Abstract
An increasing amount of evidence demonstrates that epithelial-mesenchymal transition (EMT) is important in tumor invasion and metastases. The cell-cell adhesion molecule N-cadherin and the Wnt/β-catenin cascade protein β-catenin are two biomarkers of EMT. The present study aimed to measure the expression levels of N-cadherin and β-catenin in samples from patients with nasopharyngeal carcinoma (NPC) and evaluate their prognostic significance. N-cadherin and β-catenin mRNA was evaluated using reverse transcription-quantitative polymerase chain reaction in 26 NPC tissue samples and 8 nasopharyngeal epithelium samples. Protein expression of N-cadherin and β-catenin was also detected using immunohistochemistry in 128 archival NPC paraffin-embedded specimens. Finally, associations between clinical pathological parameters and prognostic values in NPC were evaluated. The results demonstrated that both the mRNA and protein levels of N-cadherin and β-catenin were significantly increased in NPC tissues compared with the controls. Enhanced expression of N-cadherin and β-catenin protein was strongly correlated with the status of lymph node metastasis and clinical stages in patients with NPC. Notably, high expression of N-cadherin and β-catenin proteins was significantly correlated with lower overall survival (OS) rate in patients with NPC. Finally, multivariate analysis demonstrated that expression of N-cadherin protein and clinical stages were independent prognostic factors for patients with NPC. Therefore, the present study demonstrated that N-cadherin and β-catenin expression may be used as potential prognostic biomarkers for patients with NPC.
Keywords: nasopharyngeal carcinoma, N-cadherin, β-catenin, prognosis, metastasis
Introduction
Epithelial-mesenchymal transition (EMT) is important for early tumor invasion and metastases (1). During this process, high expression of N-cadherin (cell-cell adhesion molecule) and β-catenin (important Wnt/β-catenin cascade protein) are hallmarks of EMT (2). N-cadherin and β-catenin are expressed normally in mesenchymal cells, but are upregulated in a various forms of human cancer, including gastric cancer, breast tumors and nasopharyngeal carcinoma (NPC), providing these cancer cells with more invasive and motile phenotypes (3). N-cadherin and β-catenin inactivation inhibits cell migration and metastatic formation (4). Furthermore, these two proteins are often associated with unfavorable prognoses in a diverse range of human malignancies (5,6). Notably, accumulating evidence suggests that N-cadherin and β-catenin are molecular targets in current cancer gene therapy and have been evaluated in preclinical and clinical studies (7,8). Thus, N-cadherin and β-catenin may be important for tumor development and progression.
NPC is a distinctive malignant disease that is prevalent in southern China, and has an annual incidence rate of ~20 per 100,000 people (9). Unlike other types of head and neck cancer, NPC exhibits features of regional lymph node metastases, and patients with NPC often present at an advanced clinical stage for diagnosis and/or treatment (10). Thus, it is imperative to understand the molecular changes in NPC that drive tumor progression and metastasis (11,12). Positive N-cadherin and β-catenin have been reported in multiple forms of human carcinoma to date with the exception of NPC. Therefore, the present study aimed to investigate the expression of N-cadherin and β-catenin in patients with NPC and further study their prognostic value.
Materials and methods
Tumor sample collection
A total of 128 NPC paraffin-embedded specimens were collected between January 2007 and October 2009 from Xiangya Hospital and Hunan Provincial Tumor Hospital, Central South University (CSU; Changsha, China). Individuals were excluded if they had a previous history of cancer and underwent radiotherapy or chemotherapy at diagnosis. All patients were classified and staged according to the sixth edition of the Union for International Cancer Control/American Joint Committee on Cancer Tumor-Node-Metastasis staging system (13). Follow-up information was collected every 6 months after diagnosis or until mortality. The complete cohort characteristics of the 91 patients with NPC are listed in Table I.
Table I.
Correlations between protein expression of N-cadherin and β-catenin and clinicopathological parameters in patients with nasopharyngeal carcinoma.
| N-cadherin expression | β-catenin expression | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | No. | High | Low | P-value | No. | High | Low | P-value |
| Age, years | 91 | 55 | 36 | 91 | 59 | 32 | ||
| ≤48 | 46 | 25 | 21 | 46 | 32 | 14 | ||
| >48 | 45 | 30 | 15 | 0.230 | 45 | 27 | 18 | 0.339 |
| Gender | ||||||||
| Male | 54 | 29 | 25 | 54 | 34 | 20 | ||
| Female | 37 | 26 | 11 | 0.112 | 37 | 25 | 12 | 0.651 |
| Clinical stages | ||||||||
| I and II | 32 | 10 | 22 | 32 | 13 | 19 | ||
| III and IV | 59 | 45 | 14 | <0.001 | 59 | 46 | 13 | 0.001 |
| Lymph node | ||||||||
| Metastasis | 63 | 37 | 26 | 63 | 50 | 13 | ||
| No metastasis | 28 | 18 | 10 | 0.617 | 28 | 9 | 19 | <0.001 |
Additionally, 26 fresh biopsy samples from patients with NPC and 8 nasopharyngeal epithelial (NPE) tissue samples were collected from Xiangya Hospital, CSU between August 2013 and October 2013. These samples were validated and diagnosed by qualified pathologists, and were used to determine the mRNA expression of N-cadherin and β-catenin. All tissue samples were infiltrated in RNAlater® Stabilization Solution (Qiagen, Inc., Valencia, CA, USA) and snap-frozen in liquid nitrogen. Prior informed consent was obtained, and the study protocol was approved by the Ethics Committee of Xiangya Hospital, CSU.
RNA isolation
Total RNA was isolated using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's protocol. RNA was extracted using the RNeasy® Mini kit (Qiagen, Inc.) and treated with DNase I according to the manufacturer's protocol. The integrity and quality of RNA was confirmed using agarose gel electrophoresis and absorbance at 260 nm. Then, 2 µg total RNA was reverse transcribed to cDNA using a PrimeScript RT reagent kit with a DNA Eraser (Takara Biotechnology Co., Ltd., Tokyo, Japan) via the SuperScript® First-Strand Synthesis system (Thermo Fisher Scientific, Inc.) with random hexamer primers (Promega Corporation, Madison, WI, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
RT-qPCR was performed using the aforementioned cDNA. Primers for mRNA were designed and synthesized for N-cadherin (forward, 5′-GCGTCTGTAGAGGCTTCTGG-3′ and reverse, 5′-GCCACTTGCCACTTTTCCTG-3′) and β-catenin (forward, 5′-GGGAAGAGTCCGGAGGAGAT-3′ and reverse, 5′-GGCTGTCAGGTTTGATCCCA-3′). qPCR was performed using the Bio-Rad iQ™5 Multicolor Real-Time PCR Detection system (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The final PCR reaction mixture (10 µl) included 1 µl cDNA product, 4 µl DEPC water and 5 µl SYBR® Premix Ex Taq™ II (Takara Biotechnology Co., Ltd.). Reactions were incubated at 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec. All reactions were run in triplicate with GAPDH as an internal reference (forward primer, 5′-GAGAAGGCTGGGGCTCATTT-3′ and reverse primer, 5′-AGTGATGGCATGGACTGTGG-3′). Gene expression levels were quantified using the 2−ΔΔCq method (14). The data were representative of the means of three experiments.
Immunohistochemistry
Tissue samples were fixed in 4% formaldehyde for 48 h at room temperature and then dehydrated by ethanol from 75–100% for 1 h at room temperature. The tissues were subsequently infiltrated by xylene twice for 30 min at room temperature, embedded in paraffin and cut into 4 µm sections. Following this, sections were deparaffinized by washing with xylene twice and rehydrated with ethanol from 75–100%, following antigen retrieval by heating in a microwave for 4 min at 100% power followed by 16 min at 20% power. After blocking peroxidase activity with 3% H2O2 for 5 min at room temperature, sections were blocked by 5% goat serum (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) in PBS for 30 min at room temperature. Negative control slides were probed with 5% normal goat serum in PBS under the same experimental conditions. Primary antibodies were added in blocking buffer (horse serum (Santa Cruz Biotechnology, Inc.) 0.2% Triton X-100 and 0.1% bovine serum albumin in PBS) and incubated with sections at 4°C overnight. The primary antibodies were as follows: Rabbit polyclonal anti-N-cadherin (dilution, 1:300; #18203; Abcam, Cambridge, MA, USA) and rabbit monoclonal anti-β-catenin (dilution, 1:500; #32572; Abcam). Following sufficient rinses by PBS three times (5 min each), sections were immunostained with Polink-1 HRP DAB Detection system (PV-6000; Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China). A light microscope and a digital color camera were used for examination and image capture of the slides.
Immunohistochemistry results were diagnosed and scored independently by two qualified pathologists without knowledge of the information of all patients with NPC. Assessments of N-cadherin and β-catenin proteins referred to methods described by Hui et al (15). The intensity of N-cadherin and β-catenin staining was scored as 0, 1, 2 and 3. Scores of positive cell percentage were assigned as 0 (<5%), 1 (5–25%), 2 (26–50%), and 3 (≥51%). The scores of each view were multiplied to give a final score of 0–9, and the final score of one sample was the mean of 10 microscopic fields. For final statistical analysis, tumors were divided into two groups: High expression of N-cadherin and β-catenin protein (scored 6–9) and low expression of N-cadherin and β-catenin protein (scored 0–5).
Statistical analysis
Statistical procedures were analyzed using SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA). The χ2 test was used to analyze the potential associations between the expression of N-cadherin and β-catenin, and diverse NPC clinicopathological variables. The Kaplan-Meier survival method was applied to determine the prognostic value of N-cadherin and β-catenin proteins. In addition, the Cox proportional hazards model was used to perform multivariate analysis and to determine the final independent prognostic factors. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of N-cadherin and β-catenin mRNA is increased in NPC samples
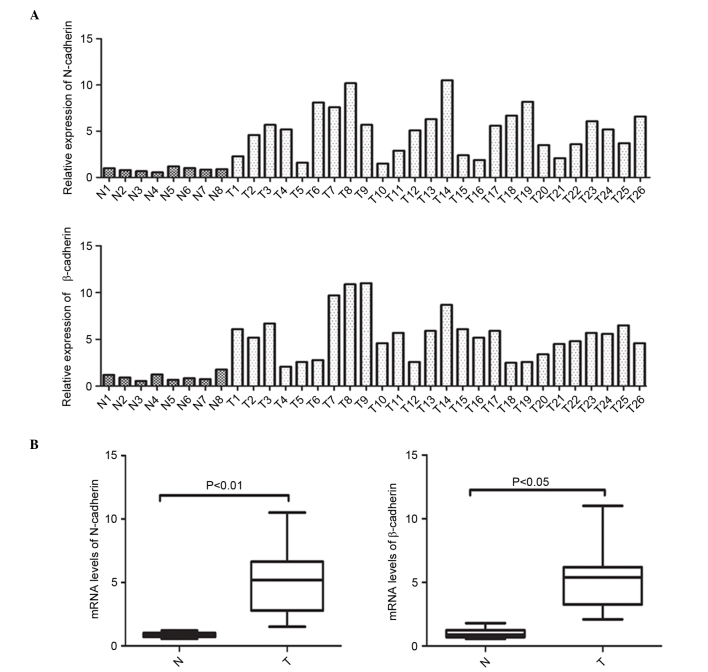
In order to clarify the abnormal expression of N-cadherin and β-catenin at the transcriptional level, mRNA expression of N-cadherin and β-catenin was initially measured in 26 NPC specimens and 8 normal NPE samples was initially measured via RT-qPCR. Fig. 1A and B depict the individual and average mRNA expression of N-cadherin and β-catenin in the NPC and NPE tissues. These data clearly demonstrate that the mRNA expression of N-cadherin (P<0.001) and β-catenin (P=0.0004) was elevated in the NPC tissues when compared with the noncancerous NPE specimens.
Figure 1.
N-cadherin and β-catenin expression in NPC samples. (A and B) Reverse transcription-quantitative polymerase chain reaction analysis of relative expression of N-cadherin and β-catenin in 26 NPC specimens and 8 nasopharyngeal epithelial specimens. Data are normalized to glyceraldehyde 3-phosphate dehydrogenase. Differences between groups were analyzed using the t-test (N-cadherin, P<0.01; β-catenin, P<0.01). NPC, nasopharyngeal carcinoma; N, normal; T, tumor.
Protein expression of N-cadherin and β-catenin is associated with NPC clinicopathological parameters
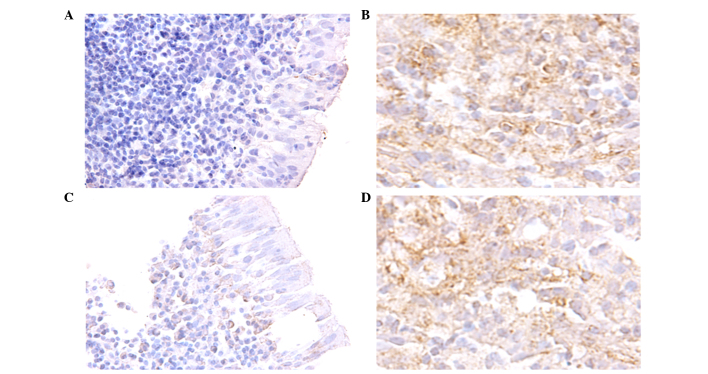
Based on the aberrant mRNA expression N-cadherin and β-catenin, the expression levels of their encoded proteins were subsequently assayed via immunohistochemistry in 91 paraffin-embedded NPC tissues. Staining of N-cadherin and β-catenin proteins demonstrated that they were primarily distributed in the cytoplasm of NPC cells (Fig. 2). Moreover, high N-cadherin (55/91 samples, 60.4%) and β-catenin (59/91 samples, 64.8%) protein expression frequently occurred in the NPC samples. Following this, correlations between N-cadherin and β-catenin expression and NPC clinicopathological variables were examined. Expression of N-cadherin and β-catenin protein was positively correlated with lymph node metastasis status and advanced clinical stages in patients with NPC (P=0.001) (Table I). However, no significant differences were identified between expression levels of these proteins and parameters, including age (P=0.339) and gender (P=0.651).
Figure 2.
Representative images of N-cadherin and β-catenin as detected in 91 paraffin-embedded NPC tissues by immunohistochemistry. Brown denotes a positive signal. Expression of N-cadherin in (A) columnar epithelia and (B) NPC. Magnification, ×200. Expression of β-catenin in (C) columnar epithelia and (D) NPC. Magnification, ×400. Nuclei were counterstained by hematoxylin. NPC, nasopharyngeal carcinoma.
Survival analysis
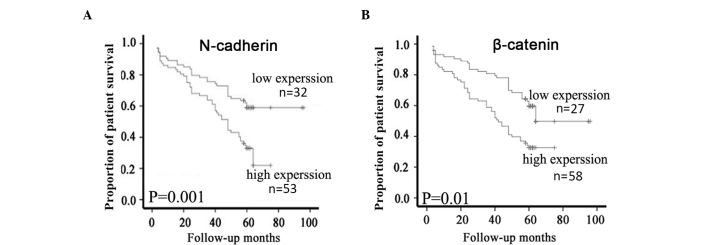
Of the 91 patients with NPC, 85 (93.4%) had intact follow-up information that was able to be further used for survival analysis. According to the expression levels of N-cadherin and β-catenin proteins, these cases were categorized into two groups containing high and low N-cadherin or β-catenin expression. Kaplan-Meier survival analyses demonstrated that patients with NPC with high N-cadherin protein expression had a significantly lower overall survival (OS) rate than those with low N-cadherin expression (P=0.001; Fig. 3A). Similarly, high β-catenin protein expression was significantly associated with a lower OS rate compared with low expression of β-catenin protein (P=0.01; Fig. 3B). Finally, Cox multivariate analysis determined that N-cadherin expression (P=0.017) and clinical stage (P=0.024) were independent factors with prognostic value in patients with NPC (Table II).
Figure 3.
Expression of N-cadherin and β-catenin were significantly correlated with survival of patients with NPC. Kaplan-Meier estimates of overall survival rate for 85 NPC patients are presented according to (A) N-cadherin or (B) β-catenin expression. P-values were obtained using the log-rank test. NPC, nasopharyngeal carcinoma.
Table II.
Cox regression analysis of the progression-free survival and overall survival rates.
| Progression-free survival | Overall survival | |||||
|---|---|---|---|---|---|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Gender (female/male) | 1.665 | 0.710–3.416 | 0.251 | 1.436 | 0.815–3.317 | 0.342 |
| Age (≤48/>48) | 1.16 | 0.816–1.221 | 0.914 | 0.875 | 0.657–1.149 | 0.764 |
| β-catenin (high/low) | 1.417 | 0.857–2.124 | 0.612 | 1.214 | 0.716–2.151 | 0.273 |
| N-cadherin (high/low) | 2.453 | 1.325–3.455 | 0.013a | 2.887 | 1.561–5.176 | 0.017a |
| Clinical stage (I and II/III and IV) | 2.612 | 1.724–3.294 | 0.021 | 2.429 | 1.816–3.169 | 0.024a |
P<0.05. HR, hazard ratio; CI, confidence interval.
Discussion
The present study measured the expression of N-cadherin and β-catenin in NPC, which indicated that the mRNA and protein expression of the two were significantly elevated in the NPC tissues. Moreover, the data demonstrated that increased protein expression of N-cadherin and β-catenin was positively correlated with NPC lymph node metastasis and predicted a poorer prognosis in patients with NPC.
The cadherin family is comprised of various proteins and includes E-cadherin (epithelial), N-cadherin (neural), P-cadherin (placental) and nonclassical cadherins, such as OB-Cadherin, VE-Cadherin, K-cadherin, LI-cadherin, BR-cadherin, M-cadherin, R-cadherin and T-cadherin (16). E-cadherin has been studied as an epithelial cell molecule in a various forms of human cancer, including NPC (17). A number of studies have demonstrated that downregulated E-cadherin is associated with cancer metastasis and poor prognosis (18–20). Studies performed in vitro and in vivo have established the hypothesis that E-cadherin is a vital molecule in the EMT process (21).
By contrast, the well-known mesenchymal molecule N-cadherin has scarcely been investigated in NPC. In the present study, mRNA and protein expression of N-cadherin were markedly increased in the NPC tissues when compared with the noncancerous NPE tissues. Moreover, N-cadherin upregulation was highly correlated with lymph node metastasis and poor survival in patients with NPC (22). The aforementioned results are consistent with previous reports examining other forms of malignancy, including breast cancer, lung cancer and oral squamous cell carcinoma (23–25). However, it is important to note that decreased expression of N-cadherin has also been reported in several human malignancies, such as osteosarcoma (26), ovarian carcinoma (27), glioblastoma (28) and renal carcinoma (29). The opposing expression profile in these forms of cancer suggests that N-cadherin has a distinct expression pattern based on the diverse background of cancer, and its exact role in different types of cancer requires further study.
β-catenin is a critical component in the cadherin-catenin complex, which is essential in connecting actin filaments of cells to the cell-cell interface at adherens junctions (30). The aberrant expression and dysfunction of the N-cadherin/β-catenin complex leads to increased malignant capacity in cancer cells, including increased cell motility and invasion (31). Multiple signaling pathways, such as phosphoinositide 3-kinase/protein kinase B and nuclear factor-κβ, have been implicated in the mechanisms behind these unfavorable alterations in the complex (32,33). However, these mechanisms require further study in different forms of human cancer.
Finally, the prognostic significance of N-cadherin and β-catenin proteins in patients with NPC was investigated. To the best of our knowledge, the present study is the first to demonstrate that high expression of N-cadherin or β-catenin is strongly correlated with adverse prognosis in patients with NPC. These results indicate that N-cadherin and β-catenin may be used as a valuable biomarker during surveillance of patients with NPC.
In conclusion, the present study demonstrated that N-cadherin and β-catenin mRNA and protein expression are positively correlated with lymph node metastasis and prognosis in patients with NPC. As the current study was a retrospective clinical association analysis based on archival tissue specimens, further in vitro and in vivo studies are required to enable a comprehensive understanding of the potential function and related mechanism of the N-cadherin/β-catenin complex in NPC.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant nos. 81170912 and 81300819).
References
- 1.Smith A, Teknos TN, Pan Q. Epithelial to mesenchymal transition in head and neck squamous cell carcinoma. Oral Oncol. 2013;49:287–292. doi: 10.1016/j.oraloncology.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 3.Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, et al. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung S, Yao J, Suyama K, Bajaj S, Qian X, Loudig OD, Eugenin EA, Phillips GR, Hazan RB. N-cadherin regulates mammary tumor cell migration through Akt3 suppression. Oncogene. 2013;32:422–430. doi: 10.1038/onc.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G, Satyamoorthy K, Herlyn M. N-cadherin-mediated intercellular interactions promote survival and migration of melanoma cells. Cancer Res. 2001;61:3819–3825. [PubMed] [Google Scholar]
- 6.Miao Y, Li AL, Wang L, Fan CF, Zhang XP, Xu HT, Yang LH, Liu Y, Wang EH. Overexpression of NEDD9 is associated with altered expression of E-cadherin, β-catenin and N-cadherin and predictive of poor prognosis in non-small cell lung cancer. Pathol Oncol Res. 2013;19:281–286. doi: 10.1007/s12253-012-9580-2. [DOI] [PubMed] [Google Scholar]
- 7.Mao J, Fan S, Ma W, Fan P, Wang B, Zhang J, Wang H, Tang B, Zhang Q, Yu X, et al. Roles of Wnt/β-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, Johnson DS, Trivett MK, Etemadmoghadam D, Locandro B, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14:5198–5208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 9.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 10.Tulalamba W, Janvilisri T. Nasopharyngeal carcinoma signaling pathway: An update on molecular biomarkers. Int J Cell Biol. 2012;2012:594681. doi: 10.1155/2012/594681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tao Q, Chan AT. Nasopharyngeal carcinoma: Molecular pathogenesis and therapeutic developments. Expert Rev Mol Med. 2007;9:1–24. doi: 10.1017/S1462399407000312. [DOI] [PubMed] [Google Scholar]
- 12.Tian Y, Tian Y, Zhang W, Wei F, Yang J, Luo X, Zhou T, Hou B, Qian S, Deng X, et al. Junctional adhesion molecule-A, an epithelial-mesenchymal transition inducer, correlates with metastasis and poor prognosis in human nasopharyngeal cancer. Carcinogenesis. 2015;36:41–48. doi: 10.1093/carcin/bgu230. [DOI] [PubMed] [Google Scholar]
- 13.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual. Springer Science & Business Media; 2002. [DOI] [Google Scholar]
- 14.Li G, Liu Y, Liu C, Su Z, Ren S, Wang Y, Deng T, Huang D, Tian Y, Qiu Y. Genome-wide analyses of long noncoding RNA expression profiles correlated with radioresistance in nasopharyngeal carcinoma via next-generation deep sequencing. BMC Cancer. 2016;16:719. doi: 10.1186/s12885-016-2755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui L, Zhang S, Dong X, Tian D, Cui Z, Qiu X. Prognostic significance of twist and N-cadherin expression in NSCLC. PLoS One. 2013;8:e62171. doi: 10.1371/journal.pone.0062171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Z, Pan J, Chu B, Wong YC, Cheung AL, Tsao SW. Downregulation and abnormal expression of E-cadherin and beta-catenin in nasopharyngeal carcinoma: Close association with advanced disease stage and lymph node metastasis. Hum Pathol. 1999;30:458–466. doi: 10.1016/S0046-8177(99)90123-5. [DOI] [PubMed] [Google Scholar]
- 18.Guilford P. E-cadherin downregulation in cancer: Fuel on the fire? Mol Med Today. 1999;5:172–177. doi: 10.1016/S1357-4310(99)01461-6. [DOI] [PubMed] [Google Scholar]
- 19.He X, Chen Z, Jia M, Zhao X. Downregulated E-cadherin expression indicates worse prognosis in Asian patients with colorectal cancer: Evidence from meta-analysis. PloS One. 2013;8:e70858. doi: 10.1371/journal.pone.0070858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang Y, Liang X, Jiang W, Li J, Xu J, Cai X. Cyclin b1 suppresses colorectal cancer invasion and metastasis by regulating e-cadherin. PloS One. 2015;10:e0126875. doi: 10.1371/journal.pone.0126875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canel M, Serrels A, Frame MC, Brunton VG. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126:393–401. doi: 10.1242/jcs.100115. [DOI] [PubMed] [Google Scholar]
- 22.Luo WR, Wu AB, Fang WY, Li SY, Yao KT. Nuclear expression of N-cadherin correlates with poor prognosis of nasopharyngeal carcinoma. Histopathology. 2012;61:237–246. doi: 10.1111/j.1365-2559.2012.04212.x. [DOI] [PubMed] [Google Scholar]
- 23.Nagi C, Guttman M, Jaffer S, Qiao R, Keren R, Triana A, Li M, Godbold J, Bleiweiss IJ, Hazan RB. N-cadherin expression in breast cancer: Correlation with an aggressive histologic variant-invasive micropapillary carcinoma. Breast Cancer Res Treat. 2005;94:225–235. doi: 10.1007/s10549-005-7727-5. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Liu G, Kang Y, Dong Z, Qian Q, Ma X. N-cadherin expression is associated with acquisition of EMT phenotype and with enhanced invasion in erlotinib-resistant lung cancer cell lines. PloS One. 2013;8:e57692. doi: 10.1371/journal.pone.0057692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Domenico M, Pierantoni GM, Feola A, Esposito F, Laino L, DE Rosa A, Rullo R, Mazzotta M, Martano M, Sanguedolce F, et al. Prognostic significance of N-cadherin expression in oral squamous cell carcinoma. Anticancer Res. 2011;31:4211–4218. [PubMed] [Google Scholar]
- 26.Marie PJ. Role of N-cadherin in bone formation. J Cell Physiol. 2002;190:297–305. doi: 10.1002/jcp.10073. [DOI] [PubMed] [Google Scholar]
- 27.Alaee M, Danesh G, Pasdar M. Plakoglobin Reduces the in vitro Growth, Migration and Invasion of Ovarian Cancer Cells Expressing N-Cadherin and Mutant p53. PLoS One. 2006;11:e0154323. doi: 10.1371/journal.pone.0154323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musumeci G, Magro G, Cardile V, Coco M, Marzagalli R, Castrogiovanni P, Imbesi R, Graziano AC, Barone F, Di Rosa M, et al. Characterization of matrix metalloproteinase-2 and −9, ADAM-10 and N-cadherin expression in human glioblastoma multiforme. Cell Tissue Res. 2015;362:45–60. doi: 10.1007/s00441-015-2197-5. [DOI] [PubMed] [Google Scholar]
- 29.Tani T, Laitinen L, Kangas L, Lehto VP, Virtanen I. Expression of E- and N-cadherin in renal cell carcinomas, in renal cell carcinoma cell lines in vitro and in their xenografts. Int J Cancer. 1995;64:407–414. doi: 10.1002/ijc.2910640610. [DOI] [PubMed] [Google Scholar]
- 30.Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 1988;7:3679. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rappl A, Piontek G, Schlegel J. EGFR-dependent migration of glial cells is mediated by reorganisation of N-cadherin. J Cell Sci. 2008;121:4089–4097. doi: 10.1242/jcs.027995. [DOI] [PubMed] [Google Scholar]
- 32.Méndez-Samperio P, Pérez A, Rivera L. Mycobacterium bovis Bacillus Calmette-Guérin (BCG)-induced activation of PI3K/Akt and NF-kB signaling pathways regulates expression of CXCL10 in epithelial cells. Cell Immunol. 2009;256:12–18. doi: 10.1016/j.cellimm.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]