Abstract
Monoamine oxidase inhibitors (MAOIs) are often ingested together with tryptamine hallucinogens, but relatively little is known about the consequences of their combined use. We have shown previously that monoamine oxidase-A (MAO-A) inhibitors alter the locomotor profile of the hallucinogen 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in rats, and enhance its interaction with 5-HT2A receptors. The goal of the present studies was to investigate the mechanism for the interaction between 5-MeO-DMT and MAOIs, and to determine whether other behavioral responses to 5-MeO-DMT are similarly affected. Hallucinogens disrupt prepulse inhibition (PPI) in rats, an effect typically mediated by 5-HT2A activation. 5-MeO-DMT also disrupts PPI but the effect is primarily attributable to 5-HT1A activation. The present studies examined whether an MAOI can alter the respective contributions of 5-HT1A and 5-HT2A receptors to the effects of 5-MeO-DMT on PPI. A series of interaction studies using the 5-HT1A antagonist WAY-100635 and the 5-HT2A antagonist MDL 11,939 were performed to assess the respective contributions of these receptors to the behavioral effects of 5-MeO-DMT in rats pretreated with an MAOI. The effects of MAO-A inhibition on the pharmacokinetics of 5-MeO-DMT and its metabolism to bufotenine were assessed using liquid chromatography–electrospray ionization–selective reaction monitoring–tandem mass spectrometry (LC-ESI-SRM-MS/MS). 5-MeO-DMT (1 mg/kg) had no effect on PPI when tested 45-min post-injection but disrupted PPI in animals pretreated with the MAO-A inhibitor clorgyline or the MAO-A/B inhibitor pargyline. The combined effect of 5-MeO-DMT and pargyline on PPI was antagonized by pretreatment with either WAY-100635 or MDL 11,939. Inhibition of MAO-A increased the level of 5-MeO-DMT in plasma and whole brain, but had no effect on the conversion of 5-MeO-DMT to bufotenine, which was found to be negligible. The present results confirm that 5-MeO-DMT can disrupt PPI by activating 5-HT2A, and indicate that MAOIs alter 5-MeO-DMT pharmacodynamics by increasing its accumulation in the central nervous system.
Keywords: ayahuasca, psychedelic, analysis, pharmacokinetics, tryptamine, locomotor activity
1. INTRODUCTION
It is not uncommon for tryptamine hallucinogens to be administered in combination with drugs that inhibit monoamine oxidase-A (MAOA). For example, the potent hallucinogenic beverage known as ayahuasca contains tryptamines such as N,N-dimethyltryptamine (DMT) and 5-methoxy-DMT (5-MeO-DMT) (Agurell et al., 1968; McKenna et al., 1984), as well as β-carbolines such as harmaline and harmine, which are reversible MAOA inhibitors (MAOIs) (Buckholtz and Boggan, 1977; Kim et al., 1997). Although DMT is normally inactive orally due to first-pass metabolism (Turner and Merlis, 1959; Riba et al., 2015), the β-carbolines contribute to the activity of ayahuasca by blocking the catabolism of DMT in the gastrointestional tract. Modern Brazilian syncretic religious groups such as União do Vegetal have adopted the use of ayahuasca as a sacrament, and this practice has now spread to Europe and North America (Labate and Feeney, 2012). DMT and 5-MeO-DMT are also used recreationally in Western societies, typically by smoking, but they can also be ingested orally in combination with an MAOI (Shulgin and Shulgin, 1997; Ott, 1999, 2001; Cakic et al., 2010). Indeed, there is evidence that tryptamine hallucinogens and MAOIs are abused together (Ott, 1996; Brush et al., 2004; Sklerov et al., 2005).
5-HT2A receptor activation is responsible for mediating the characteristic subjective and behavioral effects of hallucinogens in both humans and animals (Nichols 2004; Halberstadt 2015). All serotonergic hallucinogens bind to 5-HT2A receptors, but with differing selectivity. Phenylalkylamines, including mescaline and 2,5-dimethoxy-4-methylamphetamine (DOM), are selective for 5-HT2 sites, whereas indoleamines such as N,N-dimethyltryptamine (DMT), 5-MeO-DMT, psilocybin, and (+)-lysergic acid diethylamide (LSD), are non-selective for serotonin (5-HT) receptor subtypes.
Previous studies in this laboratory have shown that the behavioral effects of 5-MeO-DMT are modified in animals treated with an MAOI (Halberstadt et al., 2008, 2012). Rats treated with 5-MeO-DMT alone exhibit a brief reduction in locomotor activity that can be blocked by the 5-HT1A-selective antagonist WAY-100635, but not by a 5-HT2A-selective antagonist (Krebs-Thomson et al., 2006). By contrast, when 5-MeO-DMT is administered to rats pretreated with a behaviorally inactive dose of an MAOI (e.g. clorgyline, pargyline, or harmaline), it produces biphasic effects, initially suppressing locomotor activity and then increasing activity at later time points. Administration of higher doses of 5-MeO-DMT alone did not reproduce this effect. The behavioral profile of 5-MeO-DMT is altered by the MAOA inhibitors harmaline and clorgyline, as well as by the MAOA/B inhibitor pargyline, whereas the selective MAOB inhibitor (–)-deprenyl is ineffective (Halberstadt et al., 2008, 2012). Given those findings, it is likely that MAOA inhibition is responsible for the interaction with 5-MeO-DMT. Importantly, the late hyperactivity produced by 5-MeO-DMT in combination with an MAOI can be blocked by the selective 5-HT2A antagonist MDL 11,939 but not by WAY-100635 (Halberstadt et al. 2008). It appears MAOA inhibition markedly enhances the contribution that 5-HT2A receptors make to the behavioral effects of 5-MeO-DMT.
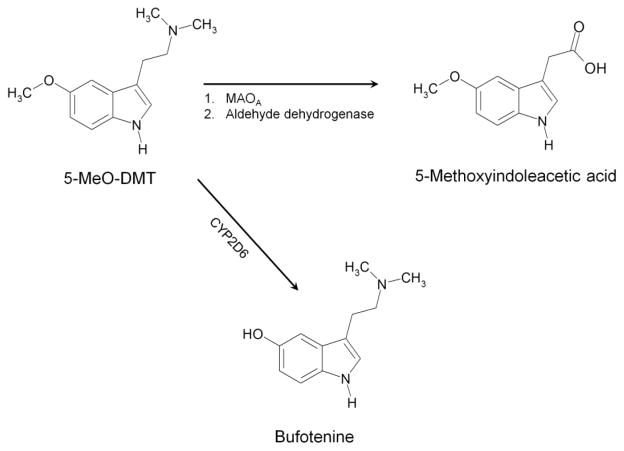
One possible explanation for the interaction between 5-MeO-DMT and MAOIs is that MAOA inhibition alters the pharmacokinetics of 5-MeO-DMT. The primary route of metabolism for 5-MeO-DMT is oxidative deamination by MAOA (Agurell et al. 1969b; Squires et al. 1975; Suzuki et al. 1981; Yu et al. 2003; Shen et al. 2010), and MAOIs are known to increase blood and brain concentrations of 5-MeO-DMT (Narasimhachari et al. 1979; Sitaram et al. 1987). Indeed, we found that α,α,β,β-tetradeutero-5-MeO-DMT, a 5-MeO-DMT isotopologue that is resistant to metabolism by MAOA due to the kinetic isotope effect, produces a biphasic locomotor profile similar to that produced by the combination of 5-MeO-DMT and an MAOI (Halberstadt et al., 2012). The finding with α,α,β,β-tetradeutero-5-MeO-DMT confirms that MAO inhibition does not directly mediate the delayed hyperactivity, which appears to be a consequence of altered 5-MeO-DMT pharmacokinetics. Nevertheless, although it is anticipated that the duration-of-action of 5-MeO-DMT would be extended by inhibiting MAOA, it is not clear why the behavioral profile of 5-MeO-DMT would be altered. It should be noted, however, that in addition to being deaminated by MAOA, 5-MeO-DMT is also O-demethylated to bufotenine (5-hydroxy-DMT) by cytochrome P450 2D6 (see Fig. 1). Inhibition of MAOA has been shown to enhance the conversion of 5-MeO-DMT to bufotenine in mice (Shen et al. 2010a,b). Since bufotenine is a potent and highly efficacious 5-HT2A agonist (Roth et al. 1997; Egan et al. 2000), we have theorized that that the delayed hyperactive phase induced by 5-MeO-DMT in the presence of an MAOI may occur as a consequence of the formation of bufotenine, which could potentially accumulate in the brain and induce hyperactivity by activating 5-HT2A (Halberstadt et al. 2012).
Figure 1.
Biotransformation of 5-MeO-DMT. The primary route of 5-MeO-DMT metabolism is oxidative deamination to 5-methoxyindoleacetic acid by monoamine oxidase-A (MAOA). Small amounts of 5-MeO-DMT are O-demethylated to bufotenine by cytochrome P-450–2D6 (CYP2D6).
The goal of the present studies was to investigate the mechanism for the interaction between 5-MeO-DMT and MAOIs, and to determine whether other behavioral responses to 5-MeO-DMT are similarly affected. Hallucinogens, including LSD and DOI, are known to inhibit prepulse inhibition (PPI) in rats by activating 5-HT2A (Sipes and Geyer 1995; Padich et al. 1996; Ouagazzal et al. 2001; Halberstadt and Geyer 2010). 5-MeO-DMT also disrupts PPI in rats (Rigdon and Weatherspoon 1992), but the effect of 5-MeO-DMT on PPI is mediated by 5-HT1A and not by 5-HT2A (Krebs-Thomson et al. 2006). Because MAOA inhibition can enhance the behavioral relevance of 5-MeO-DMT interactions with 5-HT2A, the present studies assessed whether MAOI pretreatment alters the respective contributions of 5-HT1A and 5-HT2A receptors to the effects of 5-MeO-DMT on PPI. Additional studies were conducted to determine whether inhibition of MAOA alters the pharmacokinetics of 5-MeO-DMT and its biotransformation to bufotenine.
2. MATERIALS AND METHODS
2.1. Animals
Male Sprague–Dawley rats (Harlan Industries, Indianapolis, IN, USA; initial weight 250–275 g) were housed in pairs in a temperature- and humidity-controlled vivarium under a 12-h reverse light–dark cycle (lights off at 0700 h). Food and water were available ad libitum (except during behavioral testing). Animals were allowed to acclimatize for approximately 1 week after arrival prior to behavioral testing and maintained in AALAC-approved facilities that meet all federal and state guidelines. Procedures were approved by the University of California San Diego (UCSD) institutional animal care and use committee. Principles of laboratory animal care were followed as well as specific laws of the United States.
2.2. Materials
5-Methoxy-N,N-dimethyltryptamine (5-MeO-DMT), N-methylserotonin (NMS), N-methyl-N-propargyl-3-(2,4-dichlorophenoxy)-propylamine HCl (clorgyline), pargyline HCl, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-2-pyridinylcyclohexanecarboxamide maleate (WAY-100635), ammonium acetate, sodium phosphate monobasic, and sodium phosphate dibasic were purchased from Sigma-Aldrich (St. Louis, MO). Bufotenine was purchased from Cerilliant (Red Rock, TX). α-Phenyl-1-(2-phenylethyl)-4-piperidinemethanol (MDL 11,939) was purchased from Tocris Bioscience (Ellisville, MO). LC-MS grade methanol and ethyl acetate were obtained from Honeywell-Burdick & Jackson (Muskegon, MI). Blank rat plasma used to prepare the calibrators and controls were purchased from Biochemed (Winchester, VA).
For the in vivo experiments, drug doses are expressed as the salt form of the drug, with the exception of 5-MeO-DMT and MDL 11,939, which refer to the freebase weight. All drugs were administered subcutaneously (SC) in a volume of 1 mL/kg. 5-MeO-DMT, WAY-100635, clorgyline, and pargyline were dissolved in isotonic saline. MDL 11,939 was dissolved in saline (pH 5.0) containing 0.75% Tween 80.
2.3. Apparatus
2.3.1. Behavioral Pattern Monitor
Activity was measured in the Behavioral Pattern Monitor (BPM), which assesses spatiotemporal patterns of exploratory and investigatory behavior (for details, see: Geyer et al. 1986). The rat BPM is a 30.5 × 61.0 × 28.0 cm black Plexiglas chamber equipped with 2.5 cm holes in the walls and floor. A 4 × 8 grid of infrared photobeams is used to detect the animal’s position in an X–Y plane. Infrared photobeams in each hole are used to detect investigatory nosepokes (holepokes). Rearings are detected by touchplates on the walls. Each chamber is illuminated by a 15-W red incandescent light located above the center. The status of the photobeams and the touchplate is sampled every 55 ms, digitized, and the data stored on a PC for off-line analysis.
2.3.2. Acoustic Startle
Startle chambers (SR-LAB system, San Diego Instruments, San Diego, CA) were used to measure startle reactivity (Mansbach et al. 1988). Each startle test chamber consists of a sound-attenuated, lighted, and ventilated enclosure holding a clear nonrestrictive cylindrical Plexiglas stabilimeter, 8.2 cm in diameter. The acoustic stimuli were generated by a high-frequency loudspeaker mounted 24 cm above the Plexiglas cylinder. The peak and average amplitude of the startle response were detected by a piezoelectric accelerometer, digitized, and stored on a PC. At the onset of the startling stimulus, 100 1-ms samples were recorded, and the average amplitude was used to determine the startle response. A dynamic calibration system was used to ensure comparable stabilimeter sensitivity across test chambers, and sound levels were measured using the dB(A) scale, as described previously (Mansbach et al. 1988).
Acoustic startle test sessions consisted of startle trials (PULSE-ALONE) and prepulse trials (PREPULSE+PULSE). The PULSE-ALONE trial consisted of a 40-ms 120-dB pulse of broadband white noise. PREPULSE+PULSE trials consisted of a 20-ms acoustic prepulse, an 80-ms delay, and then a 40-ms 120-dB startle pulse (100 ms onset–onset). There was an average of 15 s (range, 9–21 s) between trials. During each inter-trial interval, the movements of the rats were recorded once to measure responding when no stimulus was present (data not shown). Each startle session began with a 5-min acclimation period to a 65-dB broadband noise that was present continuously throughout the session. The startle test session included three blocks. The first and last block consisted of 5 PULSE-ALONE trials that were not used in the calculation of PPI values. The second block was designed to assess PPI; it contained 12 PULSE-ALONE trials and 30 PREPUSE+PULSE trials [10 prepulses each of 68, 71, and 77 dB (3, 6, and 12 dB above background)] presented in a pseudo-randomized order. One week after arrival, animals were tested in a brief startle/PPI session to create treatment groups matched for baseline levels of startle and PPI.
2.4. Experimental Design
2.4.1. Acoustic Startle
Animals were placed in the startle chambers 45 min after treatment with 5-MeO-DMT. The animals were tested 45 min after drug administration because the experiments were designed to assess acoustic startle and PPI during the period of time when animals treated with pargyline and 5-MeO-DMT display locomotor hyperactivity in the BPM (i.e., 40–70 min after injection of 5-MeO-DMT; see Halberstadt et al. 2012). In experiment 1, rats (n=10/group, 60 total) were treated with the nonselective MAO inhibitor pargyline (0 or 10 mg/kg) 20 min before administration of 5-MeO-DMT (0, 0.1, or 1.0 mg/kg). In experiment 2, rats (n=12/group, 48 total) were treated with the MAOA inhibitor clorgyline (0 or 0.3 mg/kg) 20 min before administration of 5-MeO-DMT (0 or 1.0 mg/kg). In experiment 3, rats (n=10/group, 60 total) were pretreated with vehicle, the selective 5- HT1A antagonist WAY-100635 (1.0 mg/kg), or the selective 5-HT2A antagonist MDL 11,939 (0.3 mg/kg), 20 min before treatment with vehicle or 1.0 mg/kg 5-MeO-DMT. For experiment 3, all the animals that were injected with 5-MeO-DMT were pretreated (20 min) with 10 mg/kg pargyline. WAY-100635 and MDL 11,939 were tested at doses previously shown to block 5-HT1A- and 5-HT2A-mediated behavioral responses in Sprague-Dawley rats (Krebs-Thomson et al., 2006; Halberstadt et al., 2008; Halberstadt and Geyer, 2010).
2.4.2. Behavioral Pattern Monitor
One day before the study, animals were taken to the BPM testing room, weighed, handled briefly, placed in a clear Plexiglas box (24 × 46 cm) for approximately 30 s, and then returned to their cages in the vivarium. On the testing day, rats were brought to the testing room and allowed to sit for 60 min before receiving injections. Injections were administered under red lights in the testing room. Animals were tested during the dark phase in darkness. Rats were divided into two groups (n = 30/group) and pretreated with vehicle or clorgyline (0.3 mg/kg) 20 min before administration of 1.0 mg/kg 5-MeO-DMT. The animals were placed in the BPM chambers 10 min after 5-MeO-DMT treatment, and locomotor activity was monitored for 10, 20, 30, 40, 50, or 60 min (n = 5/time point/group). The rats were immediately removed from the BPM chambers, anesthetized (isoflurane), decapitated, and trunk blood collected in cooled heparinized tubes. Plasma was isolated by centrifugation (3200 × g) and stored at −40°C. The brains were removed, flash frozen in isopentane at −80°C, and stored at −40°C.
2.5. Procedures for quantification of 5-MeO-DMT and bufotenine in plasma and brain tissue
2.5.1. Preparation of calibrators and controls
The calibrators and controls were prepared in silanized, 16 × 100 mm glass culture tubes containing 1 mL blank rat plasma. Working solutions containing 0.01, 0.1, 1.0, and 10 ng/μL of 5-MeO-DMT or bufotenine were prepared in methanol for spiking the analytes. Separate sets of working solutions were used for the calibrators and controls. The calibrators were prepared at 1, 2, 5, 10, 20, 50, 100, and 200 ng/mL and were run in duplicate with one set at the beginning and one set at the end of the run; controls were prepared at 5, 15, and 160 ng/mL and run at N ≥ 3 interspersed with the other samples.
2.5.2. Preparation of samples for analysis
For the plasma samples, 1 mL of each sample was transferred to a silanized 16 × 100 mm glass culture tube. For brain tissue, a 10-fold diluted tissue homogenate sample was prepared for each brain. Brain tissue was weighed, transferred to a 50-mL conical polypropylene tube, shredded with a clean wood applicator stick, and nine volumes of 50 mM sodium phosphate (pH 7.0) homogenizing buffer (i.e., 13.5 mL homogenizing buffer per 1.5 g tissue) were added. Tissue was homogenized using a Vibra Cell probe homogenizer (Sonics, Newtown, CT), using 3 × 12-s pulses at 30% amplitude, and then stored frozen prior to analysis. At the time of analysis, the homogenates were thawed, and 1 mL of each sample homogenate was transferred to a new 16 × 100 mm glass culture tube.
Prior to the analysis, 50 μL of 1 ng/μL NMS was added as internal standard to each sample, calibrator, and control tube. The tubes were vortexed, and 3 mL of 0.1 M sodium phosphate buffer (pH 6.0) was added to each tube. The tubes were briefly vortexed and then centrifuged (1200 × g). The supernatants from each tube were then applied to their respective conditioned solid phase extraction column (see below).
2.5.3. Extraction
Clean Screen ZSDAU020 solid phase extraction columns (United Chemical Technologies, Bristol, PA) were used for the extraction procedure. A separate column was utilized for each sample, standard, and control. A glass manifold device was employed for the extraction process. First, the extraction columns were conditioned by sequential addition of 3 mL of methanol, 3 mL of water, and 2 mL of 0.1 M sodium phosphate (pH 6.0), and then the supernatants from sample preparation were added. Next, the columns were sequentially washed with 3 mL of water and 3 mL of methanol. With care taken to prevent drying of the columns, the columns were eluted by the addition of 3 mL of 2% ammonium hydroxide in ethyl acetate and 4% ammonium hydroxide in methanol. The eluates from each column were collected into clean 13 × 100 mm culture tubes, and evaporated to dryness under a stream of air using a Tubovap evaporator (Caliper, Hopkinton, MA) set at 40 °C. The extracts were reconstituted with 100 μL of 10 mM ammonium acetate (pH 5.0)/methanol (80:20) and then transferred to separate 300 μL conical, polypropylene autosampler vials with snap caps.
2.5.4. LC-MS/MS analysis
The liquid chromatography-tandem mass spectrometry (LC-MS/MS) system consisted of an Agilent (Santa Clara, CA) 1100 liquid chromatograph interfaced with a Thermo-Finnegan (San Jose, CA) TSQ Quantum Access triple quadrupole mass spectrometer. The system was operated by an Xcalibur® 2.0 SR2 and Tune Master version 1.4.1 operating systems (Thermo-Finnigan). A LUNA C18(2), 150 × 4.6 mm LC column (Phenomenex, Torrance, CA) at 35 °C was used. The mobile phase was 10 mM ammonium acetate (pH 5.0)/methanol (60:40) at a flow rate of 0.2 mL/min. For the MS/MS analysis, positive ion electrospray was used for ionization. The capillary temperature was 270 °C. Nitrogen was used as the sheath gas (60 units) and the aux gas (20 units). Selected reaction monitoring was used for the analysis. Ultra high purity argon was used as the collision gas for collision induced dissociation. The following transitions were monitored: 5-MeO-DMT = 219→174; bufotenine = 205→166; NMS = 191→160). For all compounds, the precursor ions were the protonated molecular ions. A calibration curve generated from the analysis of the calibration standards was used to extrapolate the 5-MeO-DMT and bufotenine concentrations in the analytical quality controls and the extracts prepared from of the study samples. LCquan 2.5 data analysis software (Thermo-Finnigan) was used for the quantification.
2.6. Data Analysis
2.6.1. Acoustic startle
The amount of PPI was calculated as a percentage score for each PREPULSE+PULSE trial type: %PPI=100–(((startle response for PREPULSE+PULSE trial)/(startle response for PULSE-ALONE trial))×100). Startle magnitude was calculated as the average response to all of the PULSE-ALONE trials. The level of spontaneous motor activity was assessed as the average response to all NOSTIM trials. PPI data were analyzed with two- or three-factor analysis of variance (ANOVA) with pretreatment and treatment as between-subjects factors and trial type (prepulse intensity) as a repeated measure. For experiments in which there was no significant interaction between drug and prepulse intensity, PPI data were collapsed across prepulse intensity and the average PPI was used as the main dependent measure. Startle magnitude and NOSTIM data were analyzed with two-factor ANOVA. Post hoc analyses were carried out using Tukey’s studentized range method. Significance was demonstrated for these and other experiments by surpassing an alpha level of 0.05.
2.6.2. Behavioral Pattern Monitor
The raw data were reduced to the X and Y coordinates of the rat in the chamber. Locomotor activity was quantified by the number of crossings between any of eight equal square sectors within the BPM. Data were examined in 10-min time blocks. For each rat, only the behavioral data from the last 10-min time block (i.e., the test block immediately prior to collection of plasma and brain samples) were analyzed. Data were analyzed using two-way ANOVA with clorgyline pretreatment and time block as between-subject factors. Specific post hoc comparisons were made using Bonferonni’s multiple comparisons test.
2.6.3. Analysis of 5-MeO-DMT and bufotenine in plasma and brain
To facilitate data reporting in cases where 5-MeO-DMT or bufotenine concentrations fell below the limit of detection (<LOD), Helsel’s Robust Method (implemented using the UNCENSOR program: Newman and Evans 2005) was used to estimate group means and variance. However, simple substitution (null values) was used for groups with ≥ 4 censored values. The trapezoidal rule was used to calculate the area under the curve up to the last measured time point (AUC20→70 min). The density of brain tissue in rats was assumed to be 1.04 g/mL (Smith 1930; Johansson and Linder 1982; Başkaya et al. 1997). Statistical comparisons of raw analyte levels were made using Kaplan–Meier survival analysis; the nonparametric Tarone-Ware Test (SPSS Ver. 22) was used to assess equivalence between groups. AUC and Cmax values were compared using one-way ANOVA.
3. RESULTS
3.1. Effect of pargyline and 5-MeO-DMT on acoustic startle and sensorimotor gating
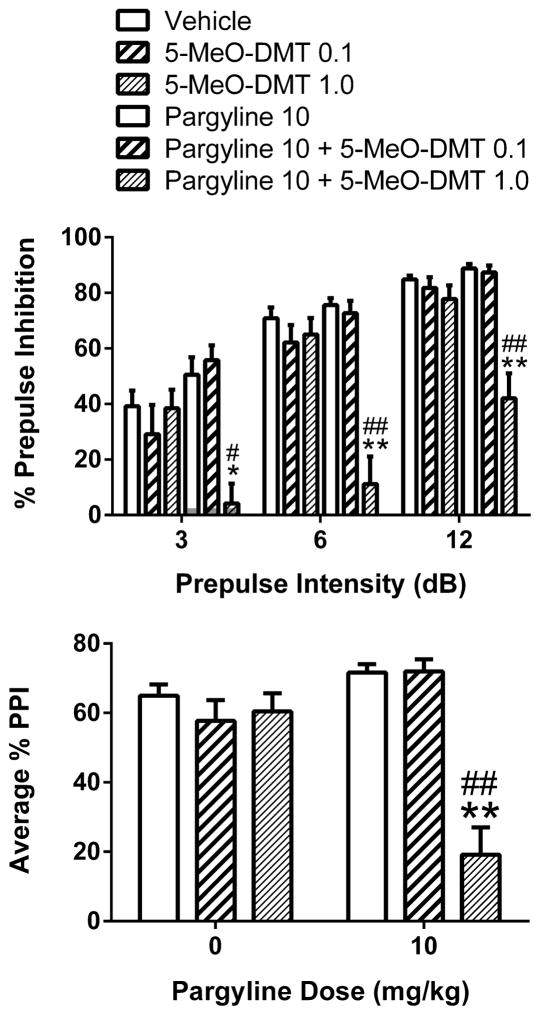
5-MeO-DMT was previously shown to disrupt PPI when administered 10 min prior to startle testing (Krebs-Thomson et al. 2006). The current studies tested whether 5-MeO-DMT alters PPI when administered 45 min prior to testing, and examined whether the response to 5-MeO-DMT is altered in animals pretreated with 10 mg/kg pargyline. As shown in Figure 2, 5-MeO-DMT did not alter PPI in saline-pretreated animals but significantly reduced PPI in animals pretreated with pargyline, resulting in an interaction between pretreatment and treatment (F(2,54)=17.59, p<0.0001) and a main effect of 5-MeO-DMT (F(2,54)=18.87, p<0.0001). There was a significant main effect of prepulse intensity (F(2,108)=162.27, p<0.0001), and pairwise comparisons revealed that 1 mg/kg 5-MeO-DMT significantly reduced PPI at all three prepulse intensities in animals pretreated with pargyline (p<0.01, Tukey’s test). There was also significant interaction between pargyline pretreatment and prepulse intensity (F(2,108)=5.05, p=0.008), but pargyline did not significantly reduce PPI at any prepulse intensity compared with vehicle pretreatment.
Figure 2.
Effect of pargyline pretreatment on the behavioral response to 5-MeO-DMT. Top panel Effect on prepulse inhibition (PP). Bottom panel Effect on PPI averaged across the three prepulse intensities. Values represent mean ± SEM for each group. Drug doses are in milligram per kilogram. *p<0.05, **p<0.01, significantly different from vehicle control. #p<0.05, ##p<0.01, significantly different from animals treated with 1 mg/kg 5-MeO-DMT alone. A total of 60 rats were used for this experiment (n=10/group).
For the startle response, there was a main effect of 5-MeO-DMT (F(2,54)=3.97, p<0.03) and an interaction between pargyline and 5-MeO-DMT (F(2,54)=5.07, p<0.01). Pretreatment with pargyline significantly reduced the amplitude of the startle response (p<0.05, Tukey’s Test; see Table 1), but the startle response was unaffected by treatment with 5-MeO-DMT. In order to confirm that the effect of pargyline and 5-MeO-DMT on PPI is independent of changes in startle magnitude, we examined the effect on PPI in subgroups of animals matched for startle level (see: Geyer and Swerdlow, 1998). We picked 7 animals from each group that had overlapping startle responses (mean±SEM = 192.5±26.3, 230.0±33.0, 232.4±37.0, 232.5±39.7, 236.2±45.4, and 304.9±29.5). Even though the magnitude of the startle response in those subgroups was unaffected by pretreatment (F(1,36)=0.09, NS), treatment (F(2,36)=1.21, NS), or pretreatment × treatment interactions (F(2,36)=1.36, NS), PPI was still significantly reduced by the combination of pargyline and 5-MeO-DMT (Pretreatment × Treatment interaction: F(2,36)=10.44, p=0.0003). This shows that the PPI disruption produced by pargyline and 5-MeO-DMT did not occur because of changes in startle magnitude.
Table 1.
Effect of drug treatment on startle magnitude
| Pretreatment (mg/kg) | Treatment (mg/kg) | n | Startle magnitude (mean±SEM) | |
|---|---|---|---|---|
| Vehicle | Vehicle | 10 | 382.43±85.91 | |
| Pargyline 10 | Vehicle | 10 | 167.66±22.30* | |
| Vehicle | 5-MeO-DMT 0.1 | 10 | 180.58±37.92 | |
| Vehicle | 5-MeO-DMT 1.0 | 10 | 274.12±56.96 | |
| Pargyline 10 | 5-MeO-DMT 0.1 | 10 | 185.69±32.15 | |
| Pargyline 10 | 5-MeO-DMT 1.0 | 10 | 371.18±40.08 | |
|
| ||||
| Vehicle | Vehicle | 12 | 425.06±67.44 | |
| Clorgyline 0.3 | Vehicle | 12 | 422.31±78.47 | |
| Vehicle | 5-MeO-DMT 1.0 | 12 | 429.21±73.77 | |
| Clorgyline 0.3 | 5-MeO-DMT 1.0 | 12 | 544.45±62.88 | |
|
| ||||
| Vehicle | Vehicle | 10 | 374.93±97.14 | |
| WAY-100635 1.0 | Vehicle | 10 | 271.30±51.26 | |
| MDL 11,939 0.3 | Vehicle | 10 | 420.59±79.88 | |
| Vehicle | Pargyline 10 + 5-MeO-DMT 1.0 | 10 | 392.24±58.82 | |
| WAY-100635 1.0 | Pargyline 10 + 5-MeO-DMT 1.0 | 10 | 172.03±47.00 | |
| MDL 11,939 0.3 | Pargyline 10 + 5-MeO-DMT 1.0 | 10 | 419.79±48.30 | |
p<0.05 versus vehicle–vehicle control
3.2. Effect of clorgyline and 5-MeO-DMT on acoustic startle and sensorimotor gating
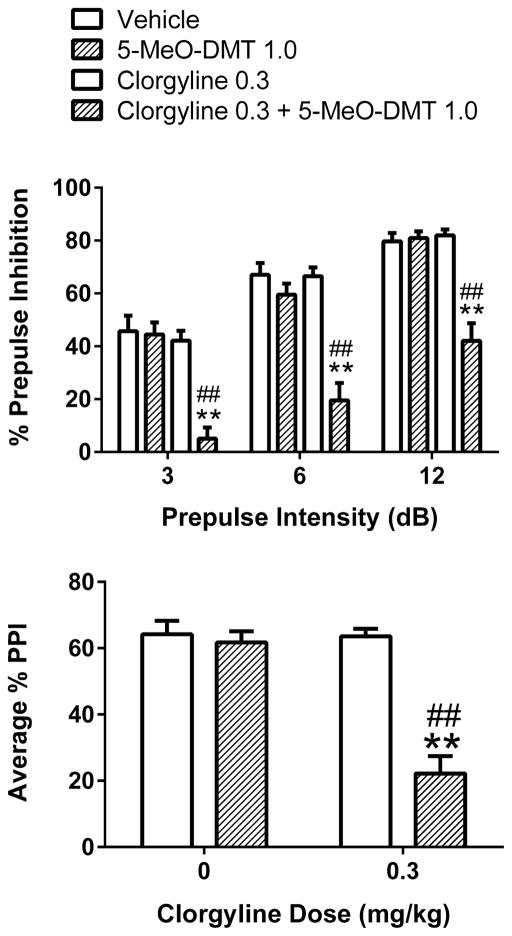
We also examined whether the response to 5-MeO-DMT is altered in animals pretreated with 0.3 mg/kg clorgyline. Similar to the experiment with pargyline, the animals were tested 45 min after administration of 5-MeO-DMT. 5-MeO-DMT did not alter PPI in saline-pretreated animals but significantly reduced PPI in animals pretreated with clorgyline (Pretreatment × Treatment: F(1,44)=24.74, p<0.0001). 5-MeO-DMT significantly reduced PPI at all three prepulse intensities in animals pretreated with clorgyline (p<0.01, Tukey’s test; Figure 3). As shown in Table 1, the startle response was not altered by treatment with 5-MeO-DMT (Main effect: F(1,44)=0.79, NS) or by pretreatment with clorgyline (Main effect: F(1,44)=0.63, NS), and there was no interaction between clorgyline and 5-MeO-DMT (F(1,44)=0.69, NS).
Figure 3.
Effect of clorgyline pretreatment on the behavioral response to 5-MeO-DMT. Top panel Effect on prepulse inhibition (PPI). Bottom panel Effect on PPI averaged across the three prepulse intensities. Values represent mean ± SEM for each group. Drug doses are in milligram per kilogram. **p<0.01, significantly different from vehicle control. ##p<0.01, significantly different from animals treated with 1 mg/kg 5-MeO-DMT alone. A total of 48 rats were used for this experiment (n=12/group).
3.3. Involvement of 5-HT1A and 5-HT2A receptors in the effects of pargyline and 5-MeO-DMT on sensorimotor gating
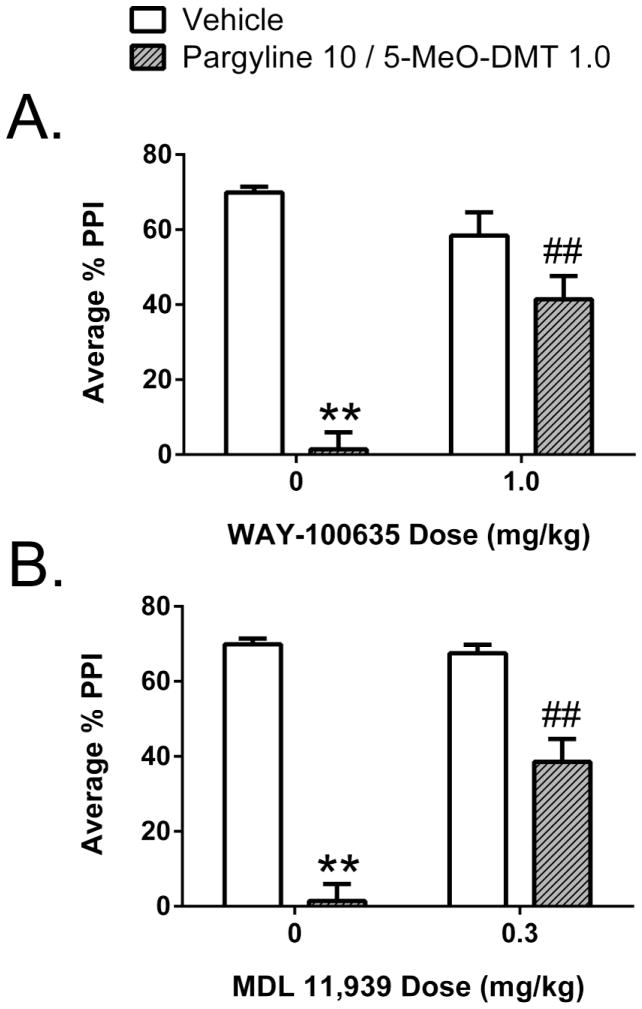
To determine whether the PPI reduction produced by 10 mg/kg pargyline and 1 mg/kg 5-MeO-DMT is mediated by interactions with 5-HT1A or 5-HT2A receptors, antagonist blockade studies were conducted with WAY-100635 and MDL 11,939, respectively. As expected, there was a main effect of treatment (F(1,54)=92.13, p<0.0001), as well as an interaction between treatment and prepulse intensity (F(2,108)=7.23, p<0.002), and the combination of pargyline and 5-MeO-DMT significantly reduced PPI at all three prepulse intensities in vehicle-pretreated animals (p<0.01, Tukey’s test). The magnitude of the startle response was unaffected by treatment with pargyline and 5-MeO-DMT (F(1,54)=0.26, NS; Table 1). The effects of pretreatment with WAY-100635 and MDL 11,939 were assessed in a single experiment, with a shared control group, but the effect of each antagonist was analyzed independently (see below).
3.3.1. WAY-100,635
For PPI, there was an interaction between pretreatment and treatment (F(1,36)=73.11, p<0.0001). The reduction of PPI produced by 10 mg/kg pargyline and 1 mg/kg 5-MeO-DMT was significantly antagonized by pretreatment with 1 mg/kg WAY-100,635 (p<0.01, Tukey’s test; Fig. 4A). There was also a trend toward a main effect of pretreatment (F(1,36)=8.17, p<0.08), but this was not confirmed by pair-wise comparisons. Although there was a main effect of pretreatment on startle magnitude (F(1,36)=5.91, p<0.03), the reduction of startle amplitude produced by 1 mg/kg WAY-100,635 did not achieve significance (see Table 1).
Figure 4.
Effects of the 5-HT1A antagonist WAY-100635 and the selective 5-HT2A antagonist MDL 11,939 on the behavioral response to pargyline and 5-MeO-DMT. (A) Effect of WAY-100635 on the disruption of prepulse inhibition (PPI). (B) Effect of MDL 11,939 on the disruption of PPI. *p<0.05, **p<0.01, significantly different from vehicle control. #p<0.05, ##p<0.01, significantly different from animals treated with 1.0 mg/kg 5-MeO-DMT alone. A total of 60 rats were used for this experiment (n=10/group).
3.3.2. MDL 11,939
The ability of pargyline and 5-MeO-DMT to reduce PPI was significantly attenuated by pretreatment with MDL 11,939 (Pretreatment × Treatment: F(1,36)=23.96, p<0.0001), and this was confirmed by post-hoc analysis (p<0.01, Tukey’s test; Fig. 4B). Pretreatment with MDL 11,939 did not alter startle amplitude (F(1,36)=0.25, NS).
3.4. Effect of clorgyline pretreatment on 5-MeO-DMT and bufotenine levels in plasma and brain
3.4.1. Analysis method
Several different extraction procedures were investigated, including liquid-liquid extraction and precipitation with methanol, but were associated with poor recovery and sensitivity (data not shown). Previous studies measuring 5-MeO-DMT and bufotenine levels in biological fluids used solid phase extraction to prepare the samples (Forsstrom et al., 2001; Martin et al., 2013). We used a different solid phase extraction method for the simultaneous detection of 5-MeO-DMT and bufotenine in plasma and brain, using liquid chromatography–electrospray ionization–selective reaction monitoring–tandem mass spectrometry (LC-ESI-SRM-MS/MS), with N-methylserotonin (NMS) as the internal standard. Performance characteristics were collected over the course of three pre-study and four study sample analytical runs. The method was selective as determined from examination of analytical results for 7 blank plasma samples fortified with internal standard; the mean detectable signal (peak area ratio) at the retention time of analytes did not exceed 0.25% and 14.7% of mean LLOQ peak area heights for 5-MeO-DMT and bufotenine, respectively. Chromatography of 5-MeO-DMT, bufotenine, and NMS was subject to ion suppression. The suppression, however, was uniform across the chromatographic effusion window of the analytes (3.5–4.5 min). Retention times were not affected by ion suppression, with little variation within or between runs (mean 5-MeO-DMT, 4.11; bufotenine, 2.60; and NMS, 2.64 minutes); the coefficient of variation (CV) for retention times relative to the NMS were 3.51% and 0.99% for 5-MeO-DMT and bufotenine, respectively. There was relatively high extraction recovery (62.7–88.0%) for all analytes. Calibration was reproducible with the use of eight calibration points in duplicate; back-calculated concentrations were within 5% of target with %CVs within 12.9% for both analytes; mean r2 values were 0.990 for 5-MeO-DMT and 0.994 for bufotenine. With the accuracy (93.3–104.7% for 5-MeO-DMT and 96.3–107.9% for bufotenine) and precision (%CV not exceeding 17.8 for 5-MeO-DMT and 19.4 for bufotenine) achieved (see Table 2; data specific for study sample runs), the conditions were adequate for reliable analysis.
Table 2.
Precision and accuracy of the method was determined from inclusion of quality control (QC) samples in 4 analytical batches that included rat plasma and brain samples. QCs prepared in rat plasma were run at n=3 per concentration per batch. QCs were acceptable in that over 2/3 total and within concentration were within ± 20% of target.
| Target | % Target | % CV | |
|---|---|---|---|
|
| |||
| 5-MeO-DMTa | 3.0 ng/mL | 93.3 | 16.1 |
| 15 ng/mL | 104.7 | 17.8 | |
| 160 ng/mL | 101.8 | 14.0 | |
|
| |||
| Bufotenineb | 3.0 ng/mL | 96.3 | 19.4 |
| 15 ng/mL | 105.1 | 12.0 | |
| 160 ng/mL | 107.9 | 12.7 | |
For 5-Methoxy-DMT, 3 low and 1 medium QCs were treated as outliers and not used in calculation of precision and accuracy.
For bufotenine, 1 medium QC was treated as an outlier and not used in calculation of precision and accuracy.
3.4.2. Analysis of 5-MeO-DMT and bufotenine in plasma and brain
To characterize the effect of MAOA inhibition on the pharmacokinetics and metabolism of 5-MeO-DMT, rats were administered 1 mg/kg 5-MeO-DMT after pretreatment with vehicle or clorgyline (0.3 mg/kg), and 5-MeO-DMT and bufotenine levels in plasma and whole brain were quantified. The concentration of 5-MeO-DMT in plasma is shown in Table 3, and the concentration in brain is shown in Table 4. The level of 5-MeO-DMT in plasma and brain peaked within the first 20 min after SC administration and then rapidly declined. Clorgyline did not significantly increase the plasma Cmax of 5-MeO-DMT (vehicle = 52.7±11.8 ng/mL; clorgyline = 78.1±9.5 ng/mL), but the clearance of 5-MeO-DMT from plasma was slowed by clorgyline, as evidenced by the fact that the concentration of 5-MeO-DMT in plasma was significantly elevated 30–70 min after administration in animals pretreated with clorgyline (see Table 3). Furthermore, clorgyline significantly increased the plasma AUC20→70 min for 5-MeO-DMT (vehicle = 12.2±1.7 ng.h/mL, clorgyline = 31.4±2.3 ng.h/mL; F(1,59)=45.6, p<0.0001). In contrast to the negligible effect of clorgyline on the plasma Cmax, clorgyline significantly increased the Cmax of 5-MeO-DMT in whole brain (vehicle: 30.3±11.2 ng/g; clorgyline: 633.8±146.5 ng/g; F(1,9)=16.8, p<0.004). As shown in Table 4, the concentration of 5-MeO-DMT in whole brain was markedly elevated in animals pretreated with clorgyline, and 5-MeO-DMT was also detectable for a longer period of time in the presence of an MAO inhibitor. It does not appear that clorgyline pretreatment significantly altered the concentration of bufotenine in plasma or brain because most of the samples were < LOD (plasma = 1–2 ng/mL; brain = 10 ng/g). The plasma Cmax for bufotenine was 1.3±0.1 ng/mL in rats pretreated with vehicle, and 1.8±0.3 ng/mL in rats pretreated with clorgyline (data not shown).
Table 3.
Effect of pretreatment with vehicle or clorgyline (0.3 mg/kg) on the concentration of 5-MeO-DMT in plasma
| Time (min)1 | Vehicle2 | Clorgyline2 | Tarone-Ware Test | ||
|---|---|---|---|---|---|
| ng/mL | nM | ng/mL | nM | ||
| 20 | 52.7±11.8 (0) | 241.6±54.1 | 78.1±9.5 (0) | 357.8±43.7 | χ2 (1, N=10) = 2.83, p=0.093) |
| 30 | 29.2±6.4 (0) | 133.8±29.4 | 62.0±6.6 (0) | 284.0±30.0 | χ2 (1, N=10) = 9.04, p=0.003) |
| 40 | 12.6±4.8 (0) | 57.6±22.1 | 42.0±9.2 (0) | 192.4±42.0 | χ2 (1, N=10) = 5.48, p<0.02) |
| 50 | 3.5±1.7 (1) | 15.8±7.6 | 23.5±5.0 (0) | 107.7±22.8 | χ2 (1, N=10) = 6.28, p<0.02) |
| 60 | 0.6±0.5 (4) | 2.7±2.3 | 15.1±2.1 (0) | 69.2±9.6 | χ2 (1, N=10) = 5.00, p<0.03) |
| 70 | 0±0 (5) | 0±0 | 10.1±4.5 (1) | 46.3±20.6 | – – |
Values are listed as mean±SEM.
Interval between injection of 5-MeO-DMT and collection of plasma.
The number of left censored values (values below the LOD) is listed in parentheses. Helsel’s Robust Method was used to estimate the mean and variance in cases with 1–3 censored values; simple substitution (null values) was used in cases with ≥4 censored values.
Table 4.
Effect of pretreatment with vehicle or clorgyline (0.3 mg/kg) on the concentration of 5-MeO-DMT in whole brain
| Time (min)1 | Vehicle2 | Clorgyline2 | Tarone-Ware Test | ||
|---|---|---|---|---|---|
| ng/g | nM | ng/g | nM | ||
| 20 | 30.3±11.2 (1) | 144.3±53.2 | 633.8±146.5 (0) | 3,019.7±698.1 | χ2 (1, N=10) = 8.53, p=0.003) |
| 30 | 16.9±3.6 (3) | 80.6±16.9 | 371.1±80.5 (0) | 1,768.1±383.3 | χ2 (1, N=10) = 6.83, p=0.009) |
| 40 | 3.7±3.3 (4) | 17.6±15.7 | 338.1±69.9 (0) | 1,610.8±333.0 | χ2 (1, N=10) = 5.00, p<0.03) |
| 50 | 0±0 (5) | 0±0 | 239.4±54.2 (0) | 1,140.5±258.2 | – – |
| 60 | 0±0 (5) | 0±0 | 267.9±22.7 (0) | 1,276.3±108.1 | – – |
| 70 | 0±0 (5) | 0±0 | 65.6±20.4 (0) | 312.5±97.2 | – – |
Values are listed as mean±SEM.
Interval between injection of 5-MeO-DMT and collection of tissue.
The number of left censored values (values below the LOD) is listed in parentheses. Helsel’s Robust Method was used to estimate the mean and variance in cases with 1–3 censored values; simple substitution (null values) was used in cases with ≥4 censored values.
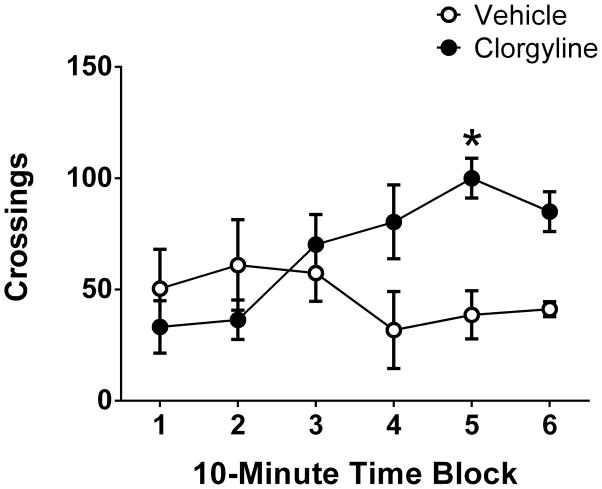
To confirm that the expected behavioral interaction occurred between 0.3 mg/kg clorgyline and 1 mg/kg 5-MeO-DMT, locomotor activity was assessed in the BPM prior to collection of analytical samples (see Figure 5). There was a significant main effect of clorgyline (F(1,48)=7.25, p<0.01) and a significant interaction between clorgyline and time block (F(5,48)=3.64, p<0.008). Importantly, compared with vehicle pretreatment, rats pretreated with clorgyline made significantly more crossings (a measure of locomotor activity) during the fifth 10-min time block (p<0.05, Bonferonni’s test), confirming the animals were hyperactive.
Figure 5.
Effect of clorgyline on the locomotor response to 5-MeO-DMT. All the animals were treated with 5-MeO-DMT (1 mg/kg SC) 10 min prior to placement in the behavioral pattern monitor, and were pretreated with either vehicle or clorgyline (0.3 mg/kg SC). Data are expressed as group means±SEM during the last 10-min block of testing immediately prior to collection of plasma and brain samples. *p<0.05, significant difference from vehicle group.
4. DISCUSSION
These studies demonstrate that pretreatment with an MAOI markedly prolongs the behavioral response to 5-MeO-DMT. Although 5-MeO-DMT has previously been shown to disrupt PPI by activating 5-HT1A receptors (Krebs-Thompson et al. 2006), the effect on PPI has a relatively short duration, and the present results confirm that 5-MeO-DMT does not alter PPI 45 min post-injection. By contrast, 5-MeO-DMT disrupted PPI at that time point in rats pretreated with an MAOI. The effect of combined treatment with 5-MeO-DMT and the MAOI pargyline on PPI was antagonized by pretreatment with the 5-HT1A antagonist WAY-100635 and the 5-HT2A antagonist MDL 11,939. Based on the findings with WAY-100635 and MDL 11,939, it appears that the PPI disruption produced by 5-MeO-DMT in animals pretreated and an MAOI is dependent on activation of 5-HT1A and 5-HT2A receptors. These findings confirm that 5-MeO-DMT, like other hallucinogens, can alter PPI in rats by activating 5-HT2A receptors. Analysis of 5-MeO-DMT pharmacokinetics revealed that systemic and central exposure to the drug is increased and prolonged after inhibition of MAOA, and showed that there is minimal conversion of 5-MeO-DMT to bufotenine in rats.
Pretreatment with the MAOA inhibitor clorgyline dramatically altered the pharmacokinetics of 5-MeO-DMT. These effects probably occurred because clorgyline blocked the metabolism of 5-MeO-DMT. 5-MeO-DMT is metabolized by deamination and clorgyline was administered at a dose that is known to inhibit MAOA. Using 5-HT deamination as a measure of MAOA activity ex vivo, Feldner and Waldmeier (1979) determined that clorgyline inhibits MAOA in liver and brain by approximately 60% and 90%, respectively, when administered to rats at 0.3 mg/kg SC. Compared to the present results, previous studies in rats and mice have yielded similar findings regarding the effects of MAOIs on 5-MeO-DMT pharmacokinetics (Narasimhachari et al. 1979; Sitaram et al. 1987; Shen et al. 2010b; Jiang et al. 2013). Sitaram and coworkers (Sitaram et al. 1987) examined the effect of pretreatment with the non-selective MAOI iproniazid phosphate on tissue levels of 5-MeO-DMT in Sprague-Dawley rats. After treatment with 5-MeO-DMT (10 mg/kg IP), blood and brain levels averaged 0.03 nmol/g (6.9 ng/mL) and 0.08 nmol/g (17.5 ng/g), respectively, when samples were collected 45 min post-injection. By contrast, in rats pretreated with iproniazid, the concentration of 5-MeO-DMT in blood was 1.2 nmol/g (275 ng/mL) and the concentration in brain was 7.8 nmol/g (1703 ng/g). In summary, iproniazid increased 5-MeO-DMT blood levels 40-fold and brain levels almost 100-fold.
One of the more interesting findings with 5-MeO-DMT is that its pharmacodynamics can be altered by inhibition of MAO. Normally, 5-MeO-DMT produces brief alterations of locomotor activity and PPI that are mediated by 5-HT1A and not by 5-HT2A receptors (Krebs-Thomson et al., 2006). Conversely, in rats pretreated with an MAOA inhibitor, the action of 5-MeO-DMT is prolonged and involves 5-HT2A activation (Halberstadt et al. 2008, 2012; Figure 4). Yu and colleagues have shown (Shen et al. 2010b) that there is a marked increase in the O-demethylation of 5-MeO-DMT in mice pretreated with an MAOI, resulting in elevated and prolonged systemic exposure to bufotenine. Based on their findings, we hypothesized that if MAOIs produce similar effects on the metabolism of 5-MeO-DMT in rats, the behavioral interaction between MAOIs and 5-MeO-DMT could be a consequence of increased formation of bufotenine, a potent and highly efficacious 5-HT2A agonist. The present results, however, reveal that there is negligible conversion of 5-MeO-DMT to bufotenine in rats, even in the presence of an MAOI. Similarly, a previous study failed to detect any bufotenine in the brains of rats treated with 5-MeO-DMT and iproniazid (Sitaram et al. 1987). Importantly, we found that inhibition of MAOA did produce a significant increase in the central accumulation of 5-MeO-DMT, and micromolar concentrations were present in the brain for at least 60 min post-injection. Therefore, it appears that MAOIs alter the behavioral response to 5-MeO-DMT by blocking its deamination, which allows 5-MeO-DMT to accumulate in the brain at concentrations sufficient to activate 5-HT2A receptors for an extended period of time. The fact that α,α,β,β-tetradeutero-5-MeO-DMT, a 5-MeO-DMT isotopologue that is resistant to metabolism by MAOA, produces a behavioral profile identical to that of 5-MeO-DMT in combination with an MAOA inhibitor (Halberstadt et al. 2012) supports the conclusion that MAOIs alter the response to 5-MeO-DMT by blocking its deamination.
Interactions between serotonergic and dopaminergic signaling may contribute to the delayed 5-HT2A-mediated behavioral response induced by 5-MeO-DMT and an MAOI. 5-HT2A receptors are expressed as heteroceptors on dopaminergic terminals, and evidence indicates that 5-HT2A can facilitate phasic dopamine release (Gobert and Millan, 1999). Activation of 5-HT2A has been observed to produce a delayed sensitization of the dopaminergic system (Marona-Lewicka and Nichols, 1997; Marona-Lewicka et al., 2009). It is possible that the high level of 5-HT2A activation produced by 5-MeO-DMT in the presence of an MAOI could induce a delayed sensitization of dopamine release, resulting in hyperactivity and disruption of PPI. The ability of 5-HT2A agonists to produce delayed effects on dopaminergic transmission would explain why 5-HT2A antagonists block the behavioral response to 5-MeO-DMT at ~45 min post-treatment, but not at earlier time-points, despite the fact that 5-MeO-DMT would occupy 5-HT2A receptors in the central nervous system immediately after administration. Future studies will examine whether the dopaminergic system contributes to the behavioral interactions between 5-MeO-DMT and MAOI.
The present findings confirm that 5-MeO-DMT can activate 5-HT2A receptors in vivo. We found the combined effect of 5-MeO-DMT and pargyline on PPI was partially dependent on 5-HT2A activation. Along the same lines, we had previously shown that in the presence of clorgyline, 5-MeO-DMT induces delayed hyperactivity via 5-HT2A (Halberstadt et al. 2008). It has also been reported that 5-MeO-DMT can elicit the head twitch response (HTR), a 5-HT2A-mediated behavior, in rats at doses ranging from 0.2–1 mg/kg (Bedard and Pycock 1977; Matthews and Smith 1980). Furthermore, although the discriminative stimulus effects of 5-MeO-DMT are primarily mediated by 5-HT1A (Spencer et al. 1987; Winter et al. 2000), 5-MeO-DMT can produce full substitution in rats trained to discriminate the 5-HT2 agonist DOM (Young et al.1980). Given that 5-MeO-DMT activates 5-HT2A in rats in vivo, it is surprising that the receptor normally does not contribute to the effects of 5-MeO-DMT on PPI and locomotor activity. One possible explanation is that activation of the 5-HT1A receptor by 5-MeO-DMT may attenuate its ability to induce behavioral responses via 5-HT2A. It is well known that the 5-HT1A receptor inhibits 5-HT2A-mediated behavioral responses (Arnt and Hyttel 1989; Darmani et al. 1990; Schreiber et al. 1995). Reports indicate that the concentration of 5-MeO-DMT required to activate 5-HT2A is on the order of several hundred nanomolar (Newton et al. 1996; Rabin et al. 2002; Kurrasch-Orbaugh et al. 2003; Strachan et al. 2010) or in the low micromolar range (Blair et al. 2000; Parrish and Nichols 2006). Therefore, the peak level of 5-MeO-DMT in the brain (144.3 nM) may not produce sufficient 5-HT2A activation to overcome the relatively potent countervailing effects mediated by 5-HT1A. It would appear, however, that in the presence of an MAO inhibitor 5-MeO-DMT accumulates in the brain at levels that are adequate to increase locomotor activity and disrupt PPI via 5-HT2A.
5-MeO-DMT can induce lateral head-weaving, tremor, and other symptoms of serotonin syndrome in rats (Grahame-Smith, 1971; Sloviter et al., 1978; Lucki et al., 1984). It has been reported that MAOIs can markedly intensify and prolong the serotonin syndrome induced by 5-MeO-DMT (Grahame-Smith, 1971; Ortman et al., 1980). It is unlikely, however, that 5-MeO-DMT-induced serotonin syndrome interfered with the assessment of the startle response in the present studies. First, the duration of the serotonin syndrome after combined administration of 5-MeO-DMT (1 mg/kg IP) and an MAOI is only 25–30 min (Ortman et al., 1980), which is shorter than the interval between drug treatment and startle testing in the present studies. Second, we have confirmed that the magnitude of the startle response is not significantly altered by treatment with pargyline and 5-MeO-DMT, demonstrating that the serotonin syndrome did not interfere with expression of the startle response.
The interaction between MAOIs and tryptamine hallucinogens is important from a toxicological perspective because these substances are used concurrently by humans. Use of ayahuasca, a hallucinogenic beverage containing DMT and MAOIs such harmaline and harmine, is currently increasing worldwide (Labate and Feeney, 2012; Lanaro et al., 2015). Furthermore, according to published accounts, 5-MeO-DMT can produce intense hallucinogenic effects when combined with harmaline and other MAOIs (Shulgin and Shulgin 1997; Ott 2001). The knowledge that MAOIs can alter the psychoactive effects of 5-MeO-DMT and other tryptamines has inspired recreational drug users to experiment with polypharmacy (Ott 1996), sometimes with tragic consequences. In one case, a man was found dead after ingesting 5-MeO-DMT, DMT, and β-carbolines in an ayahuasca-like preparation (Sklerov et al. 2005). In another instance, a 17-year old college student became extremely agitated and exhibited signs of hyperthermia, tachycardia, and rhabdomyolysis after consuming 5-MeO-DMT and an extract containing harmaline (Brush et al. 2004). The toxicity occurring in those cases may have been a consequence of MAOI effects on blood and brain levels of 5-MeO-DMT. 5-MeO-DMT inhibits 5-HT uptake at low micromolar concentrations (Berge et al. 1983; Chang et al. 1993; Nagai et al. 2007), an effect that could contribute to interactions with MAOA inhibitors and other 5-HT agonists. The present results indicate that inhibition of MAO would likely enhance the hallucinogenic effects of 5-MeO-DMT in humans by increasing 5-HT2A activation, but the high concentrations present in the brain would also interact with a variety of other targets, potentially producing adverse effects and toxicity. It is not clear to what extent the present findings with 5-MeO-DMT are relevant to DMT, but it is important to note that the latter drug is also deaminated by MAO (Suzuki et al., 1981). Although it is generally assumed that MAOIs contribute to the action of ayahuasca by allowing DMT to take effect orally, it is possible that MAO inhibition can also increase the accumulation of DMT in the central nervous system, similar to our findings with 5-MeO-DMT. Further studies are required to fully characterize the influence of MAOIs on the pharmacokinetics of DMT and the mechanism of action of ayahuasca.
Highlights.
5-MeO-DMT did not disrupt prepulse inhibition of startle (PPI) 45 min after administration to rats unless the animals were pretreated with an MAO-A inhibitor (MAOI)
The PPI disruption produced by 5-MeO-DMT and an MAOI was blocked by antagonists of 5-HT1A and 5-HT2A receptors
AOI pretreatment increased the level of 5-MeO-DMT in plasma and whole brain but had no effect on its metabolism to bufotenine
Acknowledgments
This work was supported by grants from NIMH (K01 MH100644), NIDA (R01 DA002925), and the Brain and Behavior Research Foundation. The analysis of 5-MeO-DMT and bufotenine was supported by NIDA (contracts N01DA-9-7767 and N01DA-14-7788) and was performed by Drs. David E. Moody and David M. Andrenyak at the Center for Human Toxicology at the University of Utah. I would like to thank Drs. Susan Powell and Mark Geyer for their assistance on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams LM, Geyer MA. Effects of DOM and DMT in a proposed animal model of hallucinogenic activity. Prog Neuropsychopharmacol Biol Psychiatry. 1985a;9:121–132. doi: 10.1016/0278-5846(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Adams LM, Geyer MA. A proposed animal model for hallucinogens based on LSD’s effects on patterns of exploration in rats. Behav Neurosci. 1985b;99:881–900. doi: 10.1037//0735-7044.99.5.881. [DOI] [PubMed] [Google Scholar]
- Agurell S, Holmstedt B, Lindgren JE. Alkaloid content of Banisteriopsis rusbyana. Am J Pharm Sci. 1968;140:148–151. [PubMed] [Google Scholar]
- Agurell S, Holmstedt B, Lindgren JE. Alkaloids of certain species of Virola and other South American plants of ethnopharmacologic interest. Acta Chem Scand. 1969a;23:903–916. doi: 10.3891/acta.chem.scand.23-0903. [DOI] [PubMed] [Google Scholar]
- Agurell S, Holmstedt B, Lindgren JE. Metabolism of 5-methoxy-N,-N dimethyltryptamine-14C in the rat. Biochem Pharmacol. 1969b;18:2771–2781. doi: 10.1016/0006-2952(69)90185-3. [DOI] [PubMed] [Google Scholar]
- Anonymous. Schedules of controlled substances: placement of 5-methoxy-N,N-dimethyltryptamine into Schedule I of the Controlled Substances Act. Final rule. Fed Regist. 2010;75:79296–79300. [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Facilitation of 8-OH DPAT-induced forepaw treading of rats by the 5-HT2 agonist DOI. Eur J Pharmacol. 1989;161:45–51. doi: 10.1016/0014-2999(89)90178-7. [DOI] [PubMed] [Google Scholar]
- Baisley SK, Fallace KL, Rajbhandari AK, Bakshi VP. Mutual independence of 5-HT2 and α1 noradrenergic receptors in mediating deficits in sensorimotor gating. Psychopharmacology (Berl) 2012;220:465–479. doi: 10.1007/s00213-011-2490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başkaya MK, Rao AM, Doğan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci Lett. 1997;226:33–36. doi: 10.1016/s0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- Bedard P, Pycock CJ. “Wet-dog” shake behavior in the rat: a possible quantitative model of central 5-hydroxytryptamine activity. Neuropharmacology 1977. 1977;16:663–670. doi: 10.1016/0028-3908(77)90117-4. [DOI] [PubMed] [Google Scholar]
- Berendsen HH, Jenck F, Broekkamp CL. Selective activation of 5HT1A receptors induces lower lip retraction in the rat. Pharmacol Biochem Behav. 1989;33:821–827. doi: 10.1016/0091-3057(89)90477-2. [DOI] [PubMed] [Google Scholar]
- Berge OG, Chacho D, Hole K. Inhibitory effect of 5-methoxy-N,N-dimethyltryptamine on the synaptosomal uptake of 5-hydroxytryptamine. Eur J Pharmacol. 1983;90:293–296. doi: 10.1016/0014-2999(83)90253-4. [DOI] [PubMed] [Google Scholar]
- Blair JB, Kurrasch-Orbaugh D, Marona-Lewicka D, Cumbay MG, Watts VJ, Barker EL, Nichols DE. Effect of ring fluorination on the pharmacology of hallucinogenic tryptamines. J Med Chem. 2000;43:4701–4710. doi: 10.1021/jm000339w. [DOI] [PubMed] [Google Scholar]
- Brush DE, Bird SB, Boyer EW. Monoamine oxidase inhibitor poisoning resulting from internet misinformation on illicit substances. J Toxicol Clin Toxicol. 2004;42:191–195. doi: 10.1081/clt-120030949. [DOI] [PubMed] [Google Scholar]
- Buckholtz NS, Boggan WO. Monoamine oxidase inhibition in brain and liver produced by β-carbolines: structure-activity relationships and substrate specificity. Biochem Pharmacol. 1977;26:1991–1996. doi: 10.1016/0006-2952(77)90007-7. [DOI] [PubMed] [Google Scholar]
- Cakic V, Potkonyak J, Marshall A. Dimethyltryptamine (DMT): subjective effects and patterns of use among Australian recreational users. Drug Alcohol Depend. 2010;111:30–37. doi: 10.1016/j.drugalcdep.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Chang AS, Chang SM, Starnes DM. Structure-activity relationships of serotonin transport: relevance to nontricyclic antidepressant interactions. Eur J Pharmacol. 1993;247:239–248. doi: 10.1016/0922-4106(93)90191-b. [DOI] [PubMed] [Google Scholar]
- Darmani NA, Martin BR, Pandey U, Glennon RA. Do functional relationships exist between 5-HT1A and 5-HT2 receptors? Pharmacol Biochem Behav. 1990;36:901–906. doi: 10.1016/0091-3057(90)90098-3. [DOI] [PubMed] [Google Scholar]
- Dumuis A, Sebben M, Bockaert J. Pharmacology of 5-hydroxytryptamine-1A receptors which inhibit cAMP production in hippocampal and cortical neurons in primary culture. Mol Pharmacol. 1988;33:178–186. [PubMed] [Google Scholar]
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT2A and 5-HT2C receptors. Synapse. 2000;35:144–150. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Eison AS, Wright RN. 5-HT1A and 5-HT2 receptors mediate discrete behaviors in the Mongolian gerbil. Pharmacol Biochem Behav. 1992;43:131–137. doi: 10.1016/0091-3057(92)90649-z. [DOI] [PubMed] [Google Scholar]
- Felner AE, Waldmeier PC. Cumulative effects of irreversible MAO inhibitors in vivo. Biochem Pharmacol. 1979;28:995–1002. doi: 10.1016/0006-2952(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Forsstrom T, Tuominen J, Karkkainen J. Determination of potentially hallucinogenic N-dimethylated indoleamines in human urine by HPLC/ESI-MS-MS. Scand J Clin Lab Invest. 2001;61:547–556. doi: 10.1080/003655101753218319. [DOI] [PubMed] [Google Scholar]
- Geyer MA. Approaches to the characterization of drug effects on locomotor activity in rodents. In: Adler MW, Cowan A, editors. Modern methods in pharmacology: testing and evaluation of drugs of abuse. Wiley-Liss; New York: 1990. pp. 81–99. [Google Scholar]
- Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Curr Protoc Neurosci. 1998:8.7.1–8.7.15. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Vollenweider FX. Serotonin research: contributions to understanding psychoses. Trends Pharmacol Sci. 2008;29:445–453. doi: 10.1016/j.tips.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Lyon RA, Slusher RM. N,N-di-n-propylserotonin: binding at serotonin binding sites and a comparison with 8-hydroxy-2-(di-n-propylamino)tetralin. J Med Chem. 1988;31:867–370. doi: 10.1021/jm00399a031. [DOI] [PubMed] [Google Scholar]
- Gobert A, Millan MJ. Serotonin (5-HT)2A receptor activation enhances dialysate levels of dopamine and noradrenaline, but not 5-HT, in the frontal cortex of freely-moving rats. Neuropharmacology. 1999;38:315–317. doi: 10.1016/s0028-3908(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith DG. Inhibitory effect of chlorpromazine on the syndrome of hyperactivity produced by L-tryptophan or 5-methoxy-N,N-dimethyltryptamine in rats treated with a monoamine oxidase inhibitor. Br J Pharmacol. 1971;43:856–864. doi: 10.1111/j.1476-5381.1971.tb07222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Meltzer HY. Thermoregulatory responses to serotonin (5-HT) receptor stimulation in the rat. Evidence for opposing roles of 5-HT2 and 5-HT1A receptors. Neuropharmacology. 1986a;25:1307–1313. doi: 10.1016/0028-3908(86)90101-2. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Koenig JI, Jackman H, Meltzer HY. Suppression of the hypo- and hyperthermic responses to 5-HT agonists following the repeated administration of monoamine oxidase inhibitors. Psychopharmacology (Berl) 1986b;90:403–407. doi: 10.1007/BF00179199. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL. Recent advances in the neuropsychopharmacology of serotonergic hallucinogens. Behav Brain Res. 2015;277:99–120. doi: 10.1016/j.bbr.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Buell MR, Masten VL, Risbrough VB, Geyer MA. Modification of the effects of 5-methoxy-N,N-dimethyltryptamine on exploratory behavior in rats by monoamine oxidase inhibitors. Psychopharmacology (Berl) 2008;201:55–66. doi: 10.1007/s00213-008-1247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Geyer MA. LSD but not lisuride disrupts prepulse inhibition in rats by activating the 5-HT2A receptor. Psychopharmacology (Berl) 2010;208:179–189. doi: 10.1007/s00213-009-1718-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Koedood L, Powell SB, Geyer MA. Differential contributions of serotonin receptors to the behavioral effects of indoleamine hallucinogens in mice. J Psychopharmacol. 2011;25:1548–1561. doi: 10.1177/0269881110388326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt AL, Nichols DE, Geyer MA. Behavioral effects of α,α,β,β-tetradeutero-5-MeO-DMT in rats: comparison with 5-MeO-DMT administered in combination with a monoamine oxidase inhibitor. Psychopharmacology (Berl) 2012;221:709–718. doi: 10.1007/s00213-011-2616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillegaart V, Estival A, Ahlenius S. Evidence for specific involvement of 5-HT1A and 5-HT2A/C receptors in the expression of patterns of spontaneous motor activity of the rat. Eur J Pharmacol. 1996;295:155–161. doi: 10.1016/0014-2999(95)00666-4. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in the modification of startle reaction in the rat. J Comp Physiol Psychol. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Holmstedt B, Lindgren JE. Chemical constituents and pharmacology of South American snuffs. In: Efron DH, Holmstedt B, Kline NS, editors. Ethnopharmacologic Search for Psychoactive Drugs. Washington: U.S. Goverment Printing Office; 1967. pp. 339–373. Public Health Service Publication No. 1645. [PubMed] [Google Scholar]
- Jiang XL, Shen HW, Mager DE, Yu AM. Pharmacokinetic interactions between monoamine oxidase A inhibitor harmaline and 5-methoxy-N,N-dimethyltryptamine, and the impact of CYP2D6 status. Drug Metab Dispos. 2013;41:975–86. doi: 10.1124/dmd.112.050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson C, Jackson DM, Zhang J, Svensson L. Prepulse inhibition of acoustic startle, a measure of sensorimotor gating: effects of antipsychotics and other agents in rats. Pharmacol Biochem Behav. 1995;52:649–654. doi: 10.1016/0091-3057(95)00160-x. [DOI] [PubMed] [Google Scholar]
- Johansson BB, Linder LE. Specific gravity of brain tissue during maturation. Acta Neurol Scand. 1982;66:575–581. doi: 10.1111/j.1600-0404.1982.tb03145.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Sablin SO, Ramsay RR. Inhibition of monoamine oxidase A by β-carboline derivatives. Arch Biochem Biophys. 1997;337:137–142. doi: 10.1006/abbi.1996.9771. [DOI] [PubMed] [Google Scholar]
- Kurrasch-Orbaugh DM, Watts VJ, Barker EL, Nichols DE. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J Pharmacol Exp Ther. 2003;304:229–237. doi: 10.1124/jpet.102.042184. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Paulus MP, Geyer MA. Effects of hallucinogens on locomotor and investigatory activity and patterns: influence of 5-HT2A and 5-HT2C receptors. Neuropsychopharmacology. 1998;18:339–351. doi: 10.1016/S0893-133X(97)00164-4. [DOI] [PubMed] [Google Scholar]
- Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA. The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats. Psychopharmacology (Berl) 2006;189:319–329. doi: 10.1007/s00213-006-0566-1. [DOI] [PubMed] [Google Scholar]
- Labate BC, Feeney K. Ayahuasca and the process of regulation in Brazil and internationally: implications and challenges. Int J Drug Policy. 2012;23:154–161. doi: 10.1016/j.drugpo.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Lanaro R, Calemi DB, Togni LR, Costa JL, Yonamine M, de Cazenave SO, Linardi A. Ritualistic use of ayahuasca versus street use of similar substances seized by the police: a key factor involved in the potential for intoxications and overdose? J Psychoactive Drugs. 2015;47:132–139. doi: 10.1080/02791072.2015.1013202. [DOI] [PubMed] [Google Scholar]
- Lucki I, Nobler MS, Frazer A. Differential actions of serotonin antagonists on two behavioral models of serotonin receptor activation in the rat. J Pharmacol Exp Ther. 1984;228:133–139. [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl) 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Chemel BR, Nichols DE. Dopamine D4 receptor involvement in the discriminative stimulus effects in rats of LSD, but not the phenethylamine hallucinogen DOI. Psychopharmacology (Berl) 2009;203:265–277. doi: 10.1007/s00213-008-1238-0. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Nichols DE. 5-HT2A/2C receptor agonists potentiate the discriminative cue of (+)-amphetamine in the rat. Neuropharmacology. 1997;36:1471–1475. doi: 10.1016/s0028-3908(97)00106-8. [DOI] [PubMed] [Google Scholar]
- Martin R, Schurenkamp J, Gasse A, Pfeiffer H, Kohler H. Determination of psilocin, bufotenine LSD, and its metabolites in seum, plasma, and urine by SPE-LC-MS/MS. Int J Legal Med. 2013;127:593–601. doi: 10.1007/s00414-012-0796-1. [DOI] [PubMed] [Google Scholar]
- Matthews WD, Smith CD. Pharmacological profile of a model for central serotonin receptor activation. Life Sci. 1980;26:1397–1403. doi: 10.1016/0024-3205(80)90042-9. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Repke DB, Peroutka SJ. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology. 1990;29:193–198. doi: 10.1016/0028-3908(90)90001-8. [DOI] [PubMed] [Google Scholar]
- McKenna DJ, Towers GH, Abbott F. Monoamine oxidase inhibitors in South American hallucinogenic plants: tryptamine and beta-carboline constituents of ayahuasca. J Ethnopharmacol. 1984;10:195–223. doi: 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- Nagai F, Nonaka R, Satoh Hisashi Kamimura K. The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain. Eur J Pharmacol. 2007;559:132–137. doi: 10.1016/j.ejphar.2006.11.075. [DOI] [PubMed] [Google Scholar]
- Narasimhachari N, Callison D, Lin RL. Effect of MAO inhibitors on the tissue distribution of dimethyltryptamine, 5-methoxydimethyltryptamine and bufotenin after their intraperitoneal administration in rat. Res Comm Psychol Psychiatry Behav. 1979;4:257–268. [Google Scholar]
- Newman MC, Evans D. Uncensor Program. 2005 Http://www.vims.edu/env/research/software/vims_software.html#uncensor.
- Newton RA, Phipps SL, Flanigan TP, Newberry NR, Carey JE, Kumar C, McDonald B, Chen C, Elliott JM. Characterisation of human 5-hydroxytryptamine2A and 5-hydroxytryptamine2C receptors expressed in the human neuroblastoma cell line SH-SY5Y: comparative stimulation by hallucinogenic drugs. J Neurochem. 1996;67:2521–2331. doi: 10.1046/j.1471-4159.1996.67062521.x. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ortmann R, Waldmeier PC, Radeke E, Felner A, Delini-Stula A. The effects of 5-HT uptake- and MAO-inhibitors on L-5-HTP-induced excitation in rats. Naunyn Schmiedebergs Arch Pharmacol. 1980;311:185–192. doi: 10.1007/BF00510258. [DOI] [PubMed] [Google Scholar]
- Ott J. Pharmahuasca: on phenethylamines and potentiation. MAPS Bull. 1996;6:32–34. http://www.maps.org/news-letters/v06n3/06332ott.html (retrieved September 30, 2014) [Google Scholar]
- Ott J. Pharmahuasca: human pharmacology of oral DMT plus harmine. J Psychoactive Drugs. 1999;31:171–177. doi: 10.1080/02791072.1999.10471741. [DOI] [PubMed] [Google Scholar]
- Ott J. Pharmepéna-Psychonautics: human intranasal, sublingual and oral pharmacology of 5-methoxy-N,N-dimethyl-tryptamine. J Psychoactive Drugs. 2001;33:403–407. doi: 10.1080/02791072.2001.10399925. [DOI] [PubMed] [Google Scholar]
- Padich RA, McCloskey TC, Kehne JH. 5-HT modulation of auditory and visual sensorimotor gating: II. Effects of the 5-HT2A antagonist MDL 100,907 on disruption of sound and light prepulse inhibition produced by 5-HT agonists in Wistar rats. Psychopharmacology (Berl) 1996;124:107–116. doi: 10.1007/BF02245610. [DOI] [PubMed] [Google Scholar]
- Pálenícek T, Balíková M, Bubeníková-Valesová V, Horácek J. Mescaline effects on rat behavior and its time profile in serum and brain tissue after a single subcutaneous dose. Psychopharmacology (Berl) 2008;196:51–62. doi: 10.1007/s00213-007-0926-5. [DOI] [PubMed] [Google Scholar]
- Parrish JC, Nichols DE. Serotonin 5-HT2A receptor activation induces 2-arachidonoylglycerol release through a phospholipase c-dependent mechanism. J Neurochem 2006. 2006 Nov;99(4):1164–75. doi: 10.1111/j.1471-4159.2006.04173.x. [DOI] [PubMed] [Google Scholar]
- Pehek EA, Nocjar C, Roth BL, Byrd TA, Mabrouk OS. Evidence for the preferential involvement of 5-HT2A serotonin receptors in stress- and drug-induced dopamine release in the rat medial prefrontal cortex. Neuropsychopharmacology. 2006;31:265–277. doi: 10.1038/sj.npp.1300819. [DOI] [PubMed] [Google Scholar]
- Ouagazzal A, Grottick AJ, Moreau J, Higgins GA. Effect of LSD on prepulse inhibition and spontaneous behavior in the rat. A pharmacological analysis and comparison between two rat strains. Neuropsychopharmacology. 2001;25:565–75. doi: 10.1016/S0893-133X(01)00282-2. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-induced deficits in automatic and controlled inhibition are attenuated by ketanserin in healthy human volunteers. Neuropsychopharmacology. 2012;37:630–640. doi: 10.1038/npp.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin RA, Regina M, Doat M, Winter JC. 5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens. Pharmacol Biochem Behav. 2002;72:29–37. doi: 10.1016/s0091-3057(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Riba J, McIlhenny EH, Bouso JC, Barker SA. Metabolism and urinary disposition of N,N-dimethyltryptamine after oral and smoked administration: a comparative study. Drug Test Anal 2015. 2015 May;7(5):401–6. doi: 10.1002/dta.1685. [DOI] [PubMed] [Google Scholar]
- Rigdon GC, Weatherspoon JK. 5-Hydroxytryptamine1a receptor agonists block prepulse inhibition of acoustic startle reflex. J Pharmacol Exp Ther. 1992;263:486–493. [PubMed] [Google Scholar]
- Roth BL, Choudhary MS, Khan N, Uluer AZ. High-affinity agonist binding is not sufficient for agonist efficacy at 5-hydroxytryptamine2A receptors: evidence in favor of a modified ternary complex model. J Pharmacol Exp Ther. 1997;280:576–583. [PubMed] [Google Scholar]
- Sanchez C, Arnt J, Moltzen E. Assessment of relative efficacies of 5-HT1A receptor ligands by means of in vivo animal models. Eur J Pharmacol. 1996;315:245–254. doi: 10.1016/s0014-2999(96)00621-8. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Brocco M, Audinot V, Gobert A, Veiga S, Millan MJ. (1-(2,5-Dimethoxy-4 iodophenyl)-2-aminopropane)-induced head-twitches in the rat are mediated by 5-hydroxytryptamine (5-HT)2A receptors: modulation by novel 5-HT2A/2C antagonists, D1 antagonists and 5-HT1A agonists. J Pharmacol Exp Ther. 1995;273:101–112. [PubMed] [Google Scholar]
- Shen HW, Jiang XL, Winter JC, Yu AY. Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr Drug Metabol. 2010a;11:659–666. doi: 10.2174/138920010794233495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Jiang XL, Yu AM. Nonlinear pharmacokinetics of 5-methoxy-N,N-dimethyltryptamine in mice. Drug Metab Dispos. 2011;39:1227–1234. doi: 10.1124/dmd.111.039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Wu C, Jiang XL, Yu AM. Effects of monoamine oxidase inhibitor and cytochrome P450 2D6 status on 5-methoxy-N,N-dimethyltryptamine metabolism and pharmacokinetics. Biochem Pharmacol. 2010b;80:122–128. doi: 10.1016/j.bcp.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin AT, Shulgin A. TIHKAL: the continuation. Transform Press; Berkeley: 1997. [Google Scholar]
- Sipes TA, Geyer MA. Multiple serotonin receptor subtypes modulate prepulse inhibition of the startle response in rats. Neuropharmacology. 1994;33:441–448. doi: 10.1016/0028-3908(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Sipes TE, Geyer MA. DOI disruption of prepulse inhibition of startle in the rat is mediated by 5-HT2A and not by 5-HT2C receptors. Behav Pharmacol. 1995;6:839–842. [PubMed] [Google Scholar]
- Sitaram BR, Lockett L, Talomsin R, Blackman GL, McLeod WR. In vivo metabolism of 5-methoxy-N,N-dimethyltryptamine and N,N-dimethyltryptamine in the rat. Biochem Pharmacol. 1987;36:1509–1512. doi: 10.1016/0006-2952(87)90118-3. [DOI] [PubMed] [Google Scholar]
- Sklerov J, Levine B, Moore KA, King T, Fowler D. A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an Ayahuasca preparation. J Anal Toxicol. 2005;29:838–841. doi: 10.1093/jat/29.8.838. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Drust EG, Connor JD. Specificity of a rat behavioral model for serotonin receptor activation. J Pharmacol Exp Ther. 1978;206:339–347. [PubMed] [Google Scholar]
- Smith CG. The specific gravity of the brain of the male albino rat. J Comp Neurol. 1930;50:97–108. [Google Scholar]
- Smith LM, Peroutka SJ. Differential effects of 5-hydroxytryptamine1a selective drugs on the 5-HT behavioral syndrome. Pharmacol Biochem Behav. 1986;24:1513–1519. doi: 10.1016/0091-3057(86)90477-6. [DOI] [PubMed] [Google Scholar]
- Spencer DG, Jr, Glaser T, Traber J. Serotonin receptor subtype mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N, N-dimethyltryptamine. Psychopharmacology (Berl) 1987;93:158–166. doi: 10.1007/BF00179927. [DOI] [PubMed] [Google Scholar]
- Squires RF. Evidence that 5-methoxy-N,N-dimethyltryptamine is a specific substrate for MAO-A in the rat: implications for the indoleamine dependent behavioral syndrome. J Neurochem. 1975;24:47–50. doi: 10.1111/j.1471-4159.1975.tb07626.x. [DOI] [PubMed] [Google Scholar]
- Strachan RT, Sciaky N, Cronan MR, Kroeze WK, Roth BL. Genetic deletion of p90 ribosomal S6 kinase 2 alters patterns of 5-hydroxytryptamine2A serotonin receptor functional selectivity. Mol Pharmacol. 2010;77:327–338. doi: 10.1124/mol.109.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki O, Katsumata Y, Oya M. Characterization of eight biogenic indoleamines as substrates for type A and type B monoamine oxidase. Biochem Pharmacol. 1981;30:1353–1358. [PubMed] [Google Scholar]
- Takahashi T, Takahashi K, Ido T, Yanai K, Iwata R, Ishiwata K, Nozoe S. 11C-Labeling of indolealkylamine alkaloids and the comparative study of their tissue distributions. Int J Appl Radiat Isot. 1985;36:965–969. doi: 10.1016/0020-708x(85)90257-1. [DOI] [PubMed] [Google Scholar]
- Tilson HA, Baker TG, Chamberlain JH. Behavioral and neuropharmacological analysis of amphetamine and 2,5-dimethoxy-4-methylamphetamine in rats. Psychopharmacologia. 1975;44:229–39. doi: 10.1007/BF00428899. [DOI] [PubMed] [Google Scholar]
- Tricklebank MD, Forler C, Middlemiss DN, Fozard JR. Subtypes of the 5-HT receptor mediating the behavioral responses to 5-methoxy-N,N-dimethyltryptamine in the rat. Eur J Pharmacol. 1985;117:15–24. doi: 10.1016/0014-2999(85)90467-4. [DOI] [PubMed] [Google Scholar]
- Turner WJ, Merlis S. Effect of some indolealkylamines on man. Arch Neurol Psychiatry. 1959;81:121–129. doi: 10.1001/archneurpsyc.1959.02340130141020. [DOI] [PubMed] [Google Scholar]
- van den Buuse M, Ruimschotel E, Martin S, Risbrough VB, Halberstadt AL. Enhanced effects of amphetamine but reduced effects of the hallucinogen, 5-MeO-DMT, on locomotor activity in 5-HT1A receptor knockout mice: implications for schizophrenia. Neuropharmacology. 2011;61:209–216. doi: 10.1016/j.neuropharm.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The effects of the preferential 5-HT2A agonist psilocybin on prepulse inhibition of startle in healthy human volunteers depend on interstimulus interval. Neuropsychopharmacology. 2007;32:1876–1887. doi: 10.1038/sj.npp.1301324. [DOI] [PubMed] [Google Scholar]
- Wing LL, Tapson GS, Geyer MA. 5HT-2 mediation of acute behavioral effects of hallucinogens in rats. Psychopharmacology (Berl) 1990;100:417–425. doi: 10.1007/BF02244617. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Young R, Glennon RA, Rosecrans JA. Discriminative stimulus properties of the hallucinogenic agent DOM. Commun Psychopharmacol. 1980;4:501–506. [PubMed] [Google Scholar]
- Young R, Rosecrans JA, Glennon RA. Behavioral effects of 5-methoxy-N,N-dimethyltryptamine and dose-dependent antagonism by BC-105. Psychopharmacology (Berl) 1983;80:156–160. doi: 10.1007/BF00427960. [DOI] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Herraiz T, Kupfer A, Gonzalez FJ. Screening for endogenous substrates reveals that CYP2D6 is a 5-methoxyindolethylamine O-demethylase. Pharmacogenetics. 2003;13:307–319. doi: 10.1097/01.fpc.0000054094.48725.b7. [DOI] [PubMed] [Google Scholar]
- Zhang D, Fan C, Zhang J, Zhang CH. Nonparametric methods for measurements below detection limit. Statist Med. 2009;28:700–715. doi: 10.1002/sim.3488. [DOI] [PubMed] [Google Scholar]