Abstract
Stem cells are an important tool for the study of hematopoiesis. Despite developments in cryopreservation, post-thaw cell death remains a considerable problem. Cryopreservation protocol should limit cell damage due to freezing and ensure the recovery of the functional cell characteristics after thawing. Thus, the use of cryoprotectants is essential. In particular, the efficacy of trehalose has been reported for clinical purposes in blood stem cells. The aim of the current study was to establish an efficient method for biological research based on the use of trehalose, to cryopreserve pure peripheral blood stem cells. The efficacy of trehalose was assessed in vitro and the cell viability was evaluated. The data indicate that trehalose improves cell survival after thawing compared with the standard freezing procedure. These findings could suggest the potential for future trehalose application for research purposes in cell cryopreservation.
Keywords: cryopreservation, trehalose, peripheral blood stem cells
Introduction
Stem cells in biological research provide a significant source of information and, thus, contribute to the development of novel therapeutic strategies. In particular, the ability to cultivate stem cells and human hematopoietic progenitor cells in vitro is the fundamental basis for investigating hematopoiesis. An improved understanding of the mechanisms that regulate cell proliferation and differentiation during the stages of hematopoiesis would further elucidate the molecular characteristics of diseases (which are characterized by excessive expansion or a functional defect of certain immature blood components), and facilitate the identification of substances that are able to specifically protect healthy cells from the action of cytotoxic drugs.
Hematopoietic stem and progenitor cells (HSPCs) may be isolated from peripheral blood (PB), bone marrow (BM) or umbilical cord blood (CB) (1).
Although significant improvements to cell cryopreservation procedures have been achieved (2), the improvement of current cryopreservation protocols that lead to a high mortality rate after thawing for the crystal formations that arise during freezing is a primary goal. Specifically, fast cooling forms intracellular ice crystals, which results in cell destruction and slow cooling forms ice crystals in the extracellular space, with consequent cellular dehydration. Selection of a cryoprotectant, as well as a suitable freezing rate serves to protect cells from these adverse effects (3,4).
Cryoprotectants are divided into two classes: Penetrating and nonpenetrating (5). The penetrating cryoprotectants include glycerol and 1,2-propanediol and dimethyl sulfoxide (Me2SO); the latter is commonly used for HSPC cryopreservation (6). The non penetrating cryoprotectants comprise polyvinyl pyrrolidone, trehalose, fructose, sucrose and glucose.
Trehalose is a non-toxic disaccharide of glucose that preserves the structural integrity of the cells during freezing and thawing (7). Specifically, trehalose is found in numerous organisms, such as nematodes and yeasts, which are capable of surviving during freezing and drying (8,9). Previous studies have used this disaccharide for cryopreservation of human cells, such as platelets (10), red blood cells (11), sperm (12), oocytes (13), pancreatic islets (14) and fetal skin (15). Furthermore, the use of an alternative protocol with trehalose for clinical applications to cryopreserve stem cells obtained from CB and bone marrow (BM) has previously been reported in the literature (16,17) and in a study by Scheinkonig et al (18) from mobilized PB stem cells (PBSCs). Conversely, in the current study, trehalose was administered, only for research purposes, to cryopreserve pure HSPCs, and not mobilized HSPCs, that were isolated from the PB of healthy individuals.
Materials and methods
Cytokines, antibodies and chemicals
Ficoll-Hypaque (density, 1.077 g/ml) was purchased from Pharmacia Biotech, Uppsala, Sweden. A CD34 MultiSort kit human, midiMACS separator, MACS multistand, LS columns, and anti-CD34 fluorescein isothiocyanate (FITC), human (clone: AC136; cat. no. 130-081-001), anti-CD61 PE, human (clone: Y2/51; cat. no. 130-081-501), mouse IgG2a-FITC isotype control (cat. no. 130-091-837) and mouse IgG1-PE isotype control (cat. no. 130-092-212) antibodies (all dilutions, 1:10) were purchased from Miltenyi Biotec, Inc. (Auburn, CA, USA); BIT 9500 Serum Substitute (Stem cell Technologies, Inc., Vancouver, BC, Canada); Gibco Iscove's Modified Dulbecco's Medium (IMDM) was obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA) and recombinant human TPO was from PeproTech, Inc. (Rocky Hill, NJ, USA). Me2SO, D-(+)-Trehalose dihydrate, catalase from bovine liver, lipoprotein, low density lipoproteins (LDLs) from human plasma, trypan blue and May-Grünwald-Giemsa were purchased from Sigma-Aldrich (St. Louis, MO, USA). Nalgene® Mr. Frosty® Cryo 1°C Freezing Containers were purchased from Thermo Fisher Scientific, Inc.
Purification and characterization of CD34+ cells
Hematopoietic stem and progenitor cells (HSPCs) were obtained from the PB of 80 healthy donors supplied by IOM SPA (Viagrande, Italy) under an approved Institutional Review Board protocol (project ID code: 454_1; 6 February 2008, IOM Institutional Review Board, Viagrande, Italy) and subsequent to obtaining informed consent.
Briefly, mononuclear cells (MNCs) were isolated from PB (20–30 ml) by Ficoll-Hypaque. After recovery of the cell suspension, 150–300×106 MNCs were magnetically labeled with CD34 Micro Beads and loaded onto a MACS® Column, which was placed in the magnetic field of the midi MACS separator. The CD34+ cells are retained within the column and unlabeled cells run through. After the column was removed from the magnetic field, the magnetically retained CD34+ cells were eluted as the positively selected cell fraction. The purity was evaluated by flow cytometry of the FITC-labeled anti-CD34 FITC antibody (dilution, 1:10) according to the manufacturer's instructions. Cells were analyzed by FACSCalibur (BD Biosciences) using BD CellQuest™ Pro (version 6.0; BD Biosciences) software for fluorescence intensity analysis. The CD34+ morphology was evaluated by May-Grünwald-Giemsa staining. Cells were cytocentrifuged onto glass slides, stained with May-Grünwald-Giemsa and observed by light microscopy; the percentage of CD34+ cells ranged from 89 to 98%.
Freezing media preparation for cryopreservation
Freezing media preparation for cryopreservation of human CD34+ cells with different trehalose concentrations or the classical method
Freezing media were freshly made with different D-(+)-trehalose concentrations (1.25, 1,0.6 or 0.3 M) or with a medium containing 90% fetal bovine serum (FBS) and 10% Me2SO. Dilutions were prepared using serum-free medium (IMDM) from a 2.5-M trehalose stock solution in IMDM. The freezing media and cryogenic vials (NalgeNunc International, Penfield, NY, USA) were cooled prior to use.
Freezing media preparation for cryopreservation of human CD34+ cells with different cryoprotectants for short- and long-term cryopreservation
Five freezing media were freshly prepared as follows: 10% Me2SO + 90% FBS; 1M trehalose; 1M trehalose + 10% Me2SO; 1M trehalose + 100 µg/ml catalase; and 1 M trehalose + 100 µg/ml catalase + 10% Me2SO. The freezing media and cryogenic vials were cooled prior to use. The trehalose and catalase were freshly prepared in IMDM.
Cryopreservation of human CD34+ cells
The optimal concentration of CD34+ cells (200,000) were frozen in cryogenic vials in a Nalgene® controlled-rate freezing container (NalgeNunc International) by a gradual addition of freezing medium in a final volume of 200 µl. The freezing container contained 100% isopropyl alcohol that provided a 1°C/min cooling rate when stored at-80°C in a controlled freezer for the initial freezing cycle prior to placement in a liquid nitrogen freezer (−196°C) until final use. The CD34+ cells were cryopreserved for a short (20 days) and longer (3 months) duration.
Thawing of human CD34+ cells
Frozen cells were rapidly thawed in a water bath at 37°C, gently shaken and removed when the ice crystals dissolved. Subsequently cell viability and the ability to differentiate into megakaryocytes (MKs) were evaluated. Cell viability was assessed using the dye exclusion test with trypan blue and counts were performed in triplicate. Percentages of viable cells after thawing were normalized to non-cryopreserved cells (NT, 100% viability) using the following equation: Cell viability (%) = number of recovered cells after thawing ×100/number of non-cryopreserved cells.
MK unilineage cultures
MK differentiation was induced by growing CD34+ for 14 days in BIT 9500 Serum Substitute in the presence of human LDL and 100 ng/ml thrombopoietin. Cells were incubated at 37°C for 14 days in a fully humidified atmosphere of 5% CO2. The MK differentiation stage was evaluated after thawing by flow cytometry of typical anti-CD61+ PE antibody (dilution, 1:10).
Statistical analysis
Data were analyzed using Student's t-test, and the results were expressed as the mean ± standard deviation of separate experiments. P<0.05 was considered to indicate a statistically significant difference.
Results
The aim of the current study was to develop an effective cryopreservation technique for pure HSPCs obtained from PB to replace the standard method that uses Me2SO. HSPCs were isolated from healthy individuals after acquiring informed consent. The expression levels of the CD34 surface stem marker was evaluated by flow cytometry obtaining a percentage of CD34+ cells between 89 and 98%. Morphological observations subsequent to May-Grünwald-Giemsa staining correlated with the flow cytometry data.
As the quality of the cryopreserved cells is also cell concentration-dependent (19), human CD34+ were initially frozen at different cell concentrations (100,000, 150,000 and 200,000) with a medium containing FBS and Me2SO. Subsequent to thawing, cell viability was assessed using the dye exclusion test with trypan blue. The results revealed that the optimal concentration to cryopreserve CD34+ was 200,000 (data not shown).
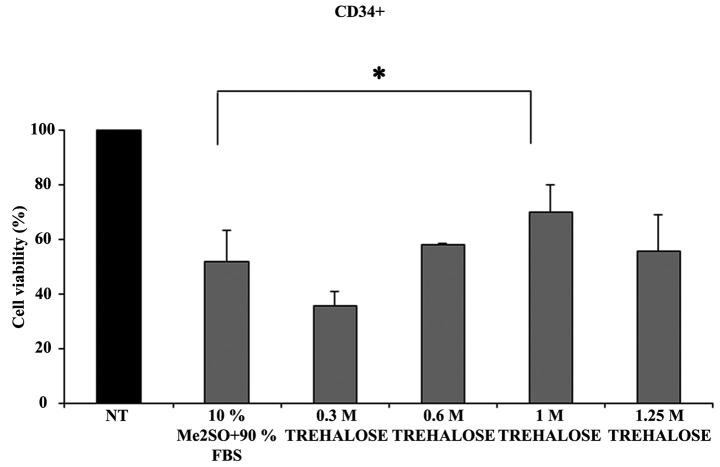
Subsequently, the effect of cryopreservation on CD34+ cells treated with four different trehalose concentrations compared with Me2SO was evaluated (Fig. 1). The findings indicated that freezing with 1M trehalose provided improved cryoprotection to CD34+ when compared with the standard freezing procedure using Me2SO.
Figure 1.
Percentage of cell viability measured after thawing of the CD34+ cells previously frozen with different concentrations of trehalose or according to the classical method. CD34+ cells were frozen with: 0.3, 0.6, 1 and 1.25M trehalose or with 10% Me2SO + 90% FBS. The viability after thawing was evaluated by Trypan Blue dye exclusion assay. Data were analyzed using Student's t-test and are expressed as the mean ± standard deviation of five separate experiments. *P<0.05. Cell viability (%) = number of recovered cells after thawing ×100/number of non-cryopreserved cells (NT, 100% viability). Me2SO, dimethyl sulfate; FBS, fetal bovine serum.
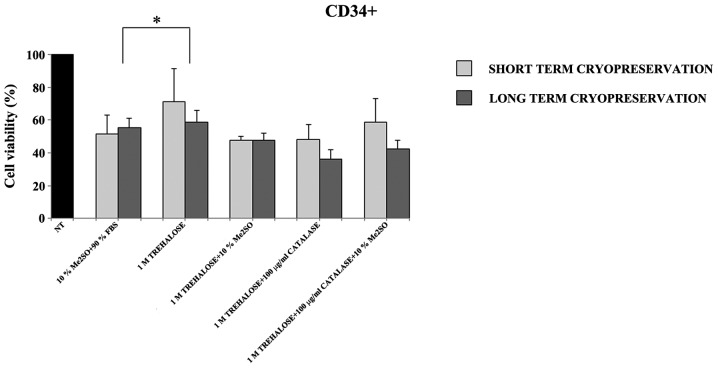
Furthermore, as it has been reported in previous studies (20,21) that freezing with trehalose in combination with an antioxidant (catalase) or with Me2SO could improve cell viability, CD34+ cells were cryopreserved for a short (20 days) and long (3 months) duration, with a medium containing different combinations of catalase, Me2SO and trehalose. The current data indicate that, after thawing, the number and viability of the CD34+ cells was higher in all the samples cryopreserved with only 1M trehalose (Fig. 2).
Figure 2.
Percentage of cell viability measured after thawing of CD34+ cells previously frozen with different cryoprotectants at short (20 days) and long (3 months) durations of cryopreservation. CD34+ were frozen with: 10% Me2SO + 90% FBS; 1M trehalose; 1M trehalose + 10% Me2SO, 1M trehalose + 100 µg/ml catalase; 1 M trehalose + 100 µg/ml catalase + 10% Me2SO. The viability after thawing was evaluated by trypan blue dye exclusion assay. Data were analyzed using Student's t-test and are expressed as the mean ± standard deviation of five separate experiments. *P<0.05. Cell viability (%) = number of recovered cells after thawing ×100/number of non-cryopreserved cells (NT, 100% viability). Me2SO, dimethyl sulfate; FBS, fetal bovine serum.
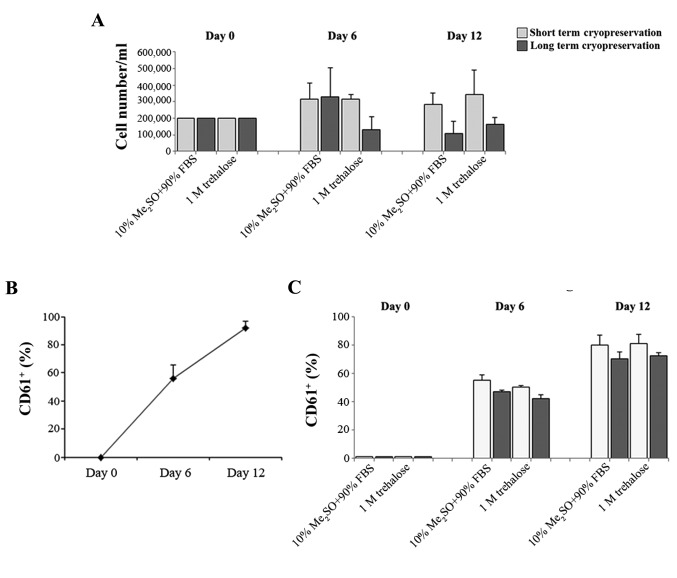
The hematopoietic stem cell is also responsible for the formation of differentiated cells in the blood that must be continually replaced through the proliferation and differentiation of immature forms. Megakaryopoiesis is a process of hematopoietic differentiation previously investigated by the current authors (22). Therefore, the ability of the fresh CD34+ cells and those obtained after thawing to proliferate and differentiate in MKs was compared. These cells were previously cryopreserved with 1M trehalose or Me2SO for short (20 days) and long (3 months) cryopreservation durations. MK differentiation was induced by growing CD34+ for 14 days and evaluated by flow cytometry of the typical anti-CD61+ antibody. CD34+ cells that were previously frozen with trehalose retained the ability to form MK subsequent to thawing (Fig. 3A). In addition the percentage of CD61+ cells obtained after thawing using the two different methods (Fig. 3C) is similar to that of MK produced from fresh CD34+ cells (Fig. 3B).
Figure 3.
Ability of the CD34+ to generate MKs subsequent to thawing. (A) Number of MKs obtained from CD34+ cells previously frozen with 1M trehalose or according to the classical method, following short (20 days) and long (3 months) durations of cryopreservation. The evaluation was performed by trypan blue dye exclusion assay on the day of thawing (day 0), and after 6 and 12 days of MK differentiation. Data are expressed as the mean ± standard deviation of three separate experiments. (B and C) Percentage of CD61+ at different stages of MK differentiation. MKs were obtained from (B) fresh CD34+ or (C) after thawing. Cells were previously cryopreserved with 1M trehalose or 10% Me2SO + 90% FBS at short and long durations of cryopreservation. The evaluation was performed by flow cytometry of typical anti-CD61+ PE antibody on the day of thawing (day 0), and after 6 and 12 days of MK differentiation. Data are expressed as the mean ± standard deviation of four separate experiments. MK, megakaryocyte; FBS, fetal bovine serum.
Discussion
Hematopoietic stem cells enable investigation of the underlying mechanisms of hematopoietic development for the ability to differentiate into different cell types. In addition, they may be used for drug development and as model systems to evaluate certain diseases for which there are no effective treatments currently available.
Cryopreservation allows cell preservation overlong periods by exposure to extremely low temperatures. Although this procedure causes suspension of metabolic reactions, during the freezing and thawing phases considerable physical changes occur, which can be detrimental to cell survival. As a result of cryopreservation, there is marked loss of cell viability and function. Therefore, the addition of a cryoprotectant to the cells is important. Among the cryoprotectants used, trehalose, which is a sugar that is utilized in cell cryopreservation, is significant (10–15). Trehalose is utilized in many fields, such as in the pharmaceutical industry (contained in various commercially available therapeutic products), the food industry (in confectionary products) and in the cosmetics industry (for example, in bath oils, creams and lotions) (23). Furthermore, the efficacy of trehalose in clinical cryopreservation of human stem cells from cord blood (24–26), bone marrow and mobilized PBSCs (18) has been widely demonstrated. Conversely, in the present study trehalose was used only for research purposes to cryopreserve pure and not mobilized PBSCs, in order to identify a more efficient protocol, which avoids cell loss that occurs during the normal course of cryopreservation. It was identified that 1M trehalose resulted in improved cryoprotection to CD34+ when compared with the standard freezing procedure that uses Me2SO. Furthermore, freezing with trehalose in combination with catalase or Me2SO did not increase cell viability. In addition, maintenance of the viability and ability of CD34+ cells to differentiate into MKs after thawing at short and long-term of cryopreservation was observed.
In conclusion the data indicate that the use of trehalose allows a greater number of hematopoietic stem cells to be obtained after thawing. The basis of a suitable cryopreservation protocol was established in the current study. However, future investigations are required to assess the repeatability on a commercial scale.
Acknowledgements
The authors would like to thank Mr. Gabriele Anastasi for his technical assistance.
References
- 1.Eaves CJ. Hematopoietic stem cells: Concepts, definitions, and the new reality. Blood. 2015;125:2605–2613. doi: 10.1182/blood-2014-12-570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berz D, McCormack EM, Winer ES, Colvin GA, Quesenberry PJ. Cryopreservation of hematopoietic stem cells. Am J Hematol. 2007;82:463–472. doi: 10.1002/ajh.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valeri CR, Pivacek LE. Effects of the temperature, the duration of frozen storage, and the freezing container on in vitro measurements in human peripheral blood mononuclear cells. Transfusion. 1996;36:303–308. doi: 10.1046/j.1537-2995.1996.36496226141.x. [DOI] [PubMed] [Google Scholar]
- 4.Cilloni D, Garau D, Regazzi E, Sammarelli G, Savoldo B, Caramatti C, Mangoni L, Rizzoli V, Carlo-Stella C. Primitive hematopoietic progenitors within mobilized blood are spared by uncontrolled rate freezing. Bone Marrow Transplant. 1999;23:497–503. doi: 10.1038/sj.bmt.1701601. [DOI] [PubMed] [Google Scholar]
- 5.McGann LE. Differing actions of penetrating and nonpenetrating cryoprotective agents. Cryobiology. 1978;15:382–390. doi: 10.1016/0011-2240(78)90056-1. [DOI] [PubMed] [Google Scholar]
- 6.Abrahamsen JF, Bakken AM, Bruserud Ø. Cryopreserving human peripheral blood progenitor cells with 5-percent rather than 10-percent DMSO results in less apoptosis and necrosis in CD34+ cells. Transfusion. 2002;42:1573–1580. doi: 10.1046/j.1537-2995.2002.00242.x. [DOI] [PubMed] [Google Scholar]
- 7.Jain NK, Roy I. Trehalose and protein stability. Curr Protoc Protein Sci. 2010 doi: 10.1002/0471140864.ps0409s59. Chapter 4, Unit 4.9. [DOI] [PubMed] [Google Scholar]
- 8.Behm CA. The role of trehalose in the physiology of nematodes. Int J Parasitol. 1997;27:215–229. doi: 10.1016/S0020-7519(96)00151-8. [DOI] [PubMed] [Google Scholar]
- 9.Sano F, Asakawa N, Inoue Y, Sakurai M. A dual role for intracellular trehalose in the resistance of yeast cells to water stress. Cryobiology. 1999;39:80–87. doi: 10.1006/cryo.1999.2188. [DOI] [PubMed] [Google Scholar]
- 10.Wolkers WF, Walker NJ, Tablin F, Crowe JH. Human platelets loaded with trehalose survive freeze-drying. Cryobiology. 2001;42:79–87. doi: 10.1006/cryo.2001.2306. [DOI] [PubMed] [Google Scholar]
- 11.Pellerin-Mendes C, Million L, Marchand-Arvier M, Labrude P, Vigneron C. In vitro study of the protective effect of trehalose and dextran during freezing of human red blood cells in liquid nitrogen. Cryobiology. 1997;35:173–186. doi: 10.1006/cryo.1997.2038. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Foote RH, Brockett CC. Effect of sucrose, trehalose, hypotaurine, taurine, and blood serum on survival of frozen bull sperm. Cryobiology. 1993;30:423–431. doi: 10.1006/cryo.1993.1042. [DOI] [PubMed] [Google Scholar]
- 13.Eroglu A, Toner M, Toth TL. Beneficial effect of microinjected trehalose on the cryosurvival of human oocytes. Fertil Steril. 2002;77:152–158. doi: 10.1016/S0015-0282(01)02959-4. [DOI] [PubMed] [Google Scholar]
- 14.Beattie GM, Crowe JH, Lopez AD, Cirulli V, Ricordi C, Hayek A. Trehalose: A cryoprotectant that enhances recovery and preserves function of human pancreatic islets after long-term storage. Diabetes. 1997;46:519–523. doi: 10.2337/diab.46.3.519. [DOI] [PubMed] [Google Scholar]
- 15.Erdag G, Eroglu A, Morgan J, Toner M. Cryopreservation of fetal skin is improved by extracellular trehalose. Cryobiology. 2002;44:218–228. doi: 10.1016/S0011-2240(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 16.Motta JP, Paraguassú-Braga FH, Bouzas LF, Porto LC. Evaluation of intracellular and extracellular trehalose as a cryoprotectant of stem cells obtained from umbilical cord blood. Cryobiology. 2014;68:343–348. doi: 10.1016/j.cryobiol.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XB, Li K, Yau KH, Tsang KS, Fok TF, Li CK, Lee SM, Yuen PM. Trehalose ameliorates the cryopreservation of cord blood in a preclinical system and increases the recovery of CFUs, long-term culture-initiating cells, and nonobese diabetic-SCID repopulating cells. Transfusion. 2003;43:265–272. doi: 10.1046/j.1537-2995.2003.00301.x. [DOI] [PubMed] [Google Scholar]
- 18.Scheinkönig C, Kappicht S, Kolb HJ, Schleuning M. Adoption of long-term cultures to evaluate the cryoprotective potential of trehalose for freezing hematopoietic stem cells. Bone Marrow Transplant. 2004;34:531–536. doi: 10.1038/sj.bmt.1704631. [DOI] [PubMed] [Google Scholar]
- 19.Alencar S, Garnica M, Luiz RR, Nogueira CM, Borojevic R, Maiolino A, Dutra HS. Cryopreservation of peripheral blood stem cell: The influence of cell concentration on cellular and hematopoietic recovery. Transfusion. 2010;50:2402–2412. doi: 10.1111/j.1537-2995.2010.02743.x. [DOI] [PubMed] [Google Scholar]
- 20.Limaye LS, Kale VP. Cryopreservation of human hematopoietic cells with membrane stabilizers and bioantioxidants as additives in the conventional freezing medium. J Hematother Stem Cell Res. 2001;10:709–718. doi: 10.1089/152581601753193931. [DOI] [PubMed] [Google Scholar]
- 21.Sasnoor LM, Kale VP, Limaye LS. A combination of catalase and trehalose as additives to conventional freezing medium results in improved cryoprotection of human hematopoietic cells with reference to in vitro migration and adhesion properties. Transfusion. 2005;45:622–633. doi: 10.1111/j.0041-1132.2005.04288.x. [DOI] [PubMed] [Google Scholar]
- 22.Vicari L, Martinetti D, Buccheri S, Colarossi C, Aiello E, Stagno F, Villari L, Cavalli M, Di Raimondo F, Gulisano M, et al. Increased phospho-mTOR expression in megakaryocytic cells derived from CD34+ progenitors of essential thrombocythaemia and myelofibrosis patients. Br J Haematol. 2012;159:237–240. doi: 10.1111/j.1365-2141.2012.09246.x. [DOI] [PubMed] [Google Scholar]
- 23.Ohtake S, Wang YJ. Trehalose: Current use and future applications. J Pharm Sci. 2011;100:2020–2053. doi: 10.1002/jps.22458. [DOI] [PubMed] [Google Scholar]
- 24.Rodrigues JP, Paraguassú-Braga FH, Carvalho L, Abdelhay E, Bouzas LF, Porto LC. Evaluation of trehalose and sucrose as cryoprotectants for hematopoietic stem cells of umbilical cord blood. Cryobiology. 2008;56:144–151. doi: 10.1016/j.cryobiol.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Motta JP, Gomes BE, Bouzas LF, Paraguassú-Braga FH, Porto LC. Evaluation of bioantioxidants in cryopreservation of umbilical cord blood using natural cryoprotectants and low concentrations of dimethylsulfoxide. Cryobiology. 2010;60:301–307. doi: 10.1016/j.cryobiol.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Chen G, Yue A, Ruan Z, Yin Y, Wang R, Ren Y, Zhu L. comparison of the effects of different cryoprotectants on stem cells from umbilical cord blood. Stem Cells Int. 2016;2016:1396783. doi: 10.1155/2016/1396783. [DOI] [PMC free article] [PubMed] [Google Scholar]