±0.10 | 1.05±0.08 | 1.07±0.09 | F3,34=0.64, NS |
| Slope (ε) | 1.495±0.264 | 1.202±0.110 | 1.094±0.084 | 1.105±0.108 | F3,34=1.35, NS |
| T50 (s) | 6.51±0.17 | 6.28±0.14 | 6.02±0.14 | 5.66±0.17** | F3,34=5.67, p<0.003 |
| r2 | 0.957±0.008 | 0.978±0.003 | 0.945±0.032 | 0.950±0.013 | F3,34=2.28, NS |
| Trials completed | 183.6±3.7 | 191.4±1.6 | 189.9±2.5 | 188.0±2.8 | F3,34=1.48, NS |
Significant difference compared to vehicle control, p<0.01 (Tukey’s test).
3.4. Effect of DOI
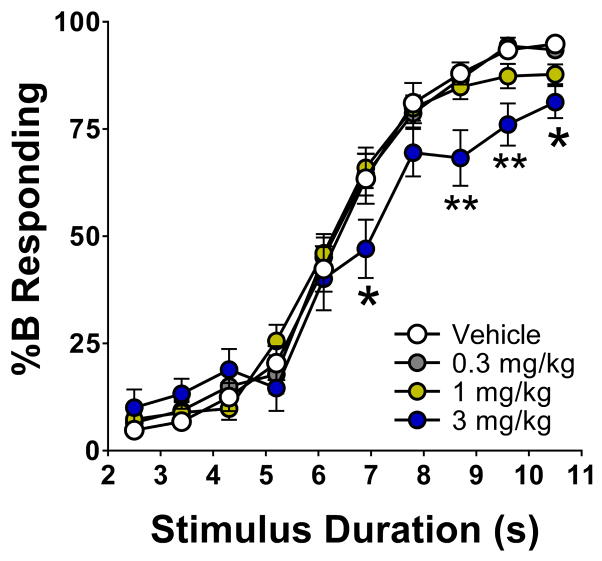
The effect of DOI on %B lever responding is shown in Fig. 4. There was a significant interaction between drug treatment and stimulus duration (F(27,315)=2.31, p=0.0003). DOI produced a flattening of the response curve, especially at longer stimulus intervals, and shifted the curve to the right. The highest dose of DOI (3 mg/kg) produced significant reductions in %B responding 6.1, 7.8, 8.7, and 10.5 s after trial onset (p<0.01, 0.05, Dunnett’s test).
Figure 4.
Effect of DOI on temporal discrimination. The data shown (group means±SEM) are the proportional choice of lever B (%B responding) as a function of stimulus duration. *Significant difference from the control group, p<0.05. **Significant difference from the control group, p<0.01.
DOI significantly increased the Weber fraction and the difference limen and reduced the slope of the curve (ε), indicating treatment with DOI reduced the precision of timing (Table 3). Although DOI tended to shift the curve to the right and 3 mg/kg DOI increased T50 from 6.43±0.18 s to 7.08±0.41s, the effect on T50 did not achieve statistical significance (Main effect: F(3,35)=1.86, NS). Administration of 3 mg/kg DOI reduced the number of trials completed during the test session from 183.2±4.3 to 137.4±6.8 (p<0.01, Dunnett’s test).
Table 3.
Effect of DOI on the discrete-trials task.
| Vehicle | 0.3 mg/kg | 1 mg/kg | 3 mg/kg | ANOVA | |
|---|---|---|---|---|---|
| (n = 10) | (n = 10) | (n = 10) | (n = 9) | ||
| Weber fraction | 0.166±0.015 | 0.192±0.022 | 0.200±0.021 | 0.275±0.030** | F3,35=4.12, p<0.02 |
| Difference limen (s) | 1.06±0.10 | 1.20±0.12 | 1.29±0.14 | 1.89±0.14** | F3,35=8.51, p=0.0002 |
| Slope (ε) | 1.109±0.096 | 1.009±0.108 | 0.968±0.133 | 0.608±0.044** | F3,35=4.34, p<0.02 |
| T50 (s) | 6.43±0.18 | 6.36±0.17 | 6.43±0.13 | 7.08±0.41 | F3,35=1.86, NS |
| r2 | 0.966±0.008 | 0.956±0.009 | 0.943±0.012 | 0.851±0.023** | F3,35=14.72, p<0.0001 |
| Trials completed | 182.3±4.3 | 188.3±2.7 | 174.6±5.4 | 137.4±6.8** | F3,35=20.66, p<0.0001 |
Significant difference versus vehicle control, p<0.01 (Dunnett’s test).
3.5. Interaction between DOI and M100907
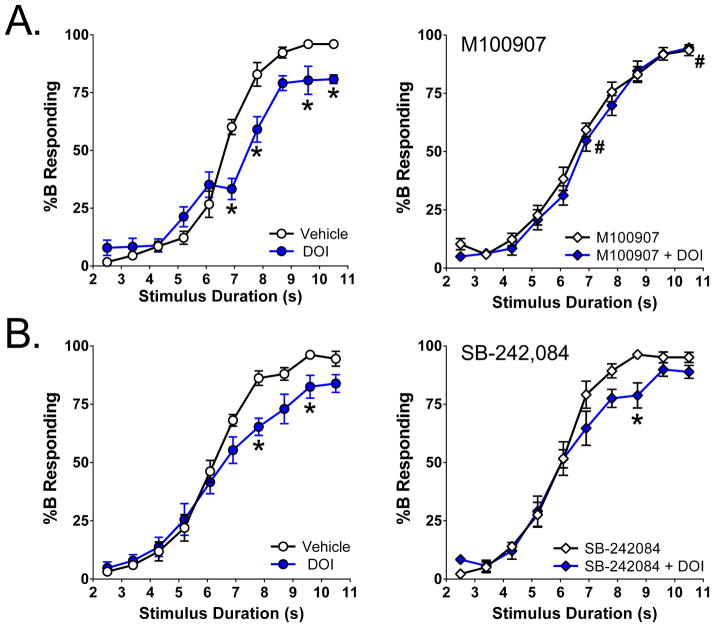
%B responding is illustrated in Figure 5A. There was a three-way interaction between M100907, DOI, and stimulus interval (F(9,279)=4.08, p<0.002), as well as a main effect of DOI (F(1,31)=10.13, p<0.004) and an interaction between DOI and stimulus interval (F(9,279)=3.93, p=0.0001). Specific comparisons revealed that 3 mg/kg DOI reduced %B responding during the 6.9, 7.8, 9.6, and 10.5 s trials (p<0.01, Tukey’s test). Pretreatment with 0.03 mg/kg M100907 blocked the effect of DOI on %B responding to the 6.9 and 10.5 s stimuli (p<0.01, Tukey’s test). There was not a significant main effect of M100907 on %B responding (F(1,31)=2.94, NS), nor was there a significant interaction between M100907 and stimulus duration (F(9,279)=1.22, NS).
Figure 5.
Effect of pretreatment with 0.03 mg/kg M100907 (A) or 0.1 mg/kg SB-242,084 (B) on the response to 3 mg/kg DOI on temporal discrimination. Vehicle pretreated mice are shown in the left panel, and antagonist pretreated mice are shown in the right panel. The data shown (group means±SEM) are the proportional choice of lever B (%B responding) as a function of stimulus duration. *Significant difference from the control group, p<0.05. #Significant difference from DOI alone, p<0.05.
As was observed in the DOI dose-response experiment (see above), treatment with 3 mg/kg DOI significantly increased the Weber fraction and the difference limen, and reduced the slope of the psychometric curve (ε), effects consistent with a reduction of the precision of timing (Table 4). DOI did not significantly alter the Weber fraction, difference limen, or ε in animals pretreated with M100907, and analysis of variance showed significant pretreatment × treatment interactions for all of those measures, but the differences between the DOI and DOI plus M100907 groups were not significantly different. However, detection of pairwise differences was confounded by effects of M100907 on baseline timing performance. For example, pretreatment with M100907 significantly increased the Weber fraction from 0.131±0.015 to 0.202±0.025 (p<0.05, Tukey’s test) and significantly reduced ε from 1.434±0.166 to 0.930±0.107 (p<0.05, Tukey’s test).
Table 4.
Effect of M100907 on the response to DOI.
| Treatmenta | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| Vehicle | DOI | M100907 | DOI + M100907 | M100907 | DOI | M100907 x DOI | |
| (n = 10) | (n = 6) | (n = 9) | (n = 10) | ||||
| Weber fraction | 0.131±0.015 | 0.233±0.018** | 0.202±0.025* | 0.177±0.017 | F1,31=0.14, NS | F1,31=3.92, p=0.0566 | F1,31=10.53, p<0.003 |
| Difference limen (s) | 0.86±0.09 | 1.71±0.14** | 1.34±0.18 | 1.21±0.12 | F1,31=0.00, NS | F1,31=6.96, p<0.02 | F1,31=12.56, p<0.002 |
| Slope (ε) | 1.434±0.166 | 0.661±0.044** | 0.930±0.107* | 0.987±0.091* | F1,31=0.50, NS | F1,31=8.08, p<0.008 | F1,31=10.83, p<0.003 |
| T50 (s) | 6.56±0.13 | 7.35±0.20* | 6.60±0.14 | 6.83±0.18 | F1,31=2.19, NS | F1,31=9.67, p=0.004 | F1,31=2.92, p=0.0975 |
| r2 | 0.977±0.007 | 0.901±0.026** | 0.962±0.008 | 0.964±0.006## | F1,31=4.92, p<0.04 | F1,31=11.46, p<0.002 | F1,31=12.71, p<0.002 |
| Trials completed | 180.6±4.3 | 148.3±8.8** | 172.6±4.6 | 170.2±6.1 | F1,31=1.40, NS | F1,31=8.76, p<0.006 | F1,31=6.54, p<0.02 |
Significant difference versus vehicle control, p<0.05 (Tukey’s test).
Significant difference versus vehicle control, p<0.01 (Tukey’s test).
Significant difference versus DOI alone, p<0.01 (Tukey’s test).
M100907 was administered at 0.03 mg/kg; DOI was administered at 3 mg/kg.
As shown in Table 4, DOI alone produced a rightward shift of the psychometric timing curve and significantly increased T50 from 6.56±0.13 s to 7.35±0.20 s (p<0.01, Tukey’s test). There was a trend toward an M100907 × DOI interaction for T50 (F(1,31)=2.92, p=0.0975), but M100907 failed to completely block the T50 increase produced by DOI.
DOI significantly reduced the number of trials completed (F(1,31)=8.76, p<0.006). Conversely, DOI had no effect on the number of trials completed when administered to mice that had been pretreated with M100907 (Pretreatment × Treatment interaction: F(1,31)=6.54, p<0.02).
3.6. Interaction between DOI and SB-242,084
In contrast to the effect of M100907, pretreatment with 0.1 mg/kg SB-242,084 did not block the ability of DOI to alter %B responding (see Fig. 5B). As expected, DOI significantly reduced %B responding at long stimulus intervals (Main effect of DOI: F(1,34)=5.36, p<0.03; DOI x Stimulus interval: F(9,306)=5.42, p<0.0001), but there was no specific interaction between SB-242,084 and DOI (SB-242,084 × DOI: F(1,34)=0.15, NS; SB-242,084 × DOI × Stimulus interval: F(9,306)=0.49, NS).
There were also no specific interactions between SB-242,084 and DOI with regard to measures of timing precision, curve slope, or clock speed (see Table 5). Although there was a significant difference between the difference limen in animals treated with DOI alone (1.74±0.16 s) compared with animals given SB-242,084 plus DOI (1.24±0.16 s; p<0.05, Tukey’s test), this difference likely occurred due to an effect of pretreatment with SB-242,084 (Main effect of SB-242,084: F(1,34)=8.49, p<0.007).
Table 5.
Effect of SB-242,084 on the response to DOI.
| Treatmenta | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| Vehicle | DOI | SB-242084 | DOI + SB-242,084 | SB-242,084 | DOI | SB-242,084 x DOI | |
| (n = 10) | (n = 10) | (n = 10) | (n = 8) | ||||
| Weber fraction | 0.160±0.017 | 0.255±0.026** | 0.131±0.006 | 0.195±0.023 | F1,34=5.30, p<0.03 | F1,34=16.94, p=0.0002 | F1,34=0.66, NS |
| Difference limen (s) | 0.98±0.08 | 1.74±0.16** | 0.78±0.03 | 1.24±0.16# | F1,34=8.49, p<0.007 | F1,34=26.49, p<0.0001 | F1,34=1.52, NS |
| Slope (ε) | 1.187±0.089 | 0.713±0.101** | 1.442±0.069 | 0.996±0.128* | F1,34=7.80, p<0.009 | F1,34=22.75, p<0.0001 | F1,34=0.03, NS |
| T50 (s) | 6.22±0.17 | 6.89±0.31 | 5.98±0.20 | 6.35±0.28 | F1,34=2.52, NS | F1,34=4.50, p<0.05 | F1,34=0.37, NS |
| r2 | 0.961±0.012 | 0.905±0.020* | 0.972±0.006 | 0.940±0.011 | F1,34=2.92, NS | F1,34=10.68, p<0.003 | F1,34=0.77, NS |
| Trials completed | 186.6±4.2 | 142.5±8.8** | 187.8±2.7 | 174.9±7.6## | F1,34=7.22, p<0.02 | F1,34=20.81, p=0.02 | F1,34=6.22, p<0.0001 |
Significant difference compared to vehicle control, p<0.05 (Tukey’s test).
Significant difference compared to vehicle control, p<0.01 (Tukey’s test).
Significant difference compared to DOI alone, p<0.05 (Tukey’s test).
Significant difference compared to DOI alone, p<0.01 (Tukey’s test).
SB-242,084 was administered at 0.1 mg/kg; DOI was administered at 3 mg/kg.
Interestingly, with regard to the number of trials completed, there was a significant SB-242084 × DOI interaction (F(1,34)=6.22, p<0.0001). Pretreatment with SB-242,084 blocked the ability of DOI to reduce the number of trials completed (p<0.01, Tukey’s test; Table 5).
3.7. Effect of 25CN-NBOH
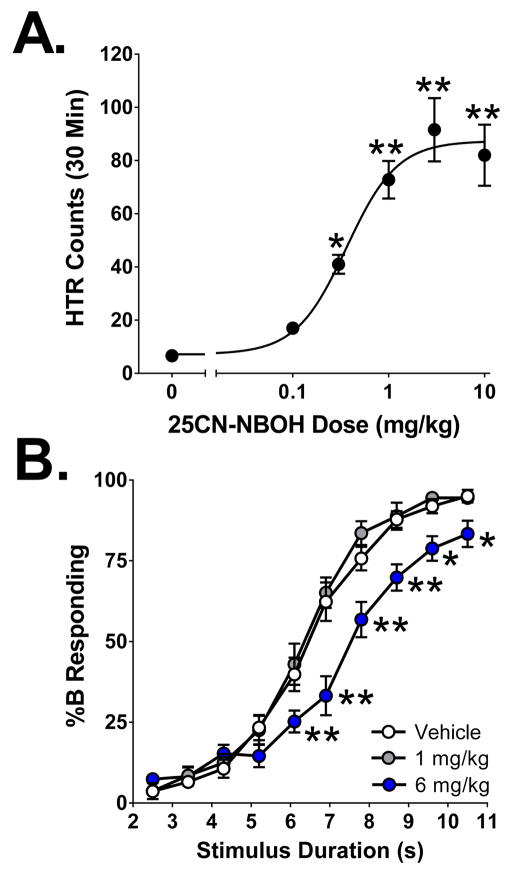
To further assess whether 5-HT2A activation can alter interval timing, we tested 25CN-NBOH, a 5-HT2A agonist (Ki = 1.3 nM) purported to be 100-fold selective versus 5-HT2C (Ki = 132 nM) (Hansen et al., 2014). To confirm the selectivity of 25CN-NBOH for 5-HT2A receptors, we submitted it to the NIMH PDSP for a comprehensive binding screen at 44 neurotransmitter binding sites and transporters. 25CN-NBOH binds to the human 5-HT2A receptor expressed in recombinant cell membranes with high affinity (Ki = 2.2 ± 0.5 nM), binding to 5-HT2B and 5-HT2C receptors with 26- and 23-fold lower affinity, respectively (see Table 6). The comprehensive binding screen confirmed that 25CN-NBOH is highly selective for 5-HT2 sites (Table S1). 25CN-NBOH exhibits moderate affinity for sigma-1 (Ki = 284 nM), α2C (Ki = 543 nM), 5-HT6 (Ki = 573 nM), sigma-2 (Ki = 575 nM), α2A (Ki = 803 nM), α2B (Ki = 1,226 nM), histaminergic H2 (Ki = 1,505 nM), and β2 (Ki = 1,609 nM) receptors, and low affinity for other sites. 25CN-NBOH was previously reported to induce the head twitch response (HTR) in NIH Swiss mice, an effect that was mediated by the 5-HT2A receptor (Fantegrossi et al., 2014). As shown in Figure 6A, 25CN-NBOH induces the HTR in C57BL/6J mice with an ED50 = 0.36 mg/kg (95% CI = 0.20–0.65) and produces a maximal response at 3 mg/kg (Drug effect: F(5,22)=18.85, p<0.0001). These findings confirm that 25CN-NBOH activates the 5-HT2A receptor in vivo.
Table 6.
Affinity of human 5-HT2A, 5-HT2B, and 5-HT2C receptors for 25CN-NBOH.
| 5-HT2A Ki (nM) [3H]ketanserin |
5-HT2B Ki (nM) [3H]LSD |
5-HT2C Ki (nM) [3H]mesulergine |
|
|---|---|---|---|
| 25CN-NBOH | 2.2 ± 0.5 | 58.0 ± 8.2 | 49.8 ± 7.4 |
Values are the mean±SEM of 3–4 independent determinations. Radioligand concentrations were 0.5 nM, 1 nM, and 0.5 nM, respectively.
We tested 25CN-NBOH in mice trained to perform the DTT at doses ranging from 0.3–3 mg/kg. At those doses, however, 25CN-NBOH did not significantly alter %B responding (Drug effect: F(3,36)=1.43, NS) or specific measures of timing (see Table S2). Nevertheless, 3 mg/kg 25CN-NBOH tended to increase T50 and the difference limen, and reduced the slope of the curve, suggesting the doses used may have been subthreshold.
A follow-up experiment confirmed that interval timing is significantly altered by a slightly higher dose of 25CN-NBOH (Fig. 6B). Similar to DOI, 6 mg/kg 25CN-NBOH reduced %B responding at long stimulus intervals (Drug effect: F(2,29)=10.37, p=0.0004; Drug x Stimulus interval: F(18,261)=4.41, p<0.0001). Administration of 6 mg/kg 25CN-NBOH also significantly increased T50 from 6.52±0.18 s to 7.63±0.24 s (p<0.01, Dunnett’s test; Table 7), increased the difference limen from 1.12±0.11 s to 1.65±0.12 s (p<0.01, Dunnett’s test), and reduced the slope of the curve from 1.088±0.109 to 0.704±0.050 (p<0.05, Dunnett’s test). There was also a trend toward a main effect of 25CN-NBOH on the Weber fraction (F(2,29)=2.62, p=0.0897) but the increase was not significant. Similar to DOI, the highest dose of 25CN-NBOH reduced the total number of trials completed (p<0.01, Dunnett’s test).
Figure 6.
(A) Effect of 25CN-NBOH on the head twitch response (HTR). Mice were treated with 25CN-NBOH and HTR was assessed for 30 minutes. Data shown are group means±SEM for the entire 30 min session. (B) Effect of 25CN-NBOH on temporal discrimination. The data shown (group means±SEM) are the proportional choice of lever B (%B responding) as a function of stimulus duration. *Significant difference from the control group, p<0.05. **Significant difference from the control group, p<0.01.
Table 7.
Effect of 25CN-NBOH on the discrete-trials task.
| Vehicle | 1 mg/kg | 6 mg/kg | ANOVA | |
|---|---|---|---|---|
| (n = 11) | (n = 9) | (n = 12) | ||
| Weber fraction | 0.171±0.015 | 0.175±0.020 | 0.217±0.015 | F2,29=2.62, p=0.0897 |
| Difference limen (s) | 1.12±0.11 | 1.09±0.11 | 1.65±0.12** | F2,29=7.89, p<0.002 |
| Slope (ε) | 1.088±0.109 | 1.098±0.125 | 0.704±0.050* | F2,29=7.89, p<0.002 |
| T50 (s) | 6.52±0.18 | 6.33±0.20 | 7.63±0.24** | F2,29=11.50, p=0.0002 |
| r2 | 0.965±0.007 | 0.965±0.009 | 0.882±0.031* | F2,29=5.52, p<0.01 |
| Trials completed | 180.2±3.9 | 182.4±3.7 | 134.5±8.4** | F2,29=19.87, p<0.0001 |
Significant difference versus vehicle control, p<0.01 (Tukey’s test).
4. DISCUSSION
We trained mice to perform a discrete-trials temporal discrimination task. Performance on the task matched previous data from rats (Body et al., 2002a; Asgari et al., 2005, 2006; Hampson et al., 2010; Valencia Torres et al., 2011). As was observed in rats, the probability of responding on the “long” lever (lever B) increased with the stimulus duration, yielding an ogival curve. A two-parameter logistic function provided an excellent fit for the data, and accounted for 90395% of the variance. Under control conditions, the indifference point (T50) was located close to the criterion of the task (6.5 s, the arithmetic mean of the range of stimulus durations), consistent with previous discrete-trials studies in rats (Body et al., 2002a; Asgari et al., 2005, 2006; Hampson et al., 2010; Valencia Torres et al., 2011). Temporal discrimination was highly precise, as demonstrated by the range of values of the Weber fraction (0.13–0.22) and difference limen (0.86–1.36 s) in control animals. We also found that temporal discrimination was disrupted by the hallucinogens DOI and 25CN-NBOH, whereas the psychometric response curve was shifted by selective blockade of 5-HT2A and 5-HT2C receptors.
The hallucinogen DOI consistently altered temporal discrimination across multiple experiments, flattening the psychometric function at long stimulus durations, displacing the curve to the right (significantly increasing T50 in 1 of 3 experiments), reducing the slope of the curve, and increasing the Weber fraction and difference limen. DOI produced the same pattern of effects in rats performing the DTT (Asgari et al., 2006; Hampson et al., 2010). According to pacemaker/accumulator models of timing, the value of T50 serves as a measure of central pacemaker speed, whereas the Weber fraction reflects the precision of temporal discrimination (Gibbon, 1977, 1991). Therefore, DOI appears to produce a disruption of temporal discrimination (increased Weber fraction) and slows the internal clock (increased T50).
The effects of DOI on temporal discrimination are likely mediated by the 5-HT2A receptor. DOI has high affinity for 5-HT2 sites but displays limited selectivity for 5-HT2A versus 5-HT2C receptors. Importantly, the effects of DOI in %B responding were completely blocked by pretreatment with the selective 5-HT2A antagonist M100907, but were not affected by pretreatment with the selective 5-HT2C antagonist SB-242,084. M100907 has subnanomolar affinity for 5-HT2A and at least 100-fold lower affinity for 5-HT2C and α1 receptors, with negligible affinity for other sites (Kehne et al., 1996). At doses comparable to those used in the present study, M100907 blocks 5-HT2A-mediated responses in C57BL/6J mice (Halberstadt and Geyer, 2014) and protects 5-HT2A receptors from inactivation with EEDQ (Canal et al., 2010), but does not interact with 5-HT2C or α1 receptors (Patel et al., 2001; da Silva and Sanders-Bush, 2008). By contrast, SB-242084 has over 100-fold selectivity for 5-HT2C receptors compared with 5-HT2A and 5-HT2B receptors (Kennett et al., 1997). Although most of the binding data published for M100907 and SB-242084 have been obtained using human and rat receptors, a recent study confirmed that M100907 is 165-fold selective for 5-HT2A vs. 5-HT2C receptors in mice, whereas SB-242,084 exhibits 960-fold selectivity for 5-HT2C over 5-HT2A in that species (Canal et al., 2013). Similar to the present results, the effect of DOI on the performance of rats in the DTT and the free-operant psychophysical procedure was reportedly blocked by pretreatment with M100907 and the 5-HT2A antagonist ketanserin (Body et al., 2003, 2006a,b; Asgari et al., 2006).
The selective 5-HT2A agonist 25CN-NBOH mimicked the effects of DOI on temporal discrimination, flattening the response curve at long stimulus durations, shifting the curve to the right (leading to a robust increase of T50) and reducing its slope, and reducing the Weber fraction and difference limen. Although 25CN-NBOH is less selective than was reported previously in the literature (Hansen et al., 2014), we confirmed it is 23-fold selective for 5-HT2A receptors vs. 5-HT2C receptors, and has negligible affinity for non-5-HT2 sites. Importantly, 25CN-NBOH is more selective than DOI, which is ~5-fold selective for 5-HT2A in humans (Almaula et al., 1996; Pigott et al., 2012; Canal et al., 2013) and 12-fold selective in mice (Canal et al., 2013). Given the results obtained with 25CN-NBOH, it is reasonable to conclude that DOI disrupts temporal discrimination by activating 5-HT2A but not 5-HT2C receptors.
Although DOI and 25CN-NBOH appear to disrupt timing, alternative explanations for the data are also possible. For example, DOI may disrupt non-temporal processes required to perform the DTT, such as attention, motivation, working memory, or decision-making. DOI is a potent hallucinogenic drug, and can produce profound perceptual and cognitive alterations in humans (Shulgin and Shulgin, 1991). High levels of 5-HT2A activation disrupt the spatial tuning of PFC pyramidal neurons during working memory tasks in animal models (Williams et al, 2002), and high doses of DOI reportedly impair attention and vigilance in operant tasks (Nakamura and Kurasawa, 2000). As noted by Asgari et al. (2006), the effects of DOI in the DTT could be caused by a breakdown of stimulus control. In other words, DOI may produce a global disruption of discriminative performance, rather than having a specific effect on temporal processing. Indeed, temporal stimulus control may be weaker at longer intervals, which could potentially explain why DOI and 25CN-NBOH preferentially alter %B responding at long durations. To determine whether DOI is producing non-specific effects on stimulus control, Hampson et al. (2010) trained rats to perform a light-intensity discrimination task that was designed to match the difficulty of the DTT. In that task, rats had to respond on two levers differentially depending on whether the intensity of a light stimulus was <22 cd/m2 or >22 cd/m2. Importantly, doses of DOI that altered timing in the DTT had no effect on performance of the light-intensity discrimination task, indicating DOI does not produce a general disruption of stimulus control in discrimination studies (Hampson et al., 2010).
Another factor indicating that DOI and 25CN-NBOH are not merely disrupting non-temporal processing is based on the strategy mice use to perform the DTT. At the beginning of a trial, rats and pigeons performing temporal discrimination tasks position themselves close to the “short” operandum, but then move to the “long” operandum as the trial progresses. At the end of the trial, the animals perform the temporal discrimination by responding on the closest operandum (Ho et al., 1995; Fetterman et al., 1998; Machado and Keen, 2003). Consistent with the findings in rats and pigeons, we have observed that mice use an identical strategy to perform the DTT (data not shown). These patterns of activity (“adjunctive behaviors”) conform to the Behavioral theory of Timing (BeT) of Killeen and Fetterman (1988), who proposed that a progression of behavioral states can serve the same role as clock pulses in pacemaker-accumulator models of timing. Importantly, although DOI and 25CN-NBOH reduced %B responding at long stimulus intervals, the mice still managed to correctly respond more than 80% of the time during the 10.5 s-trials. Such a high level of performance indicates that DOI and 25CN-NBOH did not disrupt the serial progression of adjunctive behaviors used by the mice to perform the temporal discrimination. It therefore appears unlikely that DOI and 25CN-NBOH disrupted stimulus control in the task.
There is extensive evidence that the prefrontal cortex (PFC) is involved in temporal perception and supra-second timing. A recent theory of timing, known as the Striatal Beat Frequency (SBF) model, posits that the PFC and other cortical areas serve as a source of oscillatory activity that is used by the dorsal striatum to track time (Mattell and Meck 2004; Buhusi and Meck 2005; Meck et al 2008). According to the SBF model, medium spiny neurons (MSNs) in the dorsal striatum receive input from multiple PFC pyramidal neurons that oscillate at different frequencies, and the MSNs respond to specific PFC ensemble firing patterns through a process of coincidence detection. Because pyramidal neurons fire at different frequencies, they produce periodic constructive and destructive interference, and information about duration can be extracted from their relative phase. Given the involvement of the corticostriatal pathway in interval timing, DOI and other 5-HT2A agonists (hallucinogens) may alter timing by disrupting oscillatory network activity in the PFC. 5-HT2A receptors expressed by fast spiking interneurons play a critical role in regulating PFC oscillatory activity (Puig et al., 2010). As shown by local field potential recordings in PFC, DOI reduces low-frequency oscillations in anesthetized rats (Celada et al., 2008) and inhibits gamma oscillatory activity in freely moving rats (Wood et al., 2012). Additionally, after administration of DOI, the firing of individual neurons is decoupled from the rhythmic oscillatory activity of the PFC network. It is anticipated that the effects of DOI on PFC oscillatory would cause profound alterations of timing and temporal perception.
Administration of M100907 and SB-242,084 shifted the psychometric response curve, producing a significant change in the value of T50, but had no effect on the shape of the curve or on measures of temporal precision. M100907 shifted the curve to the right and increased T50, whereas SB-242,084 shifted the curve to the left and reduced T50. Based on those findings, we have concluded that clock speed is slowed by 5-HT2A blockade and increased by blockade of 5-HT2C. It is not surprising that 5-HT2A and 5-HT2C antagonists produce countervailing effects on T50 because those 5-HT2 subtypes are known to act in a functionally antagonistic manner (Schreiber et al., 1995; Vickers et al., 2001; Halberstadt et al., 2009; Fantegrossi et al., 2010).
Similar to the present findings, other groups have reported data showing that M100907 slows the perception of time. One line of evidence is based on the effect of M100907 in differential reinforcement of low-rate (DRL) tasks, where rats must withhold responses for a certain amount of time in order to obtain reinforcement. M100907 shifts the inter-response time distribution to the right and increases the reinforcement rate in rats performing under a DRL 20-s schedule (Anastasio et al., 2011) and a DRL 72-s schedule (Marek et al., 2005), effects consistent with a reduction of clock speed. According to several previous studies, M100907 does not alter temporal differentiation or temporal discrimination in rats (Body et al., 2006a,b; Asgari et al., 2006). However, those data were not based on full dose-response experiments, but were collected in the context of antagonist blockade studies, which would likely have used low, behaviorally inactive doses M100907.
There is a relationship between dopamine (DA) levels and clock speed; SB-242,084 and M100907 may alter T50 by modulating DA release. Dopaminergic projections play an important role in interval timing (Coull et al., 2011) and are hypothesized to control the speed of the central pacemaker (Meck, 1996; Gibbon et al., 1997). The speed of the internal clock increases after administration of indirect DA agonists, including cocaine, (+)-amphetamine, and methamphetamine (Meck, 1983; Cevik, 2003; Matell et al., 2004; Cheng et al., 2006; Cheung et al., 2006; Body et al., 2009; Heilbronner and Meck, 2014), whereas haloperidol and other DA D2 receptor antagonists reduce clock speed (Meck, 1983, 1986; Drew et al., 2003; MacDonald and Meck, 2005). DA release in frontal cortex is tonically inhibited by the 5-HT2C receptor (Milan et al, 1988) and phasically facilitated by the 5-HT2A receptor (Gobert and Milan, 1999). 5-HT2C antagonists, including SB-242084, are known to increase basal DA release (Di Matteo et al., 1999; Pozzi et al., 2002; Blackburn et al., 2006). Although 5-HT2A antagonists do not influence basal DA release, they have been shown to attenuate DA efflux under conditions where release is stimulated (Schmidt et al., 1994; Pehek et al., 2001; Porras et al., 2002). By increasing DA efflux, SB-242084 should increase the speed of the internal clock, producing a proportional increase in perceived time and a reduction of T50. On the other hand, 5-HT2A blockade with M100907 would likely slow the internal clock by reducing task-related DA release, reducing perceived time and increasing T50.
Interestingly, T50 was increased regardless of whether 5-HT2A was blocked (with M100907) or activated (with DOI and 25CN-NBOH). It appears that the 5-HT2A receptor may regulate T50 in a non-monotonic fashion. There is some precedent for this type of effect: 5-HT2A activation modulates working memory with an inverted-U-shaped dose-response function (Williams et al., 2002). Additional studies are required to determine why the 5-HT2A receptor has non-linear effects on T50. Several selective 5-HT2A antagonists are currently under development as antipsychotics and hypnotics. Based on the present findings, it is possible that those agents may be capable of altering temporal perception if administered at doses producing high levels of 5-HT2A receptor blockade.
In conclusion, we have trained mice to perform a discrete-trials temporal discrimination task. Development of the task in mice enables examination of the genetic contributions to interval timing, and facilitates the use of optogenetic challenges. These experiments confirm that temporal discrimination in mice is altered by serotonergic hallucinogens via 5-HT2A receptors, consistent with the involvement of the 5-HT2A receptor in hallucinogenesis. Furthermore, as predicted, 5-HT2A and 5-HT2C receptor antagonists altered pacemaker speed, potentially explaining their effects on premature responding. 5-HT2A receptors are hypothesized to play a role in schizophrenia because they are activated by serotonergic hallucinogens and antagonized by atypical antipsychotics such as clozapine and risperidone (Geyer and Vollenweider, 2008; Quednow et al 2010). Given the evidence that temporal perception is altered in patients with schizophrenia, further studies are warranted to determine whether the 5-HT2A receptor contributes to those deficits and to their potential amelioration by antipsychotic drugs. Importantly, there are close similarities between the design and results of the DTT and the interval bisection task, a retrospective task used to assess timing in humans (Kopec and Brody, 2010). Follow-up studies are planned to assess the effects of serotonergic receptor ligands on timing in humans.
Supplementary Material
Table S1. Receptor binding data for 25CN-NBOH.
Table S2. Effect of low doses of 25CN-NBOH on the discrete-trials task.
Mice were trained to discriminate between short and long stimulus intervals.
Hallucinogens increased the variability of temporal discrimination via 5-HT2A.
T50 was increased 5-HT2A blockade and reduced by 5-HT2C blockade.
Acknowledgments
Supported by NIMH Award K01 MH100644, NIDA Award R01 DA002925, the Brain & Behavior Research Foundation, and the Veterans Affairs VISN 22 MIRECC. Receptor binding data were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program (NIMH PDSP), Contract # HHSN-271-2008-00025-C. The NIMH PDSP is directed by Dr. Bryan Roth at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almaula N, Ebersole BJ, Ballesteros JA, Weinstein H, Sealfon SC. Contribution of a helix 5 locus to selectivity of hallucinogenic and nonhallucinogenic ligands for the human 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptors: direct and indirect effects on ligand affinity mediated by the same locus. Mol Pharmacol. 1996;50:34–42. [PubMed] [Google Scholar]
- Anastasio NC, Stoffel EC, Fox RG, Bubar MJ, Rice KC, Moeller FG, Cunningham KA. Serotonin (5-hydroxytryptamine) 5-HT2A receptor: association with inherent and cocaine-evoked behavioral disinhibition in rats. Behav Pharmacol. 2011;22:248–261. doi: 10.1097/FBP.0b013e328345f90d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari K, Body S, Bak VK, Zhang ZQ, Rickard JF, Glennon JC, Fone KC, Bradshaw CM, Szabadi E. Effects of 5-HT2A receptor stimulation on the discrimination of durations by rats. Behav Pharmacol. 2006;17:51–59. doi: 10.1097/01.fbp.0000189810.69425.89. [DOI] [PubMed] [Google Scholar]
- Asgari K, Body S, Rickard JF, Zhang Z, Fone KC, Bradshaw CM, Szabadi E. Effects of quipazine and m-chlorophenylbiguanide (m-CPBG) on the discrimination of durations: evidence for the involvement of 5-HT2A but not 5-HT3 receptors. Behav Pharmacol. 2005;16:43–51. doi: 10.1097/00008877-200502000-00005. [DOI] [PubMed] [Google Scholar]
- Beringer K. Seine Geschiehte und Erseheinungsweise. Berlin: Springer; 1927. Der Meskalinrausch. [Google Scholar]
- Blackburn TP, Suzuki K, Ashby CR., Jr The acute and chronic administration of the 5-HT(2B/2C) receptor antagonist SB-200646A significantly alters the activity of spontaneously active midbrain dopamine neurons in the rat: An in vivo extracellular single cell study. Synapse. 2006;59:502–512. doi: 10.1002/syn.20263. [DOI] [PubMed] [Google Scholar]
- Body S, Asgari K, Cheung TH, Bezzina G, Fone KF, Glennon JC, Bradshaw CM, Szabadi E. Evidence that the effect of 5-HT2 receptor stimulation on temporal differentiation is not mediated by receptors in the dorsal striatum. Behav Processes. 2006a;71:258–267. doi: 10.1016/j.beproc.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Body S, Asgari K, Richard JF, Zhang Z, Fone KF, Bradshaw CM, Szabadi E. Effects of quipazine and m-chlorophenylbiganuide (m-CPBG) on temporal differentiation: evidence of the involvement of 5-HT2A but not 5-HT3 receptors in interval timing behaviour. Psychopharmacology (Berl) 2005;181:289–298. doi: 10.1007/s00213-005-2233-3. [DOI] [PubMed] [Google Scholar]
- Body S, Cheung TH, Bezzina G, Asgari K, Fone KC, Glennon JC, Bradshaw CM, Szabadi E. Effects of d-amphetamine and DOI (2,5-dimethoxy-4-iodoamphetamine) on timing behavior: interaction between D1 and 5-HT2A receptors. Psychopharmacology (Berl) 2006b;189:331–343. doi: 10.1007/s00213-006-0575-0. [DOI] [PubMed] [Google Scholar]
- Body S, Cheung TH, Hampson CL, den Boon FS, Bezzina G, Fone KC, Bradshaw CM, Szabadi E. Attenuation of the effects of d-amphetamine on interval timing behavior by central 5-hydroxytryptamine depletion. Psychopharmacology (Berl) 2009;203:547–559. doi: 10.1007/s00213-008-1400-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Body S, Chiang TJ, Mobini S, Ho MY, Bradshaw CM, Szabadi E. Effect of 8-OH-DPAT on temporal discrimination following central 5-hydroxytryptamine depletion. Pharmacol Biochem Behav. 2002a;71:787–793. doi: 10.1016/s0091-3057(01)00674-8. [DOI] [PubMed] [Google Scholar]
- Body S, Kheramin S, Ho MY, Miranda F, Bradshaw CM, Szabadi E. Effects of a 5-HT2 receptor agonist, DOI (2,5-dimethoxy-4-iodoamphetamine), and antagonist, ketanserin, on the performance of rats on a free-operant timing schedule. Behav Pharmacol. 2003;14:599–607. doi: 10.1097/00008877-200312000-00004. [DOI] [PubMed] [Google Scholar]
- Body S, Kheramin S, Ho MY, Miranda Herrera F, Bradshaw CM, Szabadi E. Effects of fenfluramine on free-operant timing behaviour: evidence for involvement of 5-HT2A receptors. Psychopharmacology (Berl) 2004;176:154–165. doi: 10.1007/s00213-004-1871-1. [DOI] [PubMed] [Google Scholar]
- Body S, Kheramin S, Mobini S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E. Antagonism by WAY-100635 of the effects of 8-OH-DPAT on performance on a free-operant timing schedule in intact and 5-HT-depleted rats. Behav Pharmacol. 2002b;13:603–614. doi: 10.1097/00008877-200212000-00001. [DOI] [PubMed] [Google Scholar]
- Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6:755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- Canal CE, Booth RG, Morgan D. Support for 5-HT2C receptor functional selectivity in vivo using structurally diverse, selective 5-HT2C receptor ligands and the 2,5-dimethoxy-4-iodoamphetamine elicited head twitch response model. Neuropharmacology. 2013;70:112–121. doi: 10.1016/j.neuropharm.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canal CE, Olaghere da Silva UB, Gresch PJ, Watt EE, Sanders-Bush E, Airey DC. The serotonin 2C receptor potently modulates the head-twitch response in mice induced by a phenethylamine hallucinogen. Psychopharmacology (Berl) 2010;209:163–174. doi: 10.1007/s00213-010-1784-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroll CA, Boggs J, O’Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia span from millisecond to several-second durations. Brain Cogn. 2009a;70:181–190. doi: 10.1016/j.bandc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Caroll CA, O’Donnell BF, Shekhar A, Hetrick WP. Timing dysfunctions in schizophrenia as measured by a repetitive finger tapping task. Brain Cogn. 2009b;71:345–353. doi: 10.1016/j.bandc.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Díaz-Mataix L, Artigas F. The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biol Psychiatry. 2008;64:392–400. doi: 10.1016/j.biopsych.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Cevik MO. Effects of methamphetamine on duration discrimination. Behav Neurosci. 2003;117:774–784. doi: 10.1037/0735-7044.117.4.774. [DOI] [PubMed] [Google Scholar]
- Cheng RK, MacDonald CJ, Meck WH. Differential effects of cocaine and ketamine on time estimation: implications for neurobiological models of interval timing. Pharmacol Biochem Behav. 2006;85:114–122. doi: 10.1016/j.pbb.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Cheung TH, Bezzina G, Asgari K, Body S, Fone KC, Bradshaw CM, Szabadi E. Evidence for a role of D1 dopamine receptors in d-amphetamine’s effect on timing behaviour in the free-operant psychophysical procedure. Psychopharmacology (Berl) 2006;185:378–388. doi: 10.1007/s00213-006-0339-x. [DOI] [PubMed] [Google Scholar]
- Cheung TH, Bezzina G, Body S, Fone KC, Bradshaw CM, Szabadi E. Tolerance to the effect of 2,5-dimethoxy-4-iodoamphetamine (DOI) on free-operant timing behaviour: interaction between behavioural and pharmacological mechanisms. Psychopharmacology (Berl) 2007;192:521–535. doi: 10.1007/s00213-007-0743-x. [DOI] [PubMed] [Google Scholar]
- Chiang TJ, Al-Ruwaitea AS, Mobini S, Ho MY, Bradshaw CM, Szabadi E. Effects of 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) on performance on two operant timing schedules. Psychopharmacology (Berl) 2000;151:379–391. doi: 10.1007/s002130000495. [DOI] [PubMed] [Google Scholar]
- Ciullo V, Spalletta G, Caltagirone C, Jorge RE, Piras F. Explicit Time Deficit in Schizophrenia: Systematic Review and Meta-Analysis Indicate it Is Primary and Not Domain Specific. Schizophr Bull. 2015 doi: 10.1093/schbul/sbv104. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantindis C, Williams GV, Goldman-Rakic PS. A role for inhibition in shaping the temporal flow of information in prefrontal cortex. Nature Neurosci. 2002;5:175–180. doi: 10.1038/nn799. [DOI] [PubMed] [Google Scholar]
- Coull JT, Cheng RK, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Vidal F, Nazarian B, Macar F. Functional anatomy of the attentional modulation of time estimation. Science. 2004;303:1506–1508. doi: 10.1126/science.1091573. [DOI] [PubMed] [Google Scholar]
- Da Silva UO, Sanders-Bush E. Specificity of 5-HT2A receptor ligands in mice. FASAB J. 2008;22:1125.3. [Google Scholar]
- Davalos DB, Rojas DC, Tregellas JR. Temporal processing in schizophrenia: effects of task-difficulty on behavioral discrimination and neuronal responses. Schizophrenia Res. 2011;127:123–130. doi: 10.1016/j.schres.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeShon HJ, Rinkel M, Solomon HC. Mental changes induced experimentally by L. S. D. (d-lysergic acid diethylamide tartrate) Psychiatr Q. 1952;26:33–53. doi: 10.1007/BF01568448. [DOI] [PubMed] [Google Scholar]
- Di Matteo V, Di Giovanni G, Di Mascio M, Esposito E. SB 242084, a selective serotonin2C receptor antagonist, increases dopaminergic transmission in the mesolimbic system. Neuropharmacology. 1999;38:1195–1205. doi: 10.1016/s0028-3908(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Drew MR, Fairhurst S, Malapani C, Horvitz JC, Balsam PD. Effects of dopamine antagonists on the timing of two intervals. Pharmacol Biochem Behav. 2003;75:9–15. doi: 10.1016/s0091-3057(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT2A and 5-HT2C receptors. Synapse. 2000;35:144–150. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Elvevåg B, McCormack T, Gilbert A, Brown GD, Weinberger DR, Goldberg TE. Duration judgements in patients with schizophrenia. Psychol Med. 2003;33:1249–1261. doi: 10.1017/s0033291703008122. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Gray BW, Bailey JM, Smith DA, Hansen M, Kristensen JL. Hallucinogen-like effects of 2-([2-(4-cyano-2,5-dimethoxyphenyl) ethylamino]methyl)phenol (25CN-NBOH), a novel N-benzylphenethylamine with 100-fold selectivity for 5-HT2A receptors, in mice. Psychopharmacology (Berl) 2014;232:1039–1047. doi: 10.1007/s00213-014-3739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetterman JG, Killeen PR. Time discrimination in Columba livia and Homo sapiens. J Exp Psychol Animal Behav Proc. 1992;18:80–94. doi: 10.1037//0097-7403.18.1.80. [DOI] [PubMed] [Google Scholar]
- Fetterman JG, Killeen PR, Hall S. Watching the clock. Behavioural Processes. 1998;44:211–222. doi: 10.1016/S0376-6357(98)00050-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492:145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. Serotonin research: Contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Gibbon J. Scalar expectancy theory and Weber’s law in animal timing. Psychological Rev. 1977;84:279–325. [Google Scholar]
- Gibbon J. Origins of scalar timing. Learn Motiv. 1991;22:3–38. [Google Scholar]
- Gibbon J, Malapani C, Dale CL, Gallistel CR. Toward a neurobiology of temporal cognition: advances and challenges. Curr Opin Neurobiol. 1997;7:170–184. doi: 10.1016/s0959-4388(97)80005-0. [DOI] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Gresch PJ, Barrett RJ, Sanders-Bush E, Smith RL. 5-Hydroxytryptamine (serotonin)2A receptors in rat anterior cingulate cortex mediate the discriminative stimulus properties of d-lysergic acid diethylamide. J Pharmacol Exp Ther. 2007;320:662–669. doi: 10.1124/jpet.106.112946. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. Recent advances in the neuropsychopharmacology of hallucinogens. Behav Brain Res. 2015;277:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Characterization of the head-twitch response induced by hallucinogens in mice: detection of the behavior based on the dynamics of head movement. Psychopharmacology (Berl) 2013;227:727–739. doi: 10.1007/s00213-013-3006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. Effect of the hallucinogen 2C-I and two superpotent N-benzyl derivatives on the head twitch response. Neuropharmacology. 2014;77:200–207. doi: 10.1016/j.neuropharm.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, van der Heijden I, Ruderman M, Risbrough VB, Gingrich JA, Geyer MA, Powell SB. Opposing effects of 5-HT2A and 5-HT2C receptors on locomotor activity in mice. Neuropsychopharmacology. 2009;34:1958–1967. doi: 10.1038/npp.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson CL, Body S, Den Boon FS, Cheung THC, Bezzina G, Langley RW, Fone KCF, Bradshaw CM, Szabadi E. Comparison of the effects of 2,5-dimethoxy-4-iodoamphetamine and D-amphetamine on the ability of rats to discriminate the durations and intensities of light stimuli. Behav Pharmacol. 2010;21:11–20. doi: 10.1097/FBP.0b013e328334707a. [DOI] [PubMed] [Google Scholar]
- Hansen M, Phonekeo K, Paine JS, Leth-Petersen S, Begtrup M, Bräuner-Osborne H, Kristensen JL. Synthesis and structure-activity relationships of N-benzyl phenethylamines as 5-HT2A/2C agonists. ACS Chem Neurosci. 2014;5:243–249. doi: 10.1021/cn400216u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner SR, Meck WH. Dissociations between interval timing and intertemporal choice following administration of fluoxetine, cocaine, or methamphetamine. Behav Processes. 2014;101:123–134. doi: 10.1016/j.beproc.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann H. Experience of time and space in model psychoses. In: Pletscher A, Ladewig D, editors. 50 Years of LSD. Current Status and Perspectives on Hallucinogens. New York: Parthenon; 1994. pp. 59–66. [Google Scholar]
- Ho MY, Al-Zahrani SSA, Velazquez-Martinez DN, Lopez Cabrera M, Bradshaw CM, Szabadi E. The role of the ascending 5-hydroxytryptaminergic pathways in timing behaviour: Further observations with the interval bisection task. Psychopharmacology (Berl) 1995;120:213–219. doi: 10.1007/BF02246196. [DOI] [PubMed] [Google Scholar]
- Ho MY, Velazquez-Martinez DN, bradshaw CM, Szabadi E. 5-Hydroxytryptamine and interval timing behavior. Pharmacol Biochem Behav. 2002;71:773–785. doi: 10.1016/s0091-3057(01)00672-4. [DOI] [PubMed] [Google Scholar]
- Hoch P, Cattell JP, Pennes HH. Effects of mescaline and lysergic acid diethylamide (d. LSD-25) Am J Psychiatry. 1952;108:579–584. doi: 10.1176/ajp.108.8.579. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, McCarty DR, Poirot M, Senyah Y, Siegel BW, Widmaier C. Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther. 1996;277:968–981. [PubMed] [Google Scholar]
- Kenna JC, Sedman G. The subjective experience of time during lysergic acid diethylamide (LSD-25) intoxication. Psychopharmacologia. 1964;5:280–288. doi: 10.1007/BF02341260. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, et al. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacology. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Killeen PR, Fetterman JG. A behavioral theory of timing. Psychol Rev. 1988;95:274–295. doi: 10.1037/0033-295x.95.2.274. [DOI] [PubMed] [Google Scholar]
- Kopec CD, Brody CD. Human performance on the temporal bisection task. Brain Cognition. 2010;74:262–272. doi: 10.1016/j.bandc.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald CJ, Meck WH. Differential effects of clozapine and haloperidol on interval timing in the supraseconds range. Psychopharmacology (Berl) 2005;182:232–244. doi: 10.1007/s00213-005-0074-8. [DOI] [PubMed] [Google Scholar]
- Machado A, Keen R. Temporal discrimination in a long operant chamber. Behav Processes. 2003;62:157–182. doi: 10.1016/s0376-6357(03)00023-8. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology. 2005;30:2205–2215. doi: 10.1038/sj.npp.1300762. [DOI] [PubMed] [Google Scholar]
- Matell MS, King GR, Meck WH. Differential modulation of clock speed in the tri-peak procedure by the chronic administration of intermittent versus continuous cocaine. Behav Neurosci. 2004;118:150–156. doi: 10.1037/0735-7044.118.1.150. [DOI] [PubMed] [Google Scholar]
- Mattell MS, Meck WH. Cortical-striatal circuits and interval timing: coincidence detection of oscillatory processes. Cogn Brain Res. 2004;21:139–170. doi: 10.1016/j.cogbrainres.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Meck WH. Selective adjustment of the speed of internal clock and memory processes. J Exp Psychol Anim Behav Processes. 1983;9:171–201. [PubMed] [Google Scholar]
- Meck WH. Affinity for the dopamine D2 receptor predicts neuroleptic potency in decreasing the speed of an internal clock. Pharmacol Biochem Behav. 1986;25:1185–1189. doi: 10.1016/0091-3057(86)90109-7. [DOI] [PubMed] [Google Scholar]
- Meck WH. Neuropharmacology of timing and time perception. Cogn Brain Res. 1996;3:227–242. doi: 10.1016/0926-6410(96)00009-2. [DOI] [PubMed] [Google Scholar]
- Meck WH, Penney TB, Pouthas V. Cortico-striatal representation of time in animals and humans. Curr Opinion Neurobiol. 2008;18:145–152. doi: 10.1016/j.conb.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. Role of serotonin in the action of atypical antipsychotic drugs. Clin Neurosci. 1995;3:64–75. [PubMed] [Google Scholar]
- Milan MJ, Dekeyne A, Gobert A. Serotonin (5-HT)2C receptors tonically inhibit dopamine (DA) and noradrenaline (NA), but not 5-HT, release in the frontal cortex in vivo. Neuropharmacology. 1988;37:953–955. doi: 10.1016/s0028-3908(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Kurasawa M. Serotonergic mechanisms involved in the attentional and vigilance task performance of rats and the palliative action of aniracetam. Naunyn Schmiedebergs Arch Pharmacol. 361:521–528. doi: 10.1007/s002100000222. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Patel S, Fernandez-Garcia E, Hutson PH, Patel S. An in vivo binding assay to determine central α1-adrenoceptor occupancy using [3H]prazocin. Brain Res Protocol. 2001;8:191–198. doi: 10.1016/s1385-299x(01)00110-6. [DOI] [PubMed] [Google Scholar]
- Pehek EA, McFarlane HG, Maguschak K, Price B, Pluto CP. M100,907, a selective 5-HT2A antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Res. 2001;888:51–59. doi: 10.1016/s0006-8993(00)03004-3. [DOI] [PubMed] [Google Scholar]
- Pigott A, Frescas S, McCorvy JD, Huang XP, Roth BL, Nichols DE. trans-2-(2,5-Dimethoxy-4-iodophenyl)cyclopropylamine and trans-2-(2,5-dimethoxy-4-bromophenyl)cyclopropylamine as potent agonists for the 5-HT(2) receptor family. Beilstein J Org Chem. 2012;8:1705–1709. doi: 10.3762/bjoc.8.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdère P, Caccia S, Esposito E, Spampinato U. 5-HT2A and 5-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharmacology. 2002;26:311–324. doi: 10.1016/S0893-133X(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Pozzi L, Acconcia S, Ceglia I, Invernizzi RW, Samanin R. Stimulation of 5-hydroxytryptamine (5-HT2C) receptors in the ventrotegmental area inhibits stress-induced but not basal dopamine release in the rat prefrontal cortex. J Neurochem. 2002;82:93–100. doi: 10.1046/j.1471-4159.2002.00947.x. [DOI] [PubMed] [Google Scholar]
- Puig MV, Celada P, Díaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y. Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci. 2010;30:2211–2222. doi: 10.1523/JNEUROSCI.3335-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Geyer MA, Halberstadt AL. Serotonin and schizophrenia. In: Muller CP, Jacobs B, editors. Handbook of the Behavioral Neurobiology of Serotonin. London: Academic Press; 2010. [Google Scholar]
- Rammsayer T. Temporal discrimination in schizophrenic and affective disorders: evidence for a dopamine-dependent internal clock. Int J Neurosci. 1990;53:111–120. doi: 10.3109/00207459008986593. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Sullivan CK, Fayadel GM. Blockade of striatal 5-hydroxytryptamine2 receptors reduces the increase in extracellular concentrations of dopamine produced by the amphetamine analogue 3,4-methylenedioxymethamphetamine. J Neurochem. 1994;62:1382–1389. doi: 10.1046/j.1471-4159.1994.62041382.x. [DOI] [PubMed] [Google Scholar]
- Serko A. Im Mescalinrausch. Jahrbücher fur Psychiatrie Neurologie. 1913;31:355–366. [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. PiHKAL: A Chemical Love Story. Berkeley, CA: Transform Press; 1991. [Google Scholar]
- Sysoeva OV, Tonevitsky AG, Wackermann J. Genetic determinants of time perception mediated by the serotonergic system. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012650. pii: e12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy JI, Monaco C, McMichael H, Tyson K, Chambliss C, Christensen HL, Celenza MA. Information-processing characteristics of explicit time estimation by patients with schizophrenia and normal controls. Percept Mot Skills. 1998;86:515–526. doi: 10.2466/pms.1998.86.2.515. [DOI] [PubMed] [Google Scholar]
- Valencia Torres L, Olarte Sánchez CM, Body S, Fone KC, Bradshaw CM, Szabadi E. Fos expression in the prefrontal cortex and nucleus accumbens following exposure to retrospective timing tasks. Behav Neurosci. 2011;125:202–214. doi: 10.1037/a0022623. [DOI] [PubMed] [Google Scholar]
- Volz HP, Nenadic I, Gaser C, Rammsayer T, Hager F, Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. NeuroReport. 2001;12:313–316. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- Wackermann J, Wittmann M, Hasler F, Vollenweider FX. Effects of varied doses of psilocybin on time interval reproduction in human subjects. Neurosci Lett. 2008;435:51–55. doi: 10.1016/j.neulet.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Ward RD, Kellendonk C, Kandel ER, Balsam PD. Timing as a window on cognition in schizophrenia. Neuropharmacology. 2011;62:1175–1181. doi: 10.1016/j.neuropharm.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Carter O, Hasler F, Cahn BR, Grimberg U, Spring P, Hell D, Flohr H, Vollenweider FX. Effects of psilocybin on time perception and temporal control of behaviour in humans. J Psychopharmacol. 2007;21:50–64. doi: 10.1177/0269881106065859. [DOI] [PubMed] [Google Scholar]
- Wood J, Kim Y, Moghaddam B. Disruption of prefrontal cortex large scale neuronal activity by different classes of psychotomimetic drugs. J Neurosci. 2012;32:3022–3031. doi: 10.1523/JNEUROSCI.6377-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Receptor binding data for 25CN-NBOH.
Table S2. Effect of low doses of 25CN-NBOH on the discrete-trials task.