Abstract
Herbal medicines have been recognized as an attractive approach for cancer therapy with minimal side effects. The present study investigated the type of interaction between a novel lipid-soluble extract from Pinellia pedatisecta Schott (PE) and cisplatin (CDDP) on human cervical cancer SiHa and CaSki cell lines in vitro. The mechanism of this combination was studied using cell proliferation, invasion and apoptosis assays, and by analyzing cell cycle distribution and protein expression, with a focus on DNA damage response (DDR) activation. Equipotent combinations of PE and CDDP were determined by isobologram analysis, in order to evaluate potential synergy. The combination index for SiHa cells was 0.43, and the index for CaSki cells was 0.68, indicating synergy. Treatment with PE and CDDP combined resulted in a significantly greater inhibition of invasion in the two cells, compared with either drug alone (SiHa, P<0.01; CaSki, P<0.001). This co-treatment induced significantly more apoptosis in the two cell lines, and arrested cells at the G0/G1 phase and G2/M phase in SiHa and CaSki, respectively, with a significant decrease (P<0.01) in S phase cells in the two cell lines. Combined PE and CDDP targeting synergistically enhanced the expression of markers of DDR (phosphorylation of ataxia-telangiectasia mutated, checkpoint kinase (Chk)-1, Chk-2, and γ-H2A histone family member X) in cells. These results suggest that PE and cisplatin act synergistically in cervical cancer cells with high DDR activation. The approach presented in the present study may have important implications for the pharmacological mechanism of Pinellia pedatisecta Schott and cervical cancer therapeutic strategies.
Keywords: Pinellia pedatisecta Schott, PE, cisplatin, cervical carcinoma, synergistic
Introduction
Cervical cancer is one of the most prevalent malignancies among women, particularly in developing countries, with ~527,600 new cases and ~265,700 mortalities in 2012 worldwide (1). In addition, the age-standardized incidence of cervical cancer has been >4 per 100,000 (1998–2002) in China (2), with age-standardized 5-year (2005–09) net survival of ~60% (3,4). It therefore remains a challenge for the Chinese government to prevent this disease. Although the first-line treatment for early stages of cervical cancer is surgical excision, concomitant platinum-based chemoradiotherapy remains a curative treatment for local advanced cervical cancer, particularly for distant control of the disease (5,6).
As one of the first-line chemotherapeutic agents in the treatment of cervical cancer, cis-dichlorodiamine platinum-II, also termed cisplatin (CDDP), exerts its cytotoxic effect predominantly by formatting an intra-strand cross-linking on DNA that blocks transcription and DNA replication, resulting in DNA damage response (DDR) (7,8). However, the chemotherapeutic use of CDDP is limited by its severe side effects and drug resistance (9,10). Combining CDDP with new anti-cancer agents has been studied to improve this clinical dilemma. Such combined treatments enable a lower cytotoxic dose of CDDP without affecting the therapeutic benefits. The purpose of the present study was to investigate a novel lipid-soluble extract (PE) from Pinellia pedatisecta Schott, which is a traditional Chinese medicine (TCM).
The research on Pedate pinellia Rhizome can be traced back to the 1970s, when 247 cases of cervical cancer were treated, and the total effective rate was 81.5% (11). The present study was focused on PE, which was extracted by the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. Unlike Western medicine, which generally uses purified compounds and aims to target a single molecule, TCM compositions usually contain multiple herbs and components that are necessary for efficacy (12,13). Initial studies identified the key constituents of the lipid-soluble extract as alkaloids, fatty acids and β-sitosterol (11,14). However, the key constituents are not necessarily efficacious or therapeutic for cervical cancer. Limited by TCM ingredient analytical technology, study of the exact curative component is progressing slowly in the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. Previous studies have evaluated the cytotoxic effect of PE on cervical cancer cell lines (15). The present study identified that PE could synergistically enhance the cytotoxicity of CDDP against CaSki cell growth in xenograft tumors in vivo. However, little is known about the effect of this combination on the malignant biological behavior of cervical cell lines and the underlying mechanisms. The present study aimed to assess the synergistic effect of PE when combined with CDDP on human cervical cancer cell lines in vitro and the potential mechanism on the DDR pathway.
Materials and methods
Reagents
Dried rhizomes of Pinellia pedatisecta Schott were purchased from Xuchang Pharmaceutical Corporation (Henan, China) in June 2013, and authenticated by Professor Jin-gui Shen of the Shanghai Institute of Materia Medica, Chinese Academy of Sciences. The extracting technique and process of PE have been described in detail in a previous study (15). A voucher specimen was deposited in the herbarium of the Shanghai Institute of Materia Medica. PE was stored in a freezer at −80°C. The PE was then dissolved in dimethyl sulfoxide at a concentration of 500 µg/µl, and stored at 4°C for a week prior to use. CDDP was purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Rabbit monoclonal antibodies directed against cleaved-PARP (dilution, 1:1,000; #9185), cleaved-caspase-3 (dilution, 1:1,000; #9664), phosphorylated ataxia-telangiectasia mutated (ATM; Ser1918; dilution, 1:1,000; #5883) phosphorylated-checkpoint kinase (Chk)-1 (Ser345) (dilution, 1:1,000; #2348), phosphorylated-Chk-2 (Ser68) (dilution, 1:1,000; #2197) and -H2A histone family member X (H2AX) (Ser139) (dilution, 1:1,000; #9718) were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). The anti-GAPDH antibody (dilution, 1:5,000; #9482) was obtained from Abcam (Cambridge, MA, USA). The Cell Counting Kit-8 (CCK-8) was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan) and the AlexaFluor 488 Annexin V/Dead Cell Apoptosis kit was obtained from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Cell culture
The human cervical cancer cell lines SiHa and CaSki were obtained from the American Type Culture Collection (Manassas, VA, USA) and resuscitated by the Cell Bank of the Chinese Academy of Science (Shanghai, China). SiHa and CaSki cells were routinely cultured in minimum essential medium and RPMI-1640 medium, respectively, with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 IU/ml penicillin G, and 100 mg/ml streptomycin sulfate (Sigma-Aldrich; Merck Millipore). The incubation conditions were 37°C under 5% CO2 and 95% air atmosphere at constant humidity. The culture medium was changed every other day.
Cell proliferation assay and isobologram analysis
To obtain the appropriate concentration of PE and CDDP in the following experiment, the half-maximal inhibitory concentration (IC50) was determined in the CaSki and SiHa cell lines. CaSki cells cultured in 96-well plates were treated with various concentrations of PE (0, 100, 200, 400 and 800 µg/ml), or CDDP (0, 1.25, 2.5, 5, 10, 20, 40, 60 and 80 µg/ml) for 24, 48 and 72 h. SiHa cells cultured in 96-well plates were treated with various concentrations of PE (0, 100, 200, 400, 800 and 1,600 µg/ml) or CDDP (0, 1.25, 2.5, 5, 10, 20, 40 and 60 µg/ml) for the same time periods. CCK-8 assay was performed to investigate the cytotoxicity of PE and CDDP on CaSki and SiHa, following treatment for different time periods. Cell viability percentage was determined relative to the control. Each experiment was performed in 6 replicate wells for each drug concentration. Cell growth curves were drawn and the IC50 values at 48 h were calculated with CalcuSyn software analysis (Version 2.1; Biosoft, Cambridge, UK).
CaSki and SiHa cells were seeded onto 96-well plates at a cell density of 50,000 cells per well in 100 µl cell culture medium, 24 h prior to being treated with different doses of PE, CDDP or a combination of the two. For the SiHa cells, six concentrations of PE (15.6, 31.2, 62.5, 125, 250 and 500 µg/ml) and CDDP (2.5, 5, 10, 20, 40 and 80 µg/ml) were assayed. However, for the CaSki cells, five concentrations of PE (15.6, 31.2, 62.5, 125 and 250 µg/ml) and CDDP (2.5, 5, 10, 20 and 40 µg/ml) were assayed. The relation ratio between the mixtures was 6:1. After treatment for 48 h, the cell culture medium was replaced with 100 µl fresh medium containing 10 µl CCK-8 solution and incubation continued at 37°C for 2 h. The absorbance was then measured at 450 nm using the Multiskan MK3 (Thermo Fisher Scientific, Inc.). The percentage of cell proliferation was determined relative to the control. Each experiment was performed in six replicate wells for each drug concentration.
Interactions between PE and CDDP were evaluated by the isobologram analysis method, which was applied to determine additive, synergistic and antagonistic effects (16).
Cell invasion assay and cell anti-proliferation assay
Cell invasion assays were conducted with 8 µm hanging cell culture inserts (Millipore Filter Corporation; Merck Millipore, Bedford, MA, USA) with polycarbonate membrane. Each insert was coated with 80 µl of Matrigel (BD Biosciences, San Jose, CA, USA), followed by incubation at 37°C for 4 h. Cells were trypsinized and adjusted to 2×105 cells/ml in serum-free medium with PE (125 µg/ml for CaSki and 250 µg/ml for SiHa), CDDP (20 µg/ml for CaSki and 40 µg/ml for SiHa), or a combination of the two. Cells (500 µl) were seeded in the upper chambers of 24-well plates, and 600 µl of 10% FBS medium was added to the bottom of the chamber and incubated for 48 h in the invasion assay. The cells were then fixed with 4% paraformaldehyde for 10 min at room temperature and the Matrigel was wiped off the upper surface. The cells were then stained with 0.5% crystal violet solution for 5 min prior to rinsing with PBS. The number of migratory cells was counted in 3 random fields for each group using a light microscope (magnification, ×100) and presented as a percentage compared with the control. For each treatment, three repetitions were performed.
Assessment of apoptosis and cell cycle distribution
Apoptosis was detected by flow cytometry via the examination of altered plasma membrane phospholipid packing, using the lipophilic dye Annexin V. Cells were treated with PE, CDDP or a combination of the two, as aforementioned in the cell invasion assay, and harvested for 48 h after treatment. The cells were successively washed with cold PBS and binding buffer, and were subsequently re-suspended in binding buffer at a concentration of 1×106 cells/ml. Thereafter, 5 µl of Annexin V-FITC and 1 µl of propidium iodide (PI) were added to 200 µl of cell suspension and incubated for 15 min at room temperature, avoiding light. Following the addition of 300 µl of binding buffer, the labeled cells were immediately counted by flow cytometry. All early apoptotic cells (Annexin V-positive, propidium iodide-negative), necrotic/late apoptotic cells (double positive) and living cells (double negative) were detected using a FACSCalibur flow cytometer (BD Biosciences) and subsequently analyzed by FlowJo software 7.6.1 (BD Biosciences).
For cell cycle analyses, treated cells were fixed in 75% ethanol for 24 h, at −20°C. Following centrifugation at 352 × g for 5 min at 4°C, the cells were collected and re-suspended in PBS (400 µl), RNase A (10 mg/ml, 50 µl), and PI (2 mg/ml, 10 µl). The mixtures were incubated in the dark at 37°C for 30 min prior to flow cytometric analysis by a Beckman Coulter CyAn ADP flow cytometer (Beckman Coulter, Inc., Brea, CA, USA).
Western blot analysis
Treated cells from a 60-mm dish were lysed in 50 µl radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 1% NP40; 0.25% Na-deoxycholate; 1 mM EDTA; 1 mM phenylmethane sulfonyl fluoride; and protease inhibitor cocktail). Following 15 min of centrifugation (2880 × g at 4°C), the supernatant was collected and the concentration of total protein was measured using a Bio-Rad (Bio-Rad Laboratories, Inc., Hercules, CA, USA) protein assay kit. An aliquot of 30 µg total protein was resolved in a 10% sodium dodecyl sulfate polyacrylamide gel and transferred to a polyvinylidene membrane. The membrane was blocked in 5% nonfat milk in PBS with Tween-20 (PBST; 20 mM Tris-HCl; 150 mM NaCl; 0.05% Tween-20) at room temperature for 60 min. The membranes were then incubated with the aforementioned primary antibodies overnight at 4°C. The membrane was then washed 3 times with PBST and incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (#7170S; Cell Signaling Technology, Inc.) at a 1:10,000 dilution for 1 h at room temperature. The membranes were then washed 3 times for 60 min. The specific protein bands were visualized using electrochemiluminescence western blotting substrate (Pierce; Thermo Fisher Scientific, Inc.) and imaged using ImageQuant™ LAS4000 (GE Healthcare Life Sciences, Chalfont, UK). Protein levels were normalized to GAPDH as a reference.
Statistical analysis
For isobologram analysis, CalcuSyn 2.1 was used, and the combination index values <1, =1 and >1 were used to indicate synergy, additivity and antagonism, respectively. Statistical analysis of the data was performed using either GraphPad Prism 6 software (GraphPad Software, La Jolla, CA, USA) or SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ± standard deviation of three independent experiments. Differences among groups were analyzed statistically with one-way analysis of variance, followed by the Bonferroni post hoc test for multiple comparisons. A unpaired two-tailed Student's t-test was applied to identify significant differences between two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Synergistic interaction between PE and CDDP
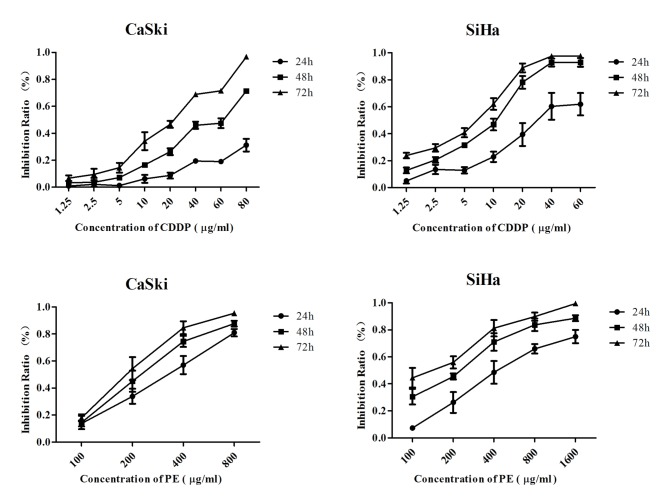
Subsequent to treatment with different concentrations of PE and CDDP for 72 h, cell growth curves were drawn. As shown in Fig. 1, cytotoxicity of PE and CDDP was in a time and dose-dependent manner on CaSki and SiHa cell lines. IC50 at 48 h was calculated by CalcuSyn software. For CaSki cells, the IC50 of CDDP was 40 µg/ml and that of PE was 250 µg/ml, while for SiHa cells, the IC50 of CDDP was 80 µg/ml and that of PE was 500 µg/ml.
Figure 1.
Cell growth curves following treatment with PE or CDDP for 24, 48 and 72 h. CDDP, cisplatin; PE, lipid-soluble extract from Pinellia pedatisecta.
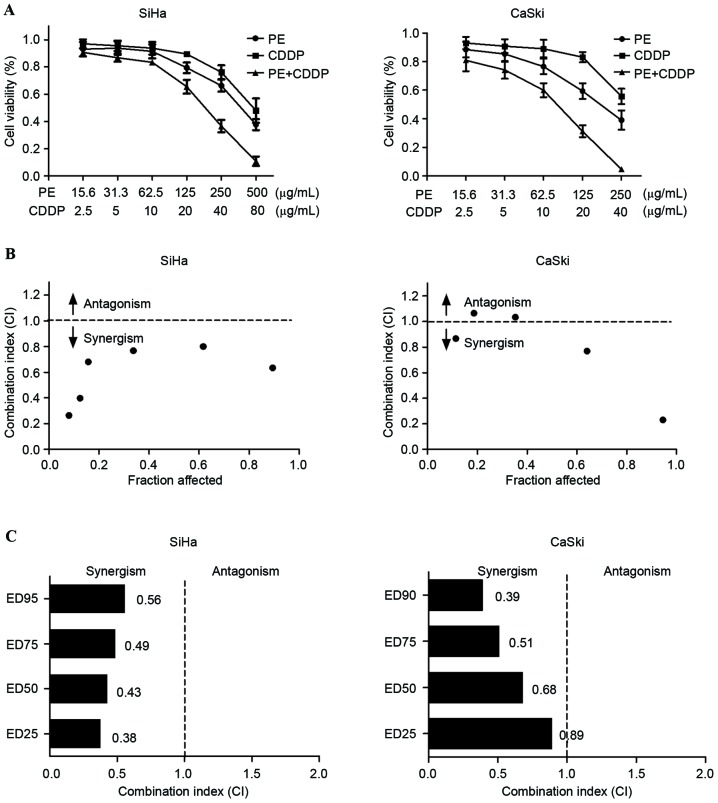
The inhibitory effects of the combined use of PE and CDDP were tested in two cervical cancer cell lines, SiHa and CaSki cells. To determine whether PE and CDDP exhibit a combined effect in cervical cancer cells, the effect of individual and combination treatment with PE and CDDP after 48 h of exposure was examined, using the CCK-8 assay. As shown in Fig. 2A, the two compounds inhibited cell growth in a dose-dependent manner in SiHa and CaSki cells. Isobologram analysis was performed to evaluate whether PE and CDDP interact synergistically (Fig. 2B). This analysis provides a combination index (CI) value that measures the degree of interaction between two or more drugs, where a CI <1 indicates synergism and CI >1 antagonism. CI values of 0.43 and 0.68 were identified for SiHa and CaSki cells when the effective dose (ED) of the two agents inhibited cell viability by 50% (Fig. 2C). Irrespective of high cytotoxicity (ED90) or low cytotoxicity (ED25), the CI value remained below 1, indicating that synergism occurs independently of the equipotency levels of PE and CDDP. Results of the present study demonstrate that PE and CDDP exhibit a synergistic interaction in cervical cancer cell lines.
Figure 2.
PE and CDDP exhibit synergistic cytotoxicity in SiHa and CaSki cells. (A) Cell viability was measured by the Cell Counting Kit-8 assay. SiHa and CaSki cells were treated with the indicated concentrations of PE and CDDP for 48 h. (B) Isobologram analysis of combination treatment with PE and CDDP in SiHa and CaSki cells. The line designates the CI, where CI =1 (additive effect), CI <1 indicates synergism and CI >1 represents antagonism. (C) The CI values for a combination of PE and CDDP at a range of ED. The CI at ED25, ED50, ED75 and ED90 indicate a synergistic interaction between PE and CDDP in SiHa and CaSki cells. CDDP, cisplatin; CI, combination index; ED, effective dose; PE, lipid-soluble extract from Pinellia pedatisecta Schott.
Combination of CDDP with PE inhibits cervical cancer cell invasion
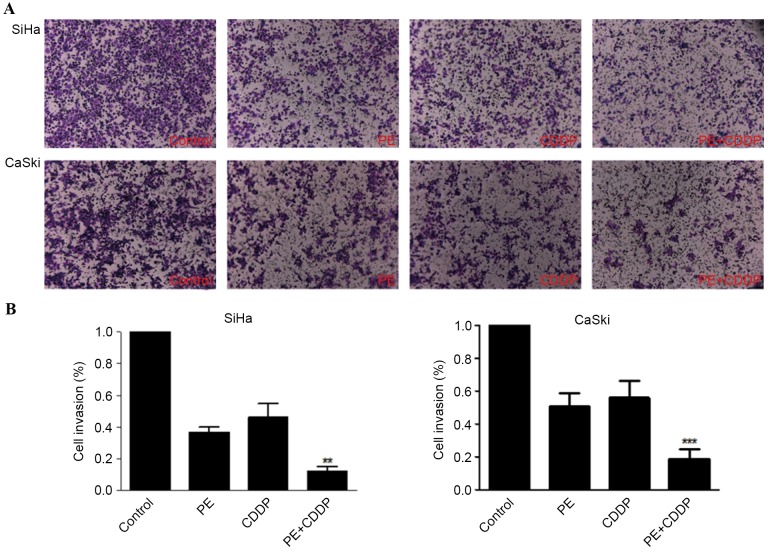
To test whether the combination of PE plus CDDP results in decreased cell invasion compared with either single agent alone, Matrigel invasion chambers were used. Results in Fig. 3 show that, for SiHa and CaSki cell lines, PE alone caused ~63 and 49% reduction in invasion compared with the control, while the combination treatment decreased cell invasion by ~88 and 81%, respectively.
Figure 3.
Combination of PE with CDDP decreases cell invasion in SiHa and CaSki cell lines. Cells were treated with PE, CDDP, and their combination. (A) Cells were plated on Matrigel invasion chambers and invading cells were counted using light microscopy (magnification, ×100). Columns represent the means of three identical wells of a single representative experiment. Bars represent the upper 95% confidence interval; **P<0.05 and ***P<0.001, for comparisons between cells treated with the combined treatment and cells treated with CDDP. CDDP, cisplatin; PE, lipid-soluble extract from Pinellia pedatisecta Schott.
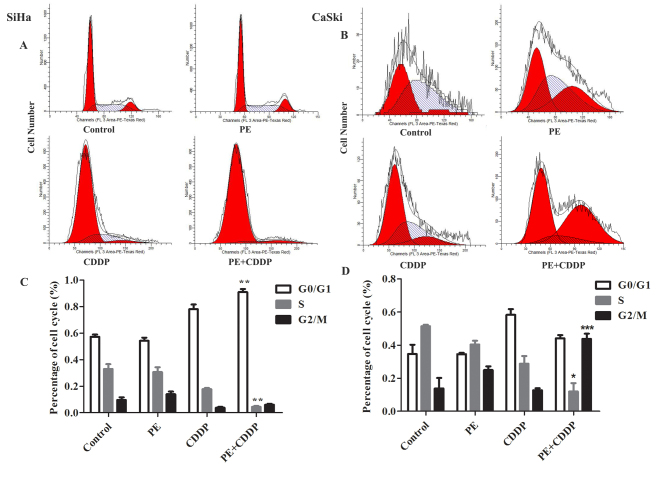
Combination of CDDP with PE increases cervical cancer cell apoptosis and G0/G1 or G2/M phase arrest
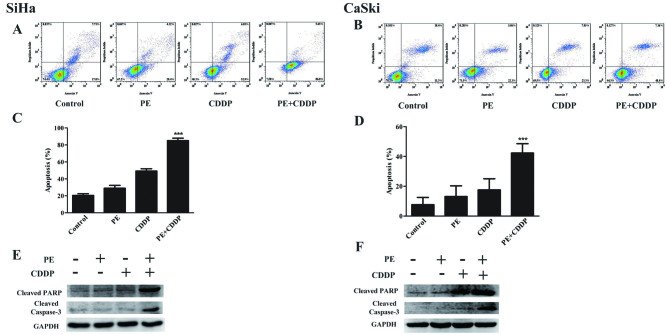
To determine whether the increased anti-proliferative effect was due to increased apoptosis and/or cell cycle alterations, apoptosis was examined using Annexin V analysis, following PE and CDDP treatment (Fig. 4). The number of apoptotic cells was quantified using flow cytometry (Fig. 4A and B). As shown in Fig. 4C and D, flow cytometric analysis of SiHa and CaSki cells revealed that PE and CDDP increased the number of apoptotic cells compared with that observed in untreated cells. Additionally, the combined targeting significantly enhanced SiHa and CaSki cell apoptosis to 86.8 and 48.4%, respectively. These results were confirmed by western blot analysis. The combination of PE with CDDP was accompanied by increased expression of cleaved PARP and caspase-3 (Fig. 4E and F). As shown in Fig. 5, a significant increase (P<0.001) in G2/M-phase cells following treatment with CDDP plus PE was observed compared with the single treatment in CaSki cells. For SiHa cells, a significant increase (P<0.01) in G0/G1-phase cells was observed. This cell cycle delay was also accompanied by a decreased percentage of S-phase cells.
Figure 4.
Effects of PE and/or CDDP on apoptosis in SiHa and CaSki cell lines. Apoptosis was evaluated as aforementioned with Annexin V staining in SiHa and CaSki cells; the cells were treated with PE, CDDP, and their combination. (A and B) Representative dot plots illustrating the data near the mean of the groups. (C and D) Columns, means of 3 identical wells of a single representative experiment; bars, upper 95% confidence interval; ***P<0.001 for comparisons between cells treated with the combined treatment and cells treated with CDDP. (E and F) Western blot analysis of PARP and caspase-3 cleavage following treatments with PE alone or with CDDP. CDDP, cisplatin; PE, lipid-soluble extract from Pinellia pedatisecta Schott; PARP, poly ADP-ribose polymerase.
Figure 5.
Effect of PE and/or CDDP on the cell cycle in SiHa and CaSki cell lines. The cell cycle was assessed as aforementioned with propidium iodide and RNase staining of SiHa and CaSki cells; the cells were treated with PE (250 µg/ml or 125 µg/ml), CCDP (40 µg/ml or 20 µg/ml), and their combination. (A and B) Representative dot plots illustrating the data near the mean of the groups. (C and D) Columns, means of 3 identical wells of a single representative experiment; *P<0.05, **P<0.01 and ***P<0.001 for comparisons between cells treated with the combined treatment and cells treated with CDDP. CDDP, cisplatin; PE, lipid-soluble extract from Pinellia pedatisecta.
Combination of PE with CDDP enhances activation of the DNA damage response
To further understand how PE and CDDP function to inhibit tumor cell growth synergistically, the phosphorylation state of crucial components of the DDR pathway was assessed in the cells, following treatment with different doses of PE, CDDP or a combination of the two. Western blot analyses were performed on proteins from SiHa cells treated with 40 µg/ml of CDDP, 250 µg/ml of PE, or a combination of the two, and the treatment was conducted for 48 h. Proteins from CaSki cells that were treated with 20 µg/ml of CDDP, 125 µg/ml of PE, or a combination of the two for 48 h were detected in the same way. As shown in Fig. 6, when co-treated with PE and CDDP, the two cell lines manifested increased phosphorylation of ATM, Chk-1, Chk-2 and H2AX compared with single agent treatment.
Figure 6.
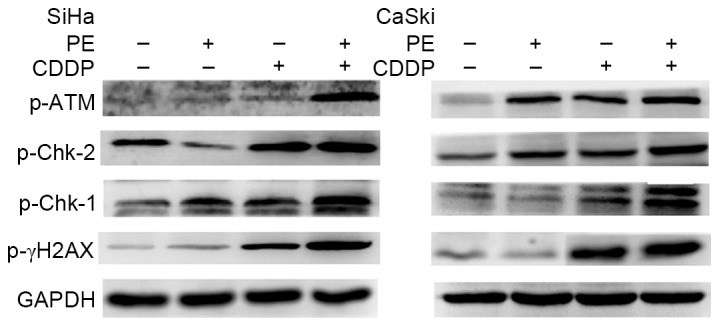
Effects on DNA damage reaction pathways following treatment with PE and CDDP in SiHa and CaSki cells. Proteins from cells treated with the indicated concentrations of PE and CDDP for 48 h were detected by western blotting of phosphorylation of ATM, Chk-1, Chk-2 and γ-H2AX. GAPDH was used to normalize protein levels. CDDP, cisplatin; PE, lipid-soluble extract from Pinellia pedatisecta Schott; p-Chk, phosphorylated checkpoint kinase; p-ATM, phosphorylated ataxia-telangiectasia mutated; γ-H2A, γ-H2AX, histone family member X.
Discussion
TCM, which has been used in China for thousands of years, has a unique theoretical system and takes a practical approach to the treatment of diseases (17). The ‘Shen Nong Ben Cao Jing’, written >2,000 years ago, was one of the earliest books on medicine in the world (17,18). The effectiveness of TCM against cancer is an area of investigation among oncologists and clinicians. There are multiple anti-cancer studies on various TCMs, including berberine (19), chrysin (20), jacarelhyperol A (21) and curcuma longa (22,23). Furthermore, evidence suggests that TCMs possess synergistic effects when combined with common chemotherapeutic drugs, enhancing toxicity against cancer cells and prolonging survival time (24,25). These combinations could also decrease the side effects of chemotherapeutic drugs and improve the quality of life in patients (26,27).
Pinellia pedatisecta rhizome has long been used as a TCM to treat Thanatophidia bites, nameless swelling, toxicum and cancer. It was recorded to exhibit efficacy at dispelling wind, relieving convulsions, drying dampness to eliminate phlegm, and eliminating stagnation (28). Previous studies have focused on Pinellia pedatisecta agglutinin, which has potential value in immunotherapy against drug-resistant cancer cells through inducing the tumoricidal activity of macrophages (29) and distinguishing among glycosylation patterns in various cancer cell lines (30). Total protein of Pinellia pedatisecta Schott has been reported to inhibit the growth of SKOV3 cells and lead to proteomic changes in the SKOV3 cell strain (31). A novel lipid-soluble extract (PE) from Pinellia pedatisecta has been investigated by the present authors for 10 years (Zhang et al, unpublished data). A previous in vivo study demonstrated that PE had a synergistic cytotoxic effect on CaSki cell growth in xenograft tumors in vivo, when combined with CDDP. This led to the investigation of whether PE has a synergistic effect when combined with CDDP on cervical cancer cell lines in vitro.
In the present study, PE and CDDP were shown individually to cause inhibition of cell growth in SiHa and CaSki cells in a dose-dependent manner. To extend analysis of the synergistic interactions between PE and CDDP, different concentrations of PE and CDDP were used to treat cervical cell lines. Drug CI analysis was used to evaluate the interactions between two drugs for a long time period. The median-effect method was created by Chou and Talalay, and CI <1, =1 and >1 indicate synergy, additivity and antagonism, respectively (16). Cell viability assays in the present study determined the CI, at quality ratios of 6:1 (PE: CDDP), for SiHa cells to be 0.43, and that for CaSki cells was 0.68. This provides evidence of the synergistic anti-proliferative effect. The synergistic effect of the combination treatment on cell apoptosis, cell cycle and cell invasion was then examined in the two cell lines. As shown in Fig. 3, although PE or CDDP alone decreased cervical cancer cell invasion, combined targeting significantly enhanced the effect compared with either treatment alone. Cells were arrested in G0/G1 or G2/M-phase in SiHa and CaSki cells, respectively, with a significant decrease (P<0.01) in S-phase cells. Similarly, combination of PE with CDDP strongly and synergistically induced apoptosis in the two cell lines (Fig. 4). The activation of caspase-3 and PARP was observed in the two cell lines treated with PE and CDDP. Caspase-3 and PARP protein serve a key role in the execution-phase of cell apoptosis (32,33). Results show that the combined use of PE and CDDP synergistically inhibited cervical cancer progression. However, the mechanisms responsible for the synergistic effects of using PE and CDDP are not understood.
It is known that the efficacy of platinum-based chemotherapy is dependent on the generation of DNA damage and the subsequent induction of apoptosis (34). DNA has been a main target of cancer therapy, since DNA damage could initiate a cascade of events that ultimately determines the fate of cancer cells (35). A DDR induced by a chemotherapeutic drug can lead to permanent cell cycle arrest or apoptosis, thus avoiding the proliferation or survival of premalignant clones (36). Modulation of the DDR network may alter the response of cancer cells to DNA damaging anticancer drugs, including CDDP and doxorubicin (37,38). This endogenous DDR involves the activation of DNA damage sensors (including ATM) and the activation of the cell cycle checkpoint kinases Chk-1 and Chk-2 together with the phosphorylation of the histone H2AX (39). To investigate the potential mechanism of the synergistic effect of PE and CDDP, western blot analysis of the primary protein in the DDR pathway was conducted. As shown in Fig. 6, the phosphorylation levels of ATM and γ-H2AX were increased more by treatment with PE plus CDDP than by either treatment alone. Therefore, the potential mechanism is possibly associated with enhancing the activation of the ATM/pChk-2 pathway to induce p53-mediated cell apoptosis in cervical cancer cells in vitro.
This phenomenon has also been observed in other TCMs. Wang et al (40) identified that curcumin could sensitize budding yeast to DNA damage by counteracting the DDR. Berberine was reported to be able to induce apoptosis and DNA damage in MG-63 cells (41). A number of studies have observed that herbal extracts are able to protect normal cells from DNA damage caused by certain unfavorable factors. It was stated that Ginkgo biloba extract (EGb 761), an antioxidant herbal medicine, can notably alleviate endothelial DNA oxidation caused by intermittent high glucose in human umbilical vein endothelial cells (42). In addition, Citri reticulatae Pericarpium can effectively protect against hydroxyl-induced DNA damage by donating a hydrogen atom/electron (43). Furthermore, the TCM compound rocaglamide protects nonmalignant primary cells from DNA damage-induced toxicity by inhibition of p53 expression, but this protective effect was not in malignant tumor cells with defective or mutant p53 (44). In the present study, it was observed that PE can induce DNA damage in cervical cancer cells, and thereby cause cell apoptosis, and it can also enhance the DDR caused by CDDP. Nevertheless, whether PE can protect normal cells from DNA damage caused by CDDP, as well as the upstream molecular reaction ATM/pChk-2 pathway in this process remains unclear and further investigation is necessary.
The present data and previous findings indicate that PE and CDDP inhibit the growth of cervical cancer cell lines synergistically. The potential mechanism may be associated with enhancing the DDR pathway to induce apoptotic signals in cervical cancer cells in vitro. The presented approach may have important implications for the pharmacological mechanism of Pinellia pedatisecta and therapeutic strategies for cervical cancer.
Acknowledgements
The present study was supported by National Natural Sciences Foundation of China (grant no. 81373867) awarded to Guiling Li.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Shi JF, Canfell K, Lew JB, Qiao YL. The burden of cervical cancer in China: Synthesis of the evidence. Int J Cancer. 2012;130:641–652. doi: 10.1002/ijc.26042. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, et al. Global surveillance of cancer survival 1995-2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferlay J, Forman D, Mathers CD, Bray F. Breast and cervical cancer in 187 countries between 1980 and 2010. Lancet. 2012;379:1390–1391. doi: 10.1016/S0140-6736(12)60595-9. [DOI] [PubMed] [Google Scholar]
- 5.Jelavić TB, Miše BP, Strikic A, Ban M, Vrdoljak E. Adjuvant chemotherapy in locally advanced cervical cancer after treatment with concomitant chemoradiotherapy-room for improvement? Anticancer Res. 2015;35:4161–4165. [PubMed] [Google Scholar]
- 6.Penson RT, Huang HQ, Wenzel LB, Monk BJ, Stockman S, HJ III Long, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, et al. Bevacizumab for advanced cervical cancer: Patient-reported outcomes of a randomised, phase 3 trial (NRG Oncology-Gynecologic Oncology Group protocol 240) Lancet Oncol. 2015;16:301–311. doi: 10.1016/S1470-2045(15)70004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-dna adducts. Chem Rev. 1999;99:2467–2498. doi: 10.1021/cr980421n. [DOI] [PubMed] [Google Scholar]
- 8.Chaney SG, Campbell SL, Bassett E, Wu Y. Recognition and processing of cisplatin- and oxaliplatin-DNA adducts. Crit Rev Oncol Hematol. 2005;53:3–11. doi: 10.1016/j.critrevonc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Sprowl JA, Lancaster CS, Pabla N, Hermann E, Kosloske AM, Gibson AA, Li L, Zeeh D, Schlatter E, Janke LJ, et al. Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin Cancer Res. 2014;20:4026–4035. doi: 10.1158/1078-0432.CCR-14-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karasawa T, Steyger PS. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett. 2015;237:219–227. doi: 10.1016/j.toxlet.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li CJ, Xu YM, Sun YL. Research on anti-cervical cancer component of Pinellia pedatisecta Schott. J Shanghai Med Univ. 1981;8:421–423. (In Chinese) [Google Scholar]
- 12.Poornima P, Kumar JD, Zhao Q, Blunder M, Efferth T. Network pharmacology of cancer: From understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Pharmacol Res. 2016;111:290–302. doi: 10.1016/j.phrs.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 13.Duan Y, Pei K, Cai H, Tu S, Cheng X, Zhang Z, Fan K, Qiao F, Qin K, Cai B. Strategy of integrated evaluation on treatment of traditional Chinese medicine as ‘interaction of system to system’ and establishment of novel fuzzy target contribution recognition with herb-pairs, a case study on Astragali Radix-FructusCorni. Mol Cell Endocrinol. 2016;434:219–237. doi: 10.1016/j.mce.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Chemistry Department of Basic Research Center, Obstetrics and Gynecology Hospital, corp-author. Research of Pinellia pedatisecta Schott on cervical cancer. Shanghai Medical Journal. 1978;1:13–16. (In Chinese) [Google Scholar]
- 15.Li GL, Jiang W, Xia Q, Chen SH, Ge XR, Gui SQ, Xu CJ. HPV E6 down-regulation and apoptosis induction of human cervical cancer cells by a novel lipid-soluble extract (PE) from Pinellia pedatisecta Schott in vitro. J Ethnopharmacol. 2010;132:56–64. doi: 10.1016/j.jep.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 16.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: The combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Reguln. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 17.Ding H, Wang Z. Experience of treatment and understanding of impediment disease in Shen nong ben cao jing (Shen-ntonz's Classic of Materia Medica) Zhonghua Yi Shi Za Zhi. 2015;45:3–6. (In Chinese) [PubMed] [Google Scholar]
- 18.Endo J, Nakamura T, Yamaki H, Miyamoto H. Studies of the fifth section in the introduction of the Qian jin yao fang-Writings quoted from the Shen nong ben cao jing and the Yao dui. Yakushigaku Zasshi. 1993;28:1–5. (In Japanese) [PubMed] [Google Scholar]
- 19.Liu X, Ji Q, Ye N, Sui H, Zhou L, Zhu H, Fan Z, Cai J, Li Q. Berberine inhibits invasion and metastasis of colorectal cancer cells via COX-2/PGE2 mediated JAK2/STAT3 signaling pathway. PLoS One. 2015;10:e0123478. doi: 10.1371/journal.pone.0123478. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Kasala ER, Bodduluru LN, Madana RM, V AK, Gogoi R, Barua CC. Chemopreventive and therapeutic potential of chrysin in cancer: Mechanistic perspectives. Toxicol Lett. 2015;233:214–225. doi: 10.1016/j.toxlet.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Li S, An FF, Liu J, Jin S, Zhang JC, Wang PC, Zhang X, Lee CS, Liang XJ. Self-carried curcumin nanoparticles for in vitroin vivo cancer therapy with real-time monitoring of drug release. Nanoscale. 2015;7:13503–13510. doi: 10.1039/C5NR03259H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devassy JG, Nwachukwu ID, Jones PJ. Curcumin and cancer: Barriers to obtaining a health claim. Nutr Rev. 2015;73:155–165. doi: 10.1093/nutrit/nuu064. [DOI] [PubMed] [Google Scholar]
- 23.Zhang S, Yin J, Li X, Zhang J, Yue R, Diao Y, Li H, Wang H, Shan L, Zhang W. Jacarelhyperol A induced apoptosis in leukaemia cancer cell through inhibition the activity of Bcl-2 proteins. BMC Cancer. 2014;14:689. doi: 10.7150/jca.9569. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Chen Q, Qin R, Fang Y, Li H. Berberine sensitizes human ovarian cancer cells to cisplatin through miR-93/PTEN/Akt signaling pathway. Cell Physiol Biochem. 2015;36:956–965. doi: 10.1159/000430270. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Wang B, Wu Q, Wei X, Zheng F, Men K, Shi H, Huang N, Wei Y, Gong C. Combined delivery and anti-cancer activity of paclitaxel and curcumin using polymeric micelles. J Biomed Nanotechnol. 2015;11:578–589. doi: 10.1166/jbn.2015.1964. [DOI] [PubMed] [Google Scholar]
- 26.Xu L, Wang S, Zhuang L, Lin J, Chen H, Zhu X, Bei W, Zhao Q, Wu H, Meng Z. Jian Pi Li Qi Decoction alleviated postembolization syndrome following transcatheter arterial chemoembolization for hepatocellular carcinoma: A randomized, double-blind, placebo-controlled trial. Integr Cancer Therpii. 2016;15:349–357. doi: 10.1177/1534735415617020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaladat AM, Atarzadeh F, Rezaeizadeh H, Mofid B, Mosalaie A, Farhan F, Amin G. Botanicals: An alternative remedy to radiotherapy-induced dysuria. Complement Ther Med. 2015;23:90–99. doi: 10.1016/j.ctim.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 28.He Y, L WL, Wang X. Pharmacological study of the rhizome powder of Pinellia pedatisecta processed by different procedures. Zhong Yao Cai. 1997;20:459–461. (In Chinese) [PubMed] [Google Scholar]
- 29.Chen K, Yang X, Wu L, Yu M, Li X, Li N, Wang S, Li G. Pinellia pedatisecta agglutinin targets drug resistant K562/ADR leukemia cells through binding with sarcolemmal membrane associated protein and enhancing macrophage phagocytosis. PLoS One. 2013;8:e74363. doi: 10.1371/journal.pone.0074363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li N, Dong G, Wang S, Zhu S, Shen Y, Li G. Pinellia pedatisecta agglutinin-based lectin blot analysis distinguishes between glycosylation patterns in various cancer cell lines. Oncol Lett. 2014;8:837–840. doi: 10.3892/ol.2014.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen XY, Zhou L, Zheng FY. Proteomic study of total protein of Pinellia pedatisecta Schott effect on human ovarian cancer SKOV3 cells. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31:1651–1656. (In Chinese) [PubMed] [Google Scholar]
- 32.Tangutoori S, Baldwin P, Sridhar S. PARP inhibitors: A new era of targeted therapy. Maturitas. 2015;81:5–9. doi: 10.1016/j.maturitas.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 33.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 34.Galluzzi L, Vitale I, Michels J, Brenner C, Szabadkai G, Harel-Bellan A, Castedo M, Kroemer G. Systems biology of cisplatin resistance: Past, present and future. Cell Death Dis. 2014;5:e1257. doi: 10.1038/cddis.2013.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hurley LH. DNA and its associated processes as targets for cancer therapy. Nat Rev Cancer. 2002;2:188–200. doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- 36.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 37.Dungl DA, Maginn EN, Stronach EA. Preventing damage limitation: Targeting DNA-PKcs and DNA double-strand break repair pathways for ovarian cancer therapy. Front Oncol. 2015;5:240. doi: 10.3389/fonc.2015.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El-Awady RA, Semreen MH, Saber-Ayad MM, Cyprian F, Menon V, Al-Tel TH. Modulation of DNA damage response and induction of apoptosis mediates synergism between doxorubicin and a new imidazopyridine derivative in breast and lung cancer cells. DNA Repair (Amst) 2015;37:1–11. doi: 10.1016/j.dnarep.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 39.O'Connor MJ. Targeting the DNA Damage Response in cancer. Mol Cell. 2015;60:547–560. doi: 10.1016/j.molcel.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 40.Wang SH, Lin PY, Chiu YC, Huang JS, Kuo YT, Wu JC, Chen CC. Curcumin-mediated HDAC inhibition suppresses the DNA Damage Response and contributes to increased DNA Damage sensitivity. PLoS One. 2015;10:e0134110. doi: 10.1371/journal.pone.0134110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu Y, Ma N, Li HX, Tian L, Ba YF, Hao B. Berberine induces apoptosis and DNA damage in MG63 human osteosarcoma cells. Mol Med Rep. 2014;10:1734–1738. doi: 10.3892/mmr.2014.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He YT, Xing SS, Gao L, Wang J, Xing QC, Zhang W. Ginkgo biloba attenuates oxidative DNA damage of human umbilical vein endothelial cells induced by intermittent high glucose. Pharmazie. 2014;69:203–207. [PubMed] [Google Scholar]
- 43.Li X, Huang YP, Chen DF. Protective effect against hydroxyl-induced DNA damage and antioxidant activity of citri reticulatae pericarpium. Adv Pharm Bull. 2013;3:175–181. doi: 10.5681/apb.2013.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Becker MS, Schmezer P, Breuer R, Haas SF, Essers MA, Krammer PH, Li-Weber M. The traditional Chinese medical compound Rocaglamide protects nonmalignant primary cells from DNA damage-induced toxicity by inhibition of p53 expression. Cell Death Dis. 2014;5:e1000. doi: 10.1038/cddis.2013.528. [DOI] [PMC free article] [PubMed] [Google Scholar]