Abstract
Exposure to solar ultraviolet B (UV-B) is a known causative factor for many skin complications such as wrinkles, black spots, shedding and inflammation. Within the wavelengths 280–320 nm, UV-B can penetrate to the epidermal level. This investigation aimed to test whether extracts from the tropical abalone [Haliotis asinina (H. asinina)] mucus-secreting tissues, the hypobranchial gland (HBG) and gills, were able to attenuate the inflammatory process, using the human keratinocyte HaCaT cell line. Cytotoxicity of abalone tissue extracts was determined using an AlamarBlue viability assay. Results showed that HaCaT cells could survive when incubated in crude HBG and gill extracts at concentrations between <11.8 and <16.9 μg/ml, respectively. Subsequently, cell viability was compared between cultured HaCaT cells exposed to serial doses of UV-B from 1 to 11 (x10) mJ/cm2 and containing 4 different concentrations of abalone extract from both the HBG and gill (0, 0.1, 2.5, 5 μg/ml). A significant increase in cell viability was observed (P<0.001) following treatment with 2.5 and 5 μg/ml extract. Without extract, cell viability was significantly reduced upon exposure to UV-B at 4 mJ/cm2. Three morphological changes were observed in HaCaT cells following UV-B exposure, including i) condensation of cytoplasm; ii) shrunken cells and plasma membrane bubbling; and iii) condensation of chromatin material. A calcein AM-propidium iodide live-dead assay showed that cells could survive cytoplasmic condensation, yet cell death occurred when damage also included membrane bubbling and chromatin changes. Western blot analysis of HaCaT cell COX-2, p38, phospho-p38, SPK/JNK and phospho-SPK/JNK following exposure to >2.5 μg/ml extract showed a significant decrease in intensity for COX-2, phospho-p38 and phospho-SPK/JNK. The present study demonstrated that abalone extracts from the HGB and gill can attenuate inflammatory proteins triggered by UV-B. Hence, the contents of abalone extract, including cellmetabolites and peptides, may provide new agents for skin anti-inflammation, preventing damage due to UV-B.
Keywords: abalone, gland, ultraviolet, inflammation
Introduction
Ultraviolet (UV) radiation is a major source of skin damage, where daylight UV is composed of three different types classified by wavelengths: UV-A (320–400 nm), UV-B (290–320 nm) and UV-C (<290 nm) (1–5). Skin inflammation after UV exposure is caused by UV-B since it easily triggers the cross-linking of macromolecules, ring-structures and repeated linear molecules (2,3,5).
UV-induced damage can be measured at the cellular level through the production of reactive oxygen species (ROS), leading to genetic mutations, the suppression of gene expression, and inhibition of peptide repair, resulting in inflammation and possibly leading to skin cancer (2,3,5–9). There are three major pathways of UV-induced skin inflammation. The first pathway involves the COX-2 mechanism whereby UV-B exposure induces arachidonic acid release from the phospholipid membrane and the COX-2 enzyme converts it to prostaglandins and free radical molecules (10–13). It has been reported that COX-2 and prostaglandin E2 elevations may be responsible for inflammation and tumorigenesis (11,13,14). The second includes the mitogen-activated protein kinase (MAPK) signaling pathways. After exposure to UV-B, phosphorylation of threonine and tyrosine leads to the activation of the MAPK protein kinase family. SPK/JNK and p38 kinase are activated (phospho-SPK/JNK and phospho-p38) in response to cellular stress and play a protective and pro-apoptotic role (5,15). Thus, UV-B is the main cause of inflammation in human keratinocytes via the MAPK pathways, JNK and p38 (16). The third includes the epidermal growth factor receptor (EGFR) pathway where UV-B-phosphorylated EGFR induces inflammation and skin tumorigenesis. Previous studies have demonstrated that EGFR regulates activation of p38 kinase leading to increased COX-2 and cytokine expressions (17,18).
The beneficial properties of mucus in UV protection have been established from research on fish. For example, coral reef fish can withstand UV due to mycosporine-like amino acids (MAAs) found in the external epithelial mucus that absorb UV (19). Relatively little is known regarding how mollusc mucus-derived compounds may mitigate UV-induced damage; yet, there is ample knowledge that molluscs, including abalone, snails, slugs, can survive in UV-exposed ecological niches. Molluscan mucus has increasingly been found to contain properties which are being exploited for use in medicines and cosmetics (20–23). For example, mucus-derived mucin compounds mixed with a honey gel were found to promote wound healing (24). Moreover, land snail (Achatina fulica) mucin promotes wound healing and provides anti-bacterial protection from Staphylococus aureus and Staphylococus epidermidis (25). Snail mucus has also been utilized as a skin cosmetic to promote skin regeneration, and as a treatment for acne, pigmentation, scarring, wrinkles, and inflammation and sun protection (23,26–29).
The abalone is a gastropod marine mollusc commonly recognized as a food source due to an edible foot muscle and constitutes an important culture industry for many countries (30,31). On the other hand, its visceral organs have not been recognized as valuable. Although the visceral organs do not appear to contain separate highly developed mucous glands as observed in snails, they do secrete copious amounts of mucus via numerous organs, including the foot muscle, hypobranchial gland (HBG) and gills (32,33). This mucus appears to be an important source for chemosensory cues required for nonspecific aggregation and larval settlement (34–37), and likely contains protective properties.
Human keratinocyte HaCaT cells have often been used for in vitro assays to understand epidermal homeostasis and processes associated with disease or injury. For example, HaCaT cells can be used to study molecular mechanisms related to abnormal human β2-defensin in response to cell cytokines (38). They have also been used to assess anti-inflammation and apoptosis from extracts derived from natural products, such as pearls (39) or pure compounds such as tectroside (40). In the present study, we explored the cell protective effects of abalone [Haliotis asinina (H. asinina)] extracts derived from the HBG and gills on HaCaT cells following UV-B exposure. For the majority of abalone species, the shell covers the entire animal, however in H. asinina the shell is reduced, leaving a significant proportion of the animal's body exposed. Its tropical distribution means that it is exposed to varying levels of UV. We demonstrated that there is significant improvement in cell survival following H. asinina tissue extract application. This is supported by our analysis of changes in the expression of inflammation-related proteins.
Materials and methods
Abalone HBG and gill extraction
Twenty adult male and female H. asinina (1–1.5 years of age) were obtained from the Coastal Aquaculture Research and Development Centre, Department of Fisheries, Prachuabkirikhan Province, Thailand. Animals were anesthetized by immersion in an ice bath for 15 min. The HBG and gills were rapidly dissected, washed and frozen in liquid nitrogen for storage at −80°C. Proteins and metabolites were extracted by homogenizing at 4°C in 0.1% trifluoroacetic acid using a Polytron homogenizer (Brinkmann Instruments, Westbury, NY, USA), followed by sonication. The extracts were centrifuged (15,000 × g for 30 min at 4°C) and then the supernatant was lyophilized.
Human keratinocyte culture
HaCaT cells were purchased from Cell Lines Service (CLS, Eppelheim, Germany). Cells were cultured under standard conditions of 5% CO2 in air at 37°C with medium renewal every 2 days. Culturing medium was Dulbecco's modified Eagle's medium (DMEM) (Gibco BRL, Gaithersburg, MD, USA) containing 5% fetal bovine serum (FBS), 10 μg/ml of penicillin/streptomycin. Cultured HaCaT cells were plated at a density of 5×105 cells/cm2 onto 60×100 cm2 tissue culture-treated Petri dishes (SPL Life Sciences Co., Ltd., Pochoen, Korea).
Cytotoxicity test of abalone HBG and gills extracts
HaCaT cells were trypsinized from subconfluent cultures by adding Trypsin-EDTA (0.25%) solution (Gibco BRL), and incubated for 10 min with regular gentle shaking. The trypsin reaction was stopped by adding 10 ml of DMEM (Gibco BRL) containing 10% FBS. The cell suspension was then centrifuged at 1,000 × g for 5 min at 25°C. The cell pellet was re-suspended in 2 ml of culturing medium with 5% FBS and mixed by vortexing. The cells were counted with a hemocytometer. Then, 1×105 cells were equally seeded into each well of a 96-well black plate and incubated at 37°C for 24 h. After that, the culturing medium was removed and replaced with 200 μl of serial abalone extracts in 1% serum-media. Serial concentrations of abalone extracts were from 0.1 to 50 mg/ml. The cells were then incubated at 37°C for 24 h and 100 μl of a 10% AlamarBlue solution (Invitrogen, Carlsbad, CA, USA) in serum-free medium was directly added to each well. The plate was incubated at 37°C for 4 h and the absorbance was measured at 540 and 630 nm with a fluorescent spectrophotometer (Epoch; BioTek, Winooski, VT, USA). The percentage of cell viability was analyzed by GraphPad Prism version 6 (GraphPad Software, Inc., San Diego, CA, USA) using one-way ANOVA at P<0.001.
UV-B irradiation of HaCaT cells
BLX-312 ultraviolet radiometer UV-B incubator emitted UV-B light with a wavelength of 300–320 nm. Irradiance was measured by UV sensor. To test UV-B irradiation, cultured HaCaT cells were divided into 4 groups: a control group and 3 experimental groups. In the control group, cells were exposed to serial dosages of UV-B from 10 to 110 mJ/cm2. In the experimental groups, cells are incubated with 10, 100 and 200 μg/ml abalone extract prior to and during the serial UV-B exposure. Then, cell viability, morphological changes and inflammatory cytokine expression levels were observed and measured by AlamarBlue, calcein AM-propidium iodide (PI) live-dead staining and western blot analysis.
AlamarBlue cell viability assay to compare the UV-B resistance of the control group with abalone extract groups
HaCaT cells were trypsinized from subconfluent cultures by adding trypsin solution, incubated for 10 min with regular gentle shaking. The trypsin reaction was stopped by adding 10 ml of DMEM culture medium containing 10% FBS. The cell suspension was then centrifuged at 1,000 × g for 5 min at 25°C. The cell pellet was re-suspended in 2 ml of culturing-medium with 5% FBS and properly mixed by vortexing. The cells were counted with a hemocytometer. Then, 1×105 cells were equally seeded into each well of a 96-well black-plate and incubated at 37°C for 24 h. After that, the culture medium was removed and replaced with 100 μl of serum-free medium. Ready to test cell plate was covered by a black sticker except one column opened for UV-B exposure. UV-B was emitted at 10 mJ/cm2, and then the UV-B exposure was stopped for 5 min and the next column sticker was removed and the next 10 mJ/cm2 UV-B dose was administered. The same protocol was repeating and continuing 10 times. Thus, the first column was exposed to 110 mJ/cm2 of UV-B, the 11th column received 1 mJ/cm2 and the last column was covered and exposed to no UV. Then, 100 μl of 10% AlamarBlue solution in serum-free medium was added to each well. The plate was incubated in 37°C for 4 h and the absorbance was measured at 540 and 630 nm with a fluorescent spectrophotometer (Epoch; BioTek). The number of viable cell was calculated as described previously (41). Next, viability of the cells in the experimental groups was tested in the same way as the control group but either the abalone HBG or gill extract was administered. In the experimental groups, the cells were incubated with abalone peptides at the concentrations of 10, 100, 200 μg/ml for 8 h prior to and during the UV-B exposure. After that the serial UV-B exposure and viability test was carried out in the same way as the control group. The percentage of cell viability was analyzed by GraphPad Prism version 6 (GraphPad Software, Inc.) using one-way ANOVA at P<0.001.
Calcein AM-PI live-dead assay and morphological change
Live-dead solution was prepared by adding: i) 250 μl of calcein AM (1 mg/ml stock solution); ii) 100 μl PI (1 mg/ml stock solution; iii) 3 ml of 5X binding buffer into serum-free medium to make 15 ml of calcein AM-PI live-dead staining solution (Molecular Probes Life Technologies, Carlsbad, CA, USA). The ready-to-use solution was maintained at 4°C. The fully grown HaCaT cells in 24-well Petri dishes were divided into two groups: i) control group; and ii) experimental group. In the control group, each dish was exposed to the serial UV-B doses started from 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, 100, 110 mJ/cm2. After every 10 mJ/cm2, the culture dish was taken out of an incubator and incubated at room temperature for 5 min and then put back in and given the next dose of 10 mJ of UV-B. In the experimental group, 100 μg of abalone HBG and gill extracts (1:1) was incubated with the cultured cells for 8 h prior and during UV-B exposure. Images were captured using phase contrast and fluorescence modes, with an inverted digital microscope (EVOS FLC, Life Technologies, Grand Island, NY, USA).
Protein determination and western blot analysis
Fully grown HaCaT cells in Petri dishes were divided into three groups: i) control group in which cells were cultured normally; ii) UV-B group in which cells were exposed to UV-B at 3×10 and 5×10 mJ/cm2; and iii) abalone peptide-treated group in which cells were incubated with 100 μg/ml peptides prior and during UV-B exposure and the cells were exposed to UV-B at 3×10 and 5×10 mJ/cm2. After that, confluent cells were scraped and sonicated in 5X lysis buffer containing Protease Inhibitor Cocktail (Merck Millipore, Darmstadt, Germany). Then, the lysates were centrifuged at 15,000 × g at 4°C for 30 min and the supernatants were frozen in liquid nitrogen. The frozen protein samples were lyophilized in a freeze dryer (Scanvac CoolSafe 110-4 PRO). Approximately 25 μg of extract was re-suspended in 0.1 M phosphate-buffered saline (PBS) and mixed with loading dye containing 50 mM Tris-HCl, 10% glycerol, 2% SDS, 100 mM DTT and 0.1% bromophenol blue. Samples were separated using 15% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were then transferred to 0.45-μm polyvinylidene fluoride membrane. The membranes were blocked for 1 h by blocking solution contained 4% non-fat dry milk, 2% bovine serum albumin (BSA) and 0.1% Tween-20 in Tris-buffered saline (TBS) pH 7.6. Polyclonal antibodies for COX-2 (ab52237), SPK/JNK (ab112501), phospho-SPK/JNK (ab4821), p38 (ab31828) and phospho-p38 (ab4822) were purchased from Abcam (Cambridge, UK) and were used at a 1:1,000 dilution to detect expression levels of the above proteins. The membranes were washed and incubated for 45 min with the secondary antibody (anti-rabbit HRP conjugated; Amersham plc, Amersham, UK) at a 1:5,000 dilution. Then, the protein bands were visualized by enhanced chemiluminescence (Amersham plc) under GENE GNOME (Syngene Bioimaging Private Ltd., Gurgaon, India). The western blot analyses were repeated in triplicate and band density was quantified using densitometry UN-SCAN-IT software (Silk Scientific, Inc., Orem, UT, USA). Protein expression levels were analyzed by GraphPad Prism version 6 (GraphPad Software, Inc.) using Dunnett's multiple comparison test.
Results
Cytotoxicity of extracts from abalone HBG and gills
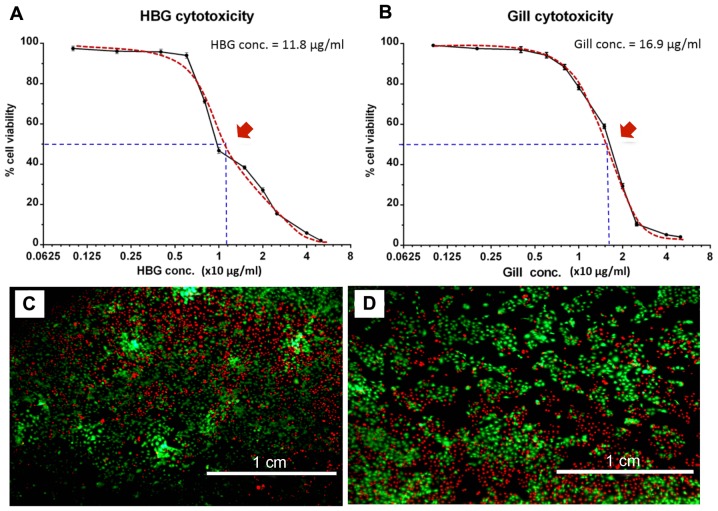
Optimal doses of abalone HBG and gill extracts were determined using an AlamarBlue cytotoxicity assay (Fig. 1A and B). For extracts derived from HBG, the maximal concentration that enabled cell survival of >50% was at 12.5 μg/ml (Fig. 1A and C). For extracts derived from gills, the concentration was at 12.5 μg/ml (Fig. 1B and D). Based on these results, subsequent experiments were performed using concentrations of 0.5, 2.5, 5.0 μg/ml of abalone tissue extracts.
Figure 1.
Analysis of the effective dose of abalone HBG and gill extracts on cell viability. Cytotoxicity test from abalone (A) HBG extract and (B) gill extract, using an AlamarBlue cell viability assay. Red arrows show the maximal concentrations of extract which allows for cell survival of >50%. (C and D) Live-dead staining (calcein AM-PI) of HaCaT cells treated with 12.5 μg/ml HBG and gill extract, respectively. HBG, hypobranchial gland; PI, propidium iodide.
Comparison of cell viability between UV-B control group and extract-treated groups
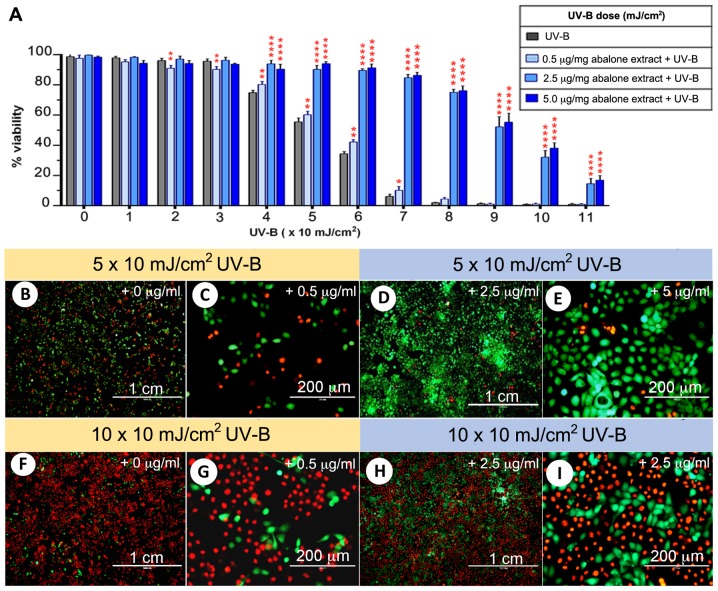
HaCaT cells were exposed to serial doses of UV-B, starting from 1×10 to 11×10 mJ/cm2, and then cell viability was compared between the four groups treated with the HBG and gill extracts (0, 0.1, 2.5 and 5.0 μg/ml with UV-B). At 1×10 to 3×10 mJ/cm2 UV-B, there was no difference in cell viability among the four groups. At 4×10 mJ/cm2 UV-B, the extract groups showed a significant increase in cell viability compared to the control group (Fig. 2A). In the control group, the cell viability was <50% at 6×10 mJ/cm2 UV-B, while in the extract groups it remained at >80%. Moreover, HaCaT cell survival was at >50% following 9×10 mJ/cm2 UV-B. HaCaT cell survival rates were further analyzed using a calcein AM-PI live-dead staining assay (Fig. 2B–I). In the 0 and 0.5 μg/ml extract-treated groups, 5×10 mJ/cm2 UV-B resulted in almost 50% cell death (Fig. 2B and C), while the survival rate was >90% in the 2.5 and 5 μg/ml extract-treated groups at the same UV-B level (Fig. 2D and E). At 10×10 mJ/cm2 UV-B, there was almost complete cell death (0.84% survival) in the control groups (Fig. 2F and G), while there was >30% cell survival when cells were incubated with 2.5 μg/ml abalone extract at the same UV-B level (Fig. 2H and I).
Figure 2.
Analysis of effective doses for abalone HBG and gill extract under UVB and HaCaT cell viability. (A) Graph comparing HaCaT cell viability among the four groups. Significant difference in cell viability when compared to the control group is shown at ****P<0.001, **P<0.01 and *P<0.05. (B-I) Representative images of HaCaT cells treated with UV-B with and without extract, showing live-dead staining as indicated by green and red, respectively. HBG, hypobranchial gland.
Changes in cell morphology in response to UV-B and abalone extracts
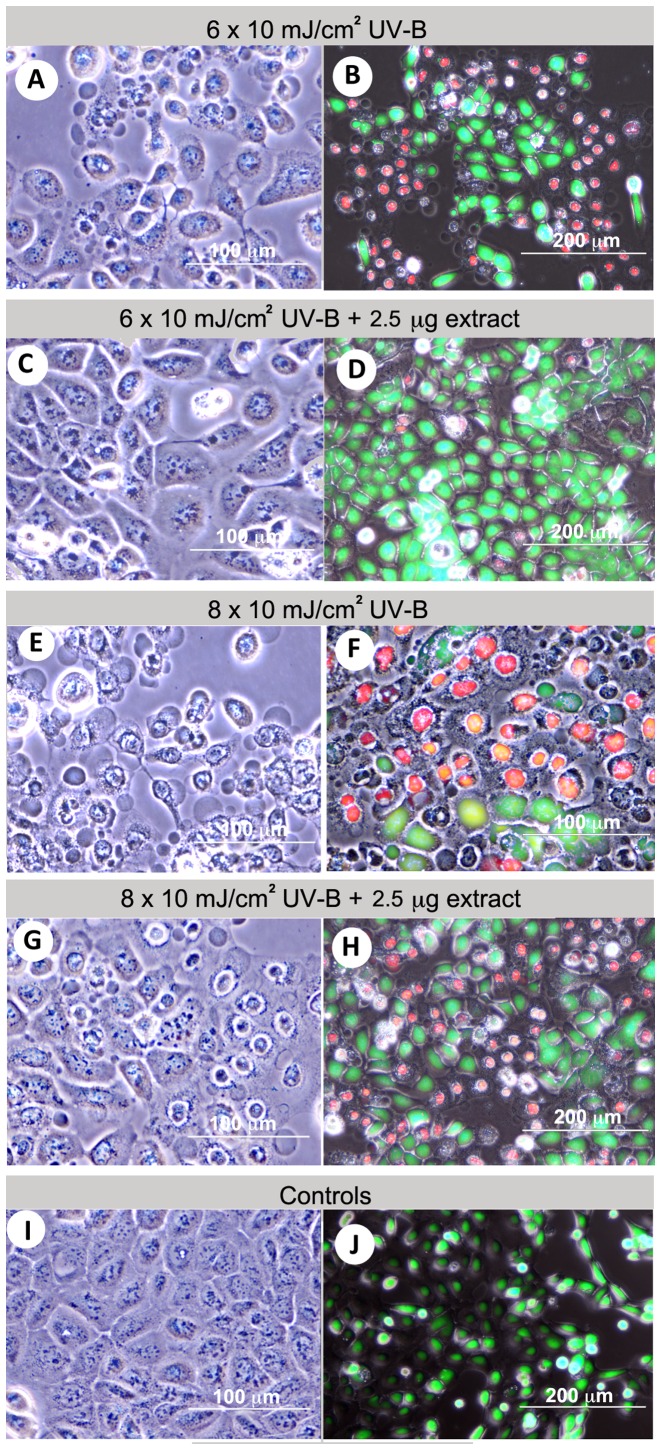
To monitor changes in cell morphology by calcein AM-PI live-dead staining, phase contrast and fluorescence microscopy was used (Fig. 3A–J). HaCaT cell morphological changes and viability following UV-B exposure (6×10 mJ/cm2) were assessed in two groups: i) control group, where HaCaT cells were exposed to UV-B alone; and ii) the experimental group where HaCaT cells received 2.5 μg/ml abalone extracts (1:1 HBG and gill extract) before UV-B exposure. UV-B exposure resulted in three obvious changes in cell morphology: i) cytoplasmic condensation; ii) cell shrinkage and membrane budding; and iii) condensation of chromatin. Fig. 3A–D shows representative micrographs of HaCaT cells with morphological changes after exposure to 6×10 mJ/cm2 UV-B alone and with the abalone extract co-treatment. In the presence of 2.5 μg/ml of abalone extract, most cells were viable with only minor morphological changes being observed. At 8×10 mJ/cm2 UV-B, most cells in the control group were dead (Fig. 3E and F) while in the experimental group there was >50% cell survival (Fig. 3G and H). This was also observed in the abalone extract-treated groups exposed to a higher UV-B dose (10×10 mJ/cm2). In the negative control (no UV-B exposure) (Fig. 3I and J), HaCaT cells appeared as round or rectangular in shape with an ~40–80 μm diameter. In addition, the nucleus occupied almost half of the total area (with 10–20 μm nucleolus), and the plasma membranes were clear, often attached to neighboring cells.
Figure 3.
Protective effect of abalone extracts following UV-B exposure, based on cell survival and morphology. Bright field (left) and fluorescence (right) images showing HaCaT cells: (A and B) after exposure to 6×10 mJ/cm2 UV-B; (C and D) pre-treated with 2.5 μg/ml abalone extract and exposed to 6×10 mJ/cm2 UV-B; (E and F) after exposure to 8×10 mJ/cm2 UV-B; (G and H) pre-treated with 2.5 μg abalone extracts and exposed to 8×10 mJ/cm2 UV-B. (I and J) Controls with no UV-B exposure or to extract. Red staining represents dead cells and green staining represents live cells. Three morphological cell types are observed, shown as (A, E and G) membrane bubbling, (A and E) condensation of cytoplasm and (A, E and G) chromatin.
Measurement of inflammatory proteins following UV-B irradiation of HaCaT cells
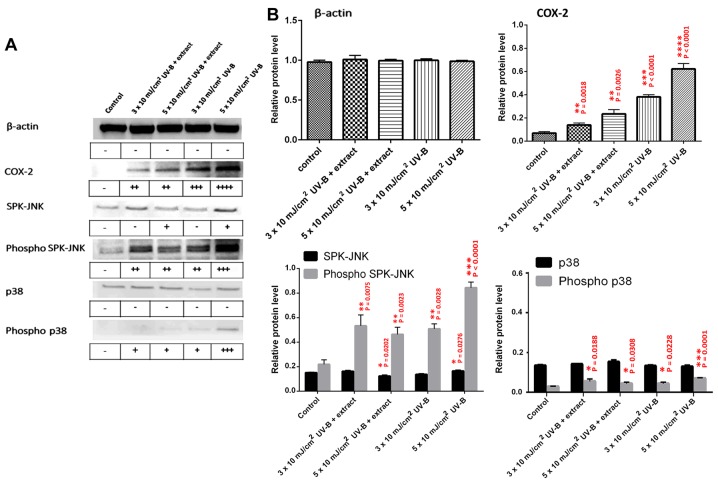
Expression of inflammation-related proteins (COX-2, SPK/JNK, phospho-SPK/JNK, p38, and phospho-p38) was analyzed by western blot analysis of HaCaT cell extracts following UV-B exposure, with or without treatment with the abalone extracts (Fig. 4A and B). The control represents HaCaT cell extracts with no UV-B and no abalone extract. HaCaT cells were also exposed to UV-B (3×10 and 5×10 mJ/cm2) with or without 2.5 μg/ml abalone extract. No significant change was observed in β-actin protein levels for any treatment. Abalone extract did show more significant inhibition of COX-2 expression when compared to the levels observed at 3×10 and 5×10 mJ/cm2 UV-B. The extract also resulted in a more significant inhibition of the inflammatory-related active forms of SPK-JNK and p38, namely phospho-SPK-JNK and phospho-p-38, when compared to these levels following exposure to 5×10 mJ/cm2 UV-B without the extract.
Figure 4.
Western blot analysis of inflammatory-related proteins present in HaCaT cells after UV-B exposure and with abalone extract. (A) Representative western blots, and (B) graphs showing relative protein levels for each protein. Densities of bands (quantified using densitometry UN-SCAN-IT software) were compared to the control group to identify relative change. −, no significant difference; +, significant difference at P<0.05; ++, significant difference at P<0.01; +++, significant difference at P<0.001; and ++++, significant difference at P<0.0001.
Discussion
In the present study, we investigated the effects of extracts derived from abalone tissues well known for secreting mucus, to ascertain whether they attenuate UV-based cell damage. We found that abalone tissue extracts attenuated the effects of UV-B on HaCaT cells, as determined by both morphological and molecular analyses of inflammatory-related proteins.
Having established that HBG and gill extract doses less than 10 μg/ml were not detrimental to HaCaT cell survival, we then tested cell survival in the presence of various concentrations of extracts with UV-B exposure. We demonstrated the capacity for abalone extracts to inhibit the process of inflammation at 4–8 mJ/cm2 UV-B, and morphological analysis and protein expression profiling supported this. In the absence of extract (control) HaCaT cells showed cytoplasmic condensation, cell shrinkage, membrane budding and accumulation of the condensation of chromatin after being exposed to UV-B. In support of the protective effects of the abalone extracts, COX-2 and phosphorylated forms of SPK-JNK and p38, were relatively less highly expressed when the extracts were applied to HaCaT cells exposed to 5×10 mJ/cm2 UV-B
The UV absorbent properties of animal mucus have been well studied in fish and corals. In corals, the algal symbionts help determine the mucus composition, including the release of MAAs which protect against UV (42). Other biomolecules that may act against photochemical damage to UV-B include cytosolic water-soluble reductants and membrane-bound lipid-soluble antioxidants.
In tropical fish species, MAAs present within the epithelial mucus are critical for absorbing UV-A and UV-B, with strong absorbance peaks between 290 and 400 nm (19). The requirement for UV-absorbent compounds in tropical water is essential, since this water is often low in UV-absorbing particulate and dissolved organic matter (43). H. asinina is distributed throughout many tropical regions worldwide, including the Great Barrier Reef (44). To date, analysis of its mucus composition has primarily focused on the existence of water-soluble peptides that may be required for non-specific communication, showing that there are three major water-soluble peptides that are released in large amounts from secretory cells and diffuse into the surrounding seawater (36). Future studies should explore the existence of MAAs, or other known and novel UV-absorbing biomolecules.
Besides the role of mucus in UV protection, other medically relevant applications should be explored. Of most interest are the bioactive compounds secreted by marine snails in the family Muricidae, and in particular their brominated indoles released from the HBG that appear to have anti-inflammatory, anticancer and steriodogenic activity (45). A recent study of the mosquito Aedes aegypti analyzed salivary gland extracts, demonstrating that semi-purified preparations can ameliorate inflammatory bowel disease (46). In addition, anti-inflammatory proteins have been discovered within the salivary gland extracts of the tick, Hyalomma anatolicumanatolicum (47) and horsefly Tabanusyao (48). Moreover, researchers discovered a free amino acid from Haliotis discus water named taurine, which has anti-inflammatory and antioxidant potentials in zebrafish (49). These types of discoveries clearly indicate that numerous other animal mucus-associated biomolecules could be used for human medicine. Moreover, numerous unpublished reports suggest the benefits of land snail mucus for skin anti-aging and wound healing. Abalone, also a gastropod, could possibly have similar, yet unexplored advantageous attributes.
In conclusion, the present study demonstrated the ability of biomolecules derived from tropical abalone gland extracts to attenuate UV-B damage. Without extract, HaCaT cell viability was significantly reduced upon exposure to UV-B at 5 mJ/cm2. This was determined based on morphological changes, live-dead staining assay and analysis of changes in the abundance of inflammatory-related proteins. Subsequent research will be carried out to determine the exact factors in the abalone extracts that are responsible for these property.
Acknowledgments
We sincerely thank the Strategic Wisdom and Research Institute (Srinakharinwirot University, Thailand) and Research and Foreign Relation Department, Faculty of Medicine (Srinakharinwirot University, Thailand) for the financial support and professional cooperation.
References
- 1.de Gruijl FR, Van der Leun JC. Estimate of the wavelength dependency of ultraviolet carcinogenesis in humans and its relevance to the risk assessment of a stratospheric ozone depletion. Health Phys. 1994;67:319–325. doi: 10.1097/00004032-199410000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Svobodova A, Walterova D, Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 3.Nichols JA, Katiyar SK. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch Dermatol Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afaq F. Natural agents: Cellular and molecular mechanisms of photoprotection. Arch Biochem Biophys. 2011;508:144–151. doi: 10.1016/j.abb.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Camarillo C, Ocampo EA, Casamichana ML, Pérez- Plasencia C, Alvarez-Sánchez E, Marchat LA. Protein kinases and transcription factors activation in response to UV-radiation of skin: Implications for carcinogenesis. Int J Mol Sci. 2012;13:142–172. doi: 10.3390/ijms13010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bender K, Göttlicher M, Whiteside S, Rahmsdorf HJ, Herrlich P. Sequential DNA damage-independent and -dependent activation of NF-kappaB by UV. EMBO J. 1998;17:5170–5181. doi: 10.1093/emboj/17.17.5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez-Pigeon H, Jean C, Charruyer A, Haure MJ, Titeux M, Tonasso L, Quillet-Mary A, Baudouin C, Charveron M, Laurent G. Human keratinocytes acquire cellular cytotoxicity under UV-B irradiation. Implication of granzyme B and perforin. J Biol Chem. 2006;281:13525–13532. doi: 10.1074/jbc.M512694200. [DOI] [PubMed] [Google Scholar]
- 8.Ali D, Verma A, Mujtaba F, Dwivedi A, Hans RK, Ray RS. UV-B-induced apoptosis and DNA damaging potential of chrysene via reactive oxygen species in human keratinocytes. Toxicol Lett. 2011;204:199–207. doi: 10.1016/j.toxlet.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 9.Wu MS, Sun DS, Lin YC, Cheng CL, Hung SC, Chen PK, Yang JH, Chang HH. Nanodiamonds protect skin from ultraviolet B-induced damage in mice. J Nanobiotechnology. 2015;13:35. doi: 10.1186/s12951-015-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devary Y, Rosette C, DiDonato JA, Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 11.Buckman SY, Gresham A, Hale P, Hruza G, Anast J, Masferrer J, Pentland AP. COX-2 expression is induced by UV-B exposure in human skin: Implications for the development of skin cancer. Carcinogenesis. 1998;19:723–729. doi: 10.1093/carcin/19.5.723. [DOI] [PubMed] [Google Scholar]
- 12.Muller-Decker K, Neufang G, Berger I, Neumann M, Marks F, Furstenberger G. Transgenic cyclooxygenase-2 overexpression sensitizes mouse skin for carcinogenesis. Proc Natl Acad Sci USA. 2002;99:12483–12488. doi: 10.1073/pnas.192323799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shibata A, Nakagawa K, Yamanoi H, Tsuduki T, Sookwong P, Higuchi O, Kimura F, Miyazawa T. Sulforaphane suppresses ultraviolet B-induced inflammation in HaCaT keratinocytes and HR-1 hairless mice. J Nutr Biochem. 2010;21:702–709. doi: 10.1016/j.jnutbio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Mizu H, Yamauchi H. Photoinflammatory responses to UV-irradiated ketoprofen mediated by the induction of ROS generation, enhancement of cyclooxygenase-2 expression, and regulation of multiple signaling pathways. Free Radic Biol Med. 2010;48:772–780. doi: 10.1016/j.freeradbiomed.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Kim JE, Kwon JY, Seo SK, Son JE, Jung SK, Min SY, Hwang MK, Heo YS, Lee KW, Lee HJ. Cyanidin suppresses ultraviolet B-induced COX-2 expression in epidermal cells by targeting MKK4, MEK1, and Raf-1. Biochem Pharmacol. 2010;79:1473–1482. doi: 10.1016/j.bcp.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 16.Englaro W, Dérijard B, Ortonne JP, Ballotti R. Solar ultraviolet light activates extracellular signal-regulated kinases and the ternary complex factor in human normal keratinocytes. Oncogene. 1998;16:661–664. doi: 10.1038/sj.onc.1201536. [DOI] [PubMed] [Google Scholar]
- 17.Ashida M, Bito T, Budiyanto A, Ichihashi M, Ueda M. Involvement of EGF receptor activation in the induction of cyclooxygenase-2 in HaCaT keratinocytes after UV-B. Exp Dermatol. 2003;12:445–452. doi: 10.1034/j.1600-0625.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 18.El-Abaseri TB, Hammiller B, Repertinger SK, Hansen LA. The epidermal growth factor receptor increases cytokine production and cutaneous inflammation in response to ultraviolet irradiation. ISRN Dermatol. 2013;2013:848705. doi: 10.1155/2013/848705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamzow JP, Losey GS. Ultraviolet radiation absorbance by coral reef fish mucus: Photo-protection and visual communication. Environ Biol Fishes. 2002;63:41–47. doi: 10.1023/A:1013846816869. [DOI] [Google Scholar]
- 20.Jacobson PB, Jacobs RS. Fuscoside: An anti-inflammatory marine natural product which selectively inhibits 5-lipoxygenase Part I: Physiological and biochemical studies in murine inflammatory models. J Pharmacol Exp Ther. 1992;262:866–873. [PubMed] [Google Scholar]
- 21.Ojika M, Kigoshi H, Yoshida Y, Ishigaki T, Nisiwaki M, Tsukada I, Arakawa M, Ekimoto H, Yamada K. Aplyronine A, a potent antitumor macrolide of marine origin, and the congeners aplyronines B and C: Isolation, structures, and bioactivities. Tetrahedron. 2007;63:3138–3167. doi: 10.1016/j.tet.2007.02.011. [DOI] [Google Scholar]
- 22.Uddin MH, Otsuka M, Muroi T, Ono A, Hanif N, Matsuda S, Higa T, Tanaka J. Deoxymanoalides from the nudibranch Chromodoris willani. Chem Pharm Bull (Tokyo) 2009;57:885–887. doi: 10.1248/cpb.57.885. [DOI] [PubMed] [Google Scholar]
- 23.Yamada K, Ojika M, Kigoshi H, Suenaga K. Aplyronine A, a potent antitumor macrolide of marine origin, and the congeners aplyronines B-H: Chemistry and biology. Nat Prod Rep. 2009;26:27–43. doi: 10.1039/B800263K. [DOI] [PubMed] [Google Scholar]
- 24.Adikwu MU, Alozie BU. Application of snail mucin dispersed in detarium gum gel in wound healing. Sci Res Essays. 2007;2:195–198. [Google Scholar]
- 25.Santana WA, Melo CM, Cardoso JC, Pereira-Filho RN, Rabelo AS, Reis FP, Albuquerque-Júnior RLC. Assessment of antimicrobial activity and healing potential of mucous secretion of Achatina fulica. Int J Morphol. 2012;30:365–373. doi: 10.4067/S0717-95022012000200001. [DOI] [Google Scholar]
- 26.Shimidzu N, Goto M, Miki W. Carotenoids as singlet oxygen quenchers in marine organisms. Fish Sci. 1996;62:134–137. [Google Scholar]
- 27.Bonnemain B. Helix and drugs: Snails for western health care from antiquity to the present. Evid Based Complement Alternat Med : eCAM. 2005;2:25–28. doi: 10.1093/ecam/neh057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Orazio N, Gammone MA, Gemello E, De Girolamo M, Cusenza S, Riccioni G. Marine bioactives: Pharmacological properties and potential applications against inflammatory diseases. Mar Drugs. 2012;10:812–833. doi: 10.3390/md10040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz MC, Sanz-Rodríguez F, Zamarrón A, Reyes E, Carrasco E, González S, Juarranz A. A secretion of the mollusc Cryptomphalus aspersa promotes proliferation, migration and survival of keratinocytes and dermal fibroblasts in vitro. Int J Cosmet Sci. 2012;34:183–189. doi: 10.1111/j.1468-2494.2011.00699.x. [DOI] [PubMed] [Google Scholar]
- 30.Chalermwat K, Szuster BW, Flaherty M. Shellfish aquaculture in Thailand. Aquac Econ Manag. 2003;7:249–261. doi: 10.1080/13657300309380343. [DOI] [Google Scholar]
- 31.Cook PA, Roy Gordon H. World abalone supply, markets, and pricing. J Shellfish Res. 2010;29:569–571. doi: 10.2983/035.029.0303. [DOI] [Google Scholar]
- 32.Wanichanon C, Laimek P, Linthong V, Sretarugsa P, Kruatrachue M, Upatham ES, Poomtong T, Sobhon P. Histology of hypobranchial glands and gills of Haliotis asinina Linnaeus. J Shellfish Res. 2004;23:1107–1112. [Google Scholar]
- 33.Naegel LCA, Aguilar-Cruz CA. The hypobranchial gland from the purple snail Plicopurpura pansa (Gould, 1853) (Prosobranchia: Muricidae) J Shellfish Res. 2006;25:391–394. doi: 10.2983/0730-8000(2006)25[391:THGFTP]2.0.CO;2. [DOI] [Google Scholar]
- 34.Laimek P, Clark S, Stewart M, Pfeffer F, Wanichanon C, Hanna P, Sobhon P. The presence of GABA in gastropod mucus and its role in inducing larval settlement. J Exp Mar Biol Ecol. 2008;354:182–191. doi: 10.1016/j.jembe.2007.11.003. [DOI] [Google Scholar]
- 35.Kuanpradit C, Cummins SF, Degnan BM, Sretarugsa P, Hanna PJ, Sobhon P, Chavadej J. Identification of an attractin-like pheromone in the mucus-secreting hypobranchial gland of the abalone Haliotis asinina linnaeus. J Shellfish Res. 2010;29:699–704. doi: 10.2983/035.029.0321. [DOI] [Google Scholar]
- 36.Kuanpradit C, Stewart MJ, York PS, Degnan BM, Sobhon P, Hanna PJ, Chavadej J, Cummins SF. Characterization of mucus-associated proteins from abalone (Haliotis) - candidates for chemical signaling. FEBS J. 2012;279:437–450. doi: 10.1111/j.1742-4658.2011.08436.x. [DOI] [PubMed] [Google Scholar]
- 37.Stewart P, Williams EA, Stewart MJ, Soonklang N, Degnan SM, Cummins SF, Hanna PJ, Sobhon P. Characterization of a GABAA receptor β subunit in the abalone Haliotis asinina that is upregulated during larval development. J Exp Mar Biol Ecol. 2011;410:53–60. doi: 10.1016/j.jembe.2011.10.005. [DOI] [Google Scholar]
- 38.Seo MD, Kang TJ, Lee CH, Lee AY, Noh M. HaCaT keratinocytes and primary epidermal keratinocytes have different transcriptional profiles of cornified envelope-associated genes to T helper cell cytokines. Biomol Ther (Seoul) 2012;20:171–176. doi: 10.4062/biomolther.2012.20.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang YL, Chang CH, Huang CC, Liu HW. Anti- inflammation and anti-apoptosis effects of pearl extract gel on UV-B irradiation HaCaT cells. Biomed Mater Eng. 2015;26(Suppl 1):S139–S145. doi: 10.3233/BME-151299. [DOI] [PubMed] [Google Scholar]
- 40.Kim SB, Kang OH, Joung DK, Mun SH, Seo YS, Cha MR, Ryu SY, Shin DW, Kwon DY. Anti-inflammatory effects of tectroside on UV-B-induced HaCaT cells. Int J Mol Med. 2013;31:1471–1476. doi: 10.3892/ijmm.2013.1343. [DOI] [PubMed] [Google Scholar]
- 41.Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum Reprod. 2007;22:1304–1309. doi: 10.1093/humrep/dem011. [DOI] [PubMed] [Google Scholar]
- 42.Shick JM, Lesser MP, Dunlap WC, Stochaj WR, Chalker BE, Won JW. Depth dependent responses to solar ultraviolet radiation and oxidative stress in the zooxanthellate coral Acropora microphthalma. Mar Biol. 1995;122:41–51. doi: 10.1007/BF00349276. [DOI] [Google Scholar]
- 43.Baker KS, Smith RC, Green AES. Middle ultraviolet radiation reaching the ocean surface. Photochem Photobiol. 1980;32:367–374. doi: 10.1111/j.1751-1097.1980.tb03776.x. [DOI] [Google Scholar]
- 44.Geiger DL. Distribution and biogeography of the recent Haliotidae (Gastropoda: Vetigastropoda) world-wide. Bollettino Malacologico. 2000;35:57–120. [Google Scholar]
- 45.Benkendorff K, Rudd D, Nongmaithem BD, Liu L, Young F, Edwards V, Avila C, Abbott CA. Are the traditional medical uses of muricidae molluscs substantiated by their pharmacological properties and bioactive compounds? Mar Drugs. 2015;13:5237–5275. doi: 10.3390/md13085237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sales-Campos H, de Souza PR, Basso PJ, Ramos AD, Nardini V, Chica JE, Capurro ML, Sá-Nunes A, de Barros Cardoso CR. Aedes aegypti salivary gland extract ameliorates experimental inflammatory bowel disease. Int Immunopharmacol. 2015;26:13–22. doi: 10.1016/j.intimp.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh M, Sangwan N, Sangwan AK. Partial characterization of a novel anti-inflammatory protein from salivary gland extract of Hyalomma anatolicum anatolicum (77Acari: Ixodidae) ticks. Vet World. 2015;8:772–776. doi: 10.14202/vetworld.2015.772-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei L, Huang C, Yang H, Li M, Yang J, Qiao X, Mu L, Xiong F, Wu J, Xu W. A potent anti-inflammatory peptide from the salivary glands of horsefly. Parasit Vectors. 2015;8:556. doi: 10.1186/s13071-015-1149-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheong SH, Hwang JW, Lee SH, Kim YS, Sim EJ, You BI, Lee SH, Park DJ, Ahn CB, Kim EK, et al. In vitro and in vivo antioxidant and anti-inflammatory activities of abalone (Haliotis discus) water extract. Adv Exp Med Biol. 2015;803:833–849. doi: 10.1007/978-3-319-15126-7_67. [DOI] [PubMed] [Google Scholar]