Abstract
The main objective of this prospective longitudinal study was to investigate bidirectional associations between adolescent cannabis use (CU) and neurocognitive performance in a community sample of 294 young men from ages 13 to 20 years. The results showed that in early adolescence, and prior to initiation to CU, poor short-term and working memory, but high verbal IQ, were associated with earlier age of onset of CU. In turn, age of CU onset and CU frequency across adolescence were associated with (a) specific neurocognitive decline in verbal IQ and executive function tasks tapping trial and error learning and reward processing by early adulthood and (b) lower rates of high-school graduation. The association between CU onset and change in neurocognitive function, however, was found to be accounted for by CU frequency. Whereas the link between CU frequency across adolescence and change in verbal IQ was explained (mediated) by high school graduation, the link between CU frequency and tasks tapping trial and error learning were independent from high school graduation, concurrent cannabis and other substance use, adolescent alcohol use, and externalizing behaviors. Findings support prevention efforts aimed at delaying onset and reducing frequency of CU.
Cannabis is the most widely used substance worldwide (Degenhardt & Hall, 2012; Degenhardt, Stockings, Patton, Hall, & Lynskey, 2016; Hall et al., 2016) and is perceived, among adolescents, as less harmful than other substances (Johnston, O’Malley, Bachman, & Schulenberg, 2013). The debate surrounding the beneficial and harmful effects following recreational or medical use still continues, especially as adolescence is viewed as a period of vulnerability for brain development, and cannabis as a threat to this development. There is evidence to suggest that the adolescent brain may be particularly vulnerable to the neurotoxic effects of substances, especially with regard to neurocognitive functioning (Rubino et al., 2009; Schweinsburg, Brown, & Tapert, 2008). This is because adolescence represents a critical period of neurodevelopment, characterized by synaptic pruning and increased myelination, particularly in cortical and frontal areas of the brain. Moreover, the endocannabinoid system appears to be involved in the regulation of the key neurodevelopmental processes of synaptic pruning and myelination (Lubman, Cheetham, & Yucel, 2015; Paus, 2005, 2007), suggesting that the introduction of exogenous cannabinoids during adolescence could disrupt normal brain development and, in turn (Lubman et al., 2015; Rubino & Parolaro, 2016), have an impact on cognitive function (Broyd, van Hell, Beale, Yucel, & Solowij, 2016; Volkow et al., 2016). Some support for this has come from animal and human studies showing that cannabis exposure during adolescence was associated with morphological and connectivity changes in brain structures that are densely populated with cannabinoid receptors (e.g., in the prefrontal cortex [PFC], hippocampus, and cerebellum; Burns et al., 2007; Realini, Rubino, & Parolaro, 2009; Rubino et al., 2015).
Although the mediating role of these structural/morphological changes resulting from cannabis use (CU) and cognitive function has yet to be demonstrated, several studies show that heavy, long-term CU is associated concurrently with impaired neurocognitive function (NCF) in animals and human adults (e.g., Fried, Watkinson, & Gray, 2005; Lubman et al., 2015; Rubino & Parolaro, 2016; Verrico, Gu, Peterson, Sampson, & Lewis, 2014). Similarly, studies comparing adolescents using cannabis on a regular basis (i.e., weekly) to controls reported that they have reduced processing speed (Gruber, Sagar, Dahlgren, Racine, & Lukas, 2012; Lisdahl & Price, 2012; Medina et al., 2007) and perform worse on tasks assessing attention (Hanson et al., 2010; Lisdahl & Price, 2012; Mathias et al., 2011; Medina et al., 2007), verbal memory (Hanson et al., 2010; Medina et al., 2007), intelligence (Harvey, Sellman, Porter, & Frampton, 2007), and executive function (Grant, Chamberlain, Schreiber, & Odlaug, 2012; Harvey et al., 2007; Lisdahl & Price, 2012; Mathias et al., 2011; Medina et al., 2007). Some suggest that these effects may persist even after extended periods of abstinence (Bolla, Brown, Eldreth, Tate, & Cadet, 2002; Broyd et al., 2016; Crean, Crane, & Mason, 2011; Lyons et al., 2004), but this is still debated as meta-analyses showed that all non-acute, residual effects of CU on cognitive performance in adults and adolescents were no longer detectable after 1 month or more of abstinence (Schreiner & Dunn, 2012; Schulte et al., 2014). Nonetheless, most human studies contributing to this debate are cross-sectional case-control or retrospective, without proper assessment of cognitive function prior to CU onset. This is an important limitation, as presubstance use performance on certain IQ and executive function tasks (assessed, e.g., at 12–14 years) have been shown to be linked with later substance use onset and increased CU frequency (Castellanos-Ryan, Séguin, Vitaro, Parent, & Tremblay, 2013; Squeglia, Jacobus, Nguyen-Louie, & Tapert, 2014; White & Batty, 2012) or be associated with change in CU frequency and severity (Cousijn et al., 2014). In the absence of clinical trials, prospective longitudinal studies that include pre- and postdrug use neurocognitive assessments are needed to clarify temporal associations between CU and neurocognitive development and guide prevention efforts.
Only a few studies have examined the prospective specific association between adolescent CU and NCF. One longitudinal study following adolescents with substance use disorders, including CU disorder, over time, found that greater cumulative CU over an 8-year follow-up period was associated with poorer attention functioning (Tapert, Granholm, Leedy, & Brown, 2002). However, although specific effects of CU were examined, authors caution that their findings cannot be attributed solely to any one substance, as polysubstance use was the norm rather than the exception in that sample. In another study, by Fried et al. (2005), concurrent chronic, but not former, heavy cannabis users showed lower scores on IQ (d ≈ 0.29) and memory (d ≈ 0.30) and faster processing speed (d ≈ 0.44) at ages 17–20 years relative to nonusers, adjusting for pre-CU onset neurocognitive performance (9–12 years). However, chronic CU was based on retrospective reports, and it is unclear if early onset of use was specifically related to neurocognitive decline beyond the effects of concurrent use. In contrast, a recent prospective longitudinal study by Meier et al. (2012) showed that persistent CU over a 20-year period across young adulthood (18–38 years) was associated with a global decline in IQ and neurocognitive performance from ages 13 to 38 years (d = 0.38). In that study, the association between persistent CU and neurocognitive decline remained when controlling for education, personality, other persistent substance use (SU) and socioeconomic status (SES; Moffitt, Meier, Caspi, & Poulton, 2013), but only for those whose persistent CU disorder was already present at 18 years. Further, secondary analyses on a small subsample showed that significant decline in NCF was still detectable in early users who reported infrequent (N = 17) CU in the year prior to testing in adulthood. Thus, in contrast to the findings of two meta-analyses (Schreiner & Dunn, 2012; Schulte et al., 2014), Meier et al.’s findings suggest that the decline in NCF was independent of frequent concurrent use (i.e., it did not recover with abstinence), and the authors question if onset of frequent use before age 19 may have detrimental and potentially irreversible effects on brain development in the long term. In sum, further studies are still needed not only because of the contrasting results between studies of adolescence and adulthood but also because the particular findings reported by Meier et al. (2012) were based on a small subsample of participants.
Furthermore, independent of potential neurotoxic effects of CU on the developing brain, the onset of CU is thought to alter a youth’s developmental trajectory in several ways. Adolescents who use cannabis regularly not only tend to have higher rates of early-onset behavioral problems (Flory, Lynam, Milich, Leukefeld, & Clayton, 2004; Heron et al., 2013; Windle & Wiesner, 2004), social adversity (von Sydow, Lieb, Pfister, Hofler, & Wittchen, 2002), and other adolescent SU (Hibell et al., 2012), but also report poor educational performance and higher school dropout (Fergusson, Boden, & Horwood, 2015; Fergusson, Horwood, & Beautrais, 2003; Flory et al., 2004; Lynskey, Coffey, Degenhardt, Carlin, & Patton, 2003; Lynskey & Hall, 2000; McCaffrey, Pacula, Han, & Ellickson, 2010; Silins et al., 2014; Stiby et al., 2015; Windle & Wiesner, 2004), all of which may confound the relationships between CU and declines in cognitive function. Measures of school achievement and high school graduation, in particular, should be taken into account as adolescent CU is hypothesized to be associated with poor school attainment through its association with cognitive impairment. However, the opposite could also be true; CU may be associated with declines in cognitive function, at least in part, through poor school attainment (e.g., Silins et al., 2014), which in turn may have an impact on cognitive development (Brinch & Galloway, 2012).
Within this context we aim to investigate the bidirectional associations between CU and cognitive function and their association with other important covariates: (a) whether pre-CU cognitive function is linked to CU onset and later frequency of use; (b) whether cognitive decline associated with adolescent onset of frequent use may be detected by young adulthood; and (c) whether it is actual adolescent-onset CU, independent of other factors (e.g., SES, high school graduation, adolescent alcohol use, externalizing problems, concurrent SU, and pre-CU cognitive function; Cousijn et al., 2014), that is associated with neurocognitive impairments. If declines in NCF are a direct result of adolescent CU, potentially through its neurotoxic effects on the developing brain, then one would hypothesize that (a) CU would precede a drop in cognitive functioning; (b) an earlier age of onset and more frequent CU would be associated with a larger cognitive decline; and (c) the association would persist after adjusting for important covariates, such as other concurrent SU, pre-CU cognitive function, externalizing problems, and academic achievement. Furthermore, one might expect that the PFCs would be especially likely to be involved, as the PFCs have a high density of cannabinoid receptors and continue to develop during adolescence. Therefore, one could hypothesize that (d) CU during adolescence would be associated with larger declines in cognitive function related to prefrontal cortices of the brain (e.g., performance on self-ordered pointing and conditional association tasks associated with mid- and posterior–dorsolateral frontal lobes, respectively) more than in function related to other brain areas (e.g., performance on paired associates learning and digit span tests associated with the hippocampus and medial temporal lobes). Alternatively, if associations between CU and cognitive decline do not persist after controlling for covariates, and there is evidence that academic achievement and/or other factors account for these associations, then results would provide support for other, more social, mechanisms by which CU and declines in cognitive function are associated. These are all important unresolved questions for planning prevention. We have addressed these questions in a prospective longitudinal design that assessed CU frequency yearly across adolescence (14–17 years) and NCF twice, in early adolescence and early adulthood.
Materials and Methods
Sample
The 294 young men who participated in this study were a sub-sample of the Montreal Longitudinal and Experimental Study of Low SES boys (MLES). In the spring of 1984, 1,037 boys attending the last year of kindergarten (M age = 6.1 years) were recruited from schools in low-SES neighborhoods of Montreal. For further information on recruitment and selection criteria of the MLES sample, see Vitaro, Brendgen, Pagani, Tremblay, and McDuff (1999) and Tremblay, Pihl, Vitaro, and Dobkin (1994). Sex (boys), ethnicity (Caucasian), and SES (low to middle SES) were homogeneous as a result of the selection procedure. Boys were then assessed annually from 10 to 17 years and again at 20 years. This study was approved by the University of Montreal Institutional Review Board, with participation in the study requiring both parental consent and child assent. The 294 young men included in this study were selected for neurocognitive testing when they were on average 20 years of age (range = 18.47–22.02), based on trajectories of teacher-rated physical aggression and hyperactivity scores across childhood and adolescence; an attempt was made to recruit equal numbers of high and low scorers. Four hundred and ninety-four participants were identified and contacted over a period of 18 months until a target number of 300 participants were recruited. Three hundred and four young men (151 low aggressive and hyperactive, 87 high aggressive and hyperactive, and 66 high aggressive or high hyperactive) originally provided consent for neurocognitive testing. However, one participant violated drug/alcohol abstinence restrictions for testing and could not be rescheduled (for further information about selection and recruitment of this subsample, see Séguin, Nagin, Assaad, & Tremblay, 2004). Another 4 participants were excluded from the current analyses because of insufficient CU data; all participants included in this study provided reliable cannabis and other SU data at a minimum of three time points across adolescence (out of five: 13–17 years), and completed at least two of the neurocognitive tasks of interest at 20 years: digit span backward (n = 300), self-ordered pointing task (SOP, n = 300), conditional association task (CAT, n = 298), and a card playing task (CAPT, n = 232). A further 5 participants were excluded because they reported using cannabis before the first measurement of NCF, that is, 13 years, which served to control for NCF prior to CU. This subsample did not differ significantly from the larger sample for whom SU data was available on alcohol use frequency, t (886) =0.12, p = .91; CU frequency, t (886) =0.42, p =.68; or number of substances tried, t (886) = 0.12, p = .91, across adolescence (averaged across 13 to 17 years). Séguin et al. (2004) previously showed that this subsample was also comparable to the larger sample on measures of hyperactivity, conduct disorder, and delinquency across adolescence. Within this sub-sample, missing data across adolescence was only predicted by verbal IQ at 13 years (odds ratio [OR] =0.82, p < .05), with lower IQ scores being associated with higher likelihood of missing data, but not by other early adolescence cognitive variables, family risk, academic achievement, or externalizing problems. Finally, 43% of the boys in this sample used cannabis at some point during adolescence. Mean use among adolescent cannabis users was 3.19 (SD = 1.50, range = 1–7), equivalent to using cannabis 3 to 5 times in the past year, and 51% used cannabis at 20 years of age (mean use among adult cannabis users = 3.54, SD = 0.50, range = 1–4), equivalent to using cannabis “very often.” See Table 1 for further sample characteristics and descriptive variables.
Table 1.
Neurocognitive scores, demographic, substance use, and control variables in whole sample (N = 294)
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| Age 13 | ||||
| Verbal IQ, correct | 9.23 | 1.93 | 2 | 13 |
| Number randomization, trials | 3.41 | 1.57 | 0 | 8 |
| DS forward, correct | 6.52 | 1,73 | 1 | 11 |
| DS back, correct | 5.93 | 1.44 | 3 | 12 |
| Age 14 | ||||
| SOP, errors | 12.01 | 4.37 | 3 | 29 |
| PAL, correct | 24.03 | 4.47 | 5 | 32 |
| Conditional, errors | 78.68 | 28.23 | 22 | 183 |
| CAPT at 13, cards played | 71.02 | 19.89 | 26 | 100 |
| Age 20 | ||||
| Verbal IQ, correct | 44.06 | 11.26 | 11 | 76 |
| Number randomization, trials | 5.11 | 2.27 | 0 | 10 |
| DS forward, correct | 8.20 | 2.17 | 2 | 12 |
| DS back, correct | 6.89 | 2.38 | 2 | 12 |
| SOP, errors | 8.39 | 4.83 | 0 | 24 |
| PAL, correct | 26.40 | 3.63 | 6 | 30 |
| Conditional, errors | 49.48 | 37.43 | 3 | 153 |
| CAPT, cards played | 69.80 | 22.86 | 26 | 100 |
| Family risk 6 years | 0.32 | 0.25 | 0 | 1 |
| Age at adult testing | 20.5 | 0.65 | 19.8 | 22.2 |
| 13–15 years | ||||
| Academic achievement | 3.34 | 1.33 | 1 | 5 |
| ADHD symptoms | 11.5 | 8.17 | 0 | 33 |
| CD symptoms | 1.53 | 2.17 | 0 | 14 |
| 14–17 years | ||||
| Tobacco use frequency | 1.69 | 0.89 | 1 | 4 |
| Alcohol frequency | 2.19 | 0.71 | 1 | 4 |
| 20 years | ||||
| Cannabis frequency | 2.47 | 1.22 | 1 | 4 |
| Stimulant/hallucinogen use | 1.53 | 0.83 | 1 | 4 |
| Drunkenness | 2.58 | 0.91 | 1 | 4 |
|
| ||||
| Rates | N | % | ||
|
| ||||
| High school graduation | 171 | 58 | ||
| Adolescent cannabis users (14–17 years) | 142 | 43 | ||
| Cannabis users at 20 years | 155 | 53 | ||
| Tobacco smokers at 20 years | 137 | 46 | ||
| Alcohol use before 15 years | 198 | 67 | ||
Note: DS, Digit span; SOP, self-ordered pointing; PAL, paired associates learning; CAPT, card playing task; ADHD, attention-deficit/hyperactivity disorder; CD, conduct disorder.
Instruments and procedure
Adolescent and adult cannabis and other SU frequency
Cannabis, tobacco use, and alcohol use frequency over the last 12 months were assessed annually from ages 13 to 17 years using the Personal Experience Screening Questionnaire (PESQ; Winters, 1992); each rated on a 7-point scale: from never (0) to 40 or more times (6; see Table 2 for CU frequency by age; skewness ranged between 1.00 and 2.23; kurtosis ranged between –0.68 and 3.84). The PESQ is one of the most widely used adolescent SU screening tools worldwide, and has been shown to have good content, construct and criterion validity in normative, juvenile offending and drug-abusing populations (Winters, 1992, 2003). An age of onset variable was computed identifying the age participants were when they first used cannabis; participants who had not reported having used cannabis as of the final adolescent assessment (age 17) were coded as initiating CU in the following year (i.e., 18 years). This procedure is preferable to dropping these participants from the analysis, and is commonly used in prospective longitudinal studies of SU (e.g., Hawkins et al., 1997). Alcohol use and tobacco use were averaged across 13 to 17 years and used as covariates in the final model (Model 4). Cannabis and other SU at 20 years were also assessed with the PESQ (Winters, 1992), and tobacco use frequency over the last 12 months was assessed on a 4-point scale: never, sometimes, once a week, and many times per week.
Table 2.
Endorsement of cannabis use frequency over the last 12 month, and mean cannabis use in full sample and cannabis users by age
| Age
|
||||
|---|---|---|---|---|
| 14 (N = 289) | 15 (N = 285) | 16 (N = 280) | 17 (N = 273) | |
| No use (0) | 262 (90.7%) | 210 (73.7%) | 172 (61.4%) | 152 (55.7% |
| Once or twice (1) | 8 (2.8%) | 27 (9.5%) | 25 (8.9%) | 32 (11.7%) |
| 3–5 times (2) | 1 (0.3%) | 13 (4.6%) | 18 (6.1%) | 9 (3.3%) |
| 6–9 times (3) | 6 (2.1%) | 5 (1.8%) | 13 (4.4%) | 11 (4.0%) |
| 10–19 times (4) | 5 (1.7%) | 12 (4.1%) | 17 (5.8%) | 16 (5.9%) |
| 20–39 times (5) | 3 (1.0%) | 3 (1.1%) | 10 (3.6%) | 13 (4.8%) |
| 40+ times (6) | 4 (1.4%) | 15 (5.3%) | 25 (8.5%) | 40 (14.7%) |
| Mean (SD) | ||||
| In full sample | 0.30 (1.09) | 0.78 (1.64) | 1.31 (2.03) | 2.66 (2.30) |
| In cannabis users | 3.22 (1.81) | 3.00 (1.95) | 3.41 (1.89) | 3.74 (2.04) |
Note: Cannabis use frequency was rated on the 7-point scale: 0 = no use, 1 = used once or twice, 2 = 3–5 times, 3 = 6–9 times, 4 = 10–19 times, 5 = 20–39 times, 6 = 40 or more times. Percentages are computed within age.
Because age of onset and frequency measures of CU at each time point were strongly correlated in this sample (r > –.65), a residual score of age of onset (adjusting for its covariance with average CU frequency from 14 to 17 years) was created and entered into models in which CU frequency and age of onset were entered together as predictors.
NCF
NCF was indexed through a series of validated measures assessing abilities related to executive function, such as short-term and working memory, planning, trial and error learning, and IQ. To evaluate differential effects CU may have on distinct brain areas, the neurocognitive battery included a number of test tapping cognitive functioning related to different regions of the brain: working memory, planning, and trial and error learning are functions associated with frontal brain areas (Petrides, 1990; Petrides, Alivisatos, Evans, & Meyer, 2013); short-term memory is associated with functioning in the hippocampus and medial temporal lobes; and IQ is associated with broader functioning in a wider brain network, which includes the frontal and parietal lobes (Colom, Karama, Jung, & Haier, 2010).
The paired associates learning (PAL) and digit span (DS) subtests (forward and backward) of the Wechsler Memory Scales—Revised (Wechsler, 1987) tap short-term memory functions often associated with the hippocampus and medial temporal lobe. During the PAL, participants are asked to listen to easy or difficult word pairs, and then asked, after the first word of a pair is provided, to cue recall for the second word. The DS task requires repeating digits in increasing spans, first in the right order (forward), followed by a backward order.
Number randomization (NR) and the SOP (Petrides et al., 1993), tap working memory functions associated with the middorsolateral frontal lobe. In the NR task, a range of numbers is provided, and participants must select all numbers without repeating a digit, using a pattern, or sequence more than two consecutive numbers. During the SOP, participants are shown 12 arrays of the same 12 stimuli arranged differently in each array, and asked to select a new stimulus in each array. Repetitions are counted as errors. CATs (Petrides, 1990) tap working memory, strategic planning functions, and an ability to learn associations between two arbitrary visual stimuli by trial and error: functions related to the posterior–dorsolateral frontal lobe. The CAT requires inductive reasoning, or learning by trial and error, to discover predetermined patterns of association between a color and an abstract symbol or a button and a light. The SOP (abstract and concrete forms) and CAT tests were administered in computerized form at age 20 years (Séguin et al., 2004), which differ from the noncomputerized versions administered at age 14 years (Séguin, Pihl, Harden, Tremblay, & Boulerice, 1995). The main difference lies in that scoring accuracy is increased and experimental error is reduced in the computerized forms, but versions do not differ in the construct and outcomes measured, as exemplified by their strong correlations across versions (and time): SOP r = .55, and CAT r = .52 (correlations among all neurocognitive tasks across time can be found in online-only supplementary Table S.1).
A trial and error learning CAPT, adapted by Newman, Patterson, and Kosson (1987), assessing response perseveration (i.e., the inability to modulate default responding in light of motivationally significant cues) and reward/punishment processing, functions related to the dorsolateral and orbital–frontal cortices (OFC), was also completed. That task has been extensively validated in the study of externalizing behavior problems and in this sample (Séguin, Arseneault, Boulerice, Harden, & Tremblay, 2002; Séguin, Arseneault, & Tremblay, 2007). The CAPT is a computer-controlled behavioral laboratory task in which participants are asked to turn over cards, one at a time, from a deck of cards. Each time they turn over a card they will either win or lose money (5 cents). The task is designed to create a response set with the help of an initially high rate of rewards, but as the game progresses, responding is gradually followed by monetary loss. Participants were instructed to play until they decided to stop and were not given any goals or suggestions that could have biased their playing strategy.
Finally, a verbal IQ estimate at age 20 was obtained with the French equivalent of the vocabulary subtests of the Wechsler Adult Intelligence Scale, the Épreuve Individuelle d’Habile Mentale (Chevrier, 1989), and the age 13 years verbal IQ estimate was obtained from a sentence completion task (Veroff, McClelland, & Marquis, 1971).
The variables that code for errors (SOP, CAT, and CAPT) were sign reversed in regression and path analyses so that higher scores on any measure indicates better ability. Task instructions for the vocabulary, DS, PAL, NR, and CAT have been published elsewhere (Barker et al., 2011; Séguin et al., 2004), as have instructions for the card-playing task (Séguin et al., 2002) and for verbal IQ (Séguin, Boulerice, Harden, Tremblay, & Pihl, 1999).
Demographic variables and potential confounders
A measure of family risk at age 6 was included as a covariate index of SES in all analyses, because it has been associated with both SU (Hayatbakhsh, Najman, Jamrozik, Mamun, & Alati, 2006) and NCF (Lovallo et al., 2013). This index tapped parental age at birth of first child, occupational status, education, and family status (intact or nonintact; Tremblay et al., 1991). Academic achievement from ages 13 to 15 was assessed with teacher-reported global academic performance, and in adulthood, with a dichotomous measure of whether participants had been awarded a high school diploma, which was confirmed by the Ministry of Education of Quebec (see Boisjoli, Vitaro, Lacourse, Barker, & Tremblay, 2007, for further details). Teacher-rated externalizing problems (i.e., attention-deficit/hyperactivity disorder and conduct disorder symptoms, including physical aggression) were assessed with the Social Behavior Questionnaire (Tremblay et al., 1991), with which teachers were asked to rate participant symptoms on a 3-point scale (never–often) at yearly intervals from ages 13 to 15. An average score across 13–15 years was computed for each set of symptoms. Table S.2 in the online-only supplementary materials shows correlations between cognitive measures and these covariates, indicating that performance on all cognitive measures in early adolescence and at 20 years, except for the CAPT, was associated negatively with family risk and externalizing problems and positively with academic achievement measures. Poor performance on the CAPT at 13 years was associated with higher family risk and lower academic achievement at 13 to 15 years, but not with externalizing problems or high school graduation; performance on the CAPT at 20 years was not significantly associated with any non-SU covariates.
Statistical analyses
An unconditional latent growth curve model of CU frequency was conducted to estimate the initial level (intercept) and the systematic change (slope) in CU frequency between the ages of 14 and 17 years. In the latent growth curve model framework, when a model is centered at the first time point (i.e., 14 years), the initial levels of CU frequency are modeled with the intercept (which varies across participants), and the systematic change over time; for instance a linear increase in CU frequency from 14 to 17 years, is modeled by a slope (which also varies across participants).
Next, a conditional latent growth model was conducted in which CU age of onset and CU frequency intercept and slope factors were associated with early adolescent NCF (at 13–14 years) indexed through the measures described earlier. Finally, several path models were conducted to examine whether variability in cannabis age of onset, CU frequency at 14 years (intercept factor), and increases in CU frequency from 14 to 17 years (slope factor) were associated with change in NCF in early adulthood (20 years) from early adolescence (13–14 years; using change scores; analyses using neurocognitive residualized scores and baseline scores as covariates were also conducted, yielding equivalent results). CU age of onset and CU frequency were entered into models separately (Models 1 and 2) and simultaneously (Models 3 and 4) to examine general and unique links of these aspects of CU on change in cognitive function. All models were adjusted for the effect of potential confounders: childhood adversity, academic achievement from 13 to 15 years verbal IQ at 13 years (and any cognitive function in early adolescence that was shown to be associated with later CU), age at adult testing, and concurrent cannabis, tobacco, and other SU in adulthood (20 years; Models 1–4). The last model further adjusted for high school graduation, adolescent alcohol use, and tobacco use frequency and externalizing problems (Model 4).
Analyses were conducted using Mplus version 6.11 (Muthén & Muthén, 1998–2009) using maximum likelihood estimation with robust standard errors (MLR). There is ongoing debate about how ordinal variables of more than five ordered categories, with skewness and kurtosis ≤2 and ≤7, respectively, should be handled. Thus, several latent growth curve models of CU frequency were conducted using MLR and weighted least squares estimators. As results were similar across methods, mirroring previous simulation studies (e.g., Rhemtulla, Brosseau-Liard, & Savalei, 2012), only models using MLR are reported. Full information maximum likelihood was used to account for missing data. Tests of goodness of fit included the comparative fit index (Bentler, 1990), the root mean square error of approximation (Browne & Cudeck, 1993), and the standardized root mean residual. Finally, the Benjamini–Hochberg procedure (Thissen, Steinberg, & Kuang, 2002) was used to correct for multiple testing in all analyses. Measures, data preparation, and analyses are further described in the Method section of supplementary online material.
Results
Preliminary analysis: Unconditional latent growth model of CU frequency
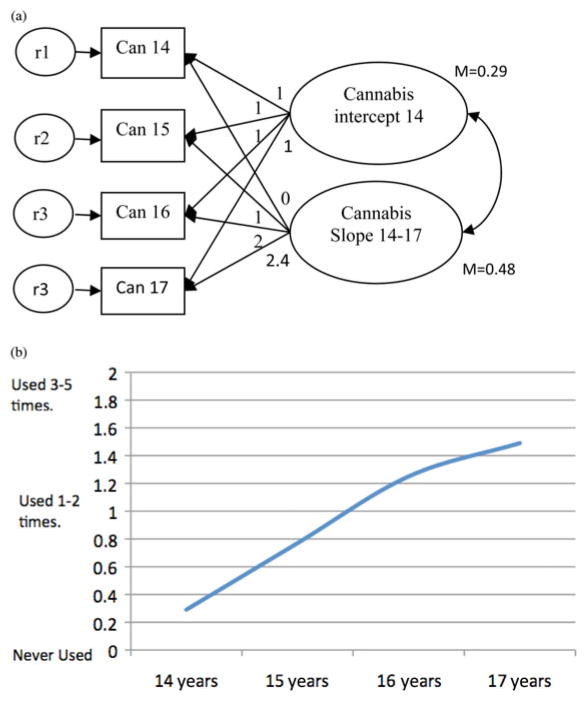
Several growth curve models were conducted to assess which growth function fit the CU frequency data best (see the Preliminary analysis section in the supplementary material). A growth model where growth was assumed to be linear across the first three time points (14–16 years), but left to be freely estimated for the last time point (17 years; see Figure 1), fit the CU data best (χ2 =10.6, df = 4, comparative fit index = 0.97, root mean square error of approximation = 0.07, standardized root mean square residual = 0.06) and showed that growth between time points 3 (16 years) and 4 (17 years) was smaller than at other time points (with the freely estimated loading resulting in 2.40 instead of a loading of 3.00, which is to be expected if growth was linear). Growth curve factor means (intercept = 0.29, slope = 0.48) were significantly different from zero at p <.001 and showed that there was an overall tendency for CU frequency to increase from 14 to 17 years. There was also significant individual variability in the mean initial level of CU at 14 years (intercept = 0.61, p < .01) and its pattern of change over time (slope =0.39, p < .01). Initial CU at age 14 years did not correlate significantly with change of CU frequency across adolescence (r = .32, p = .15).
Figure 1.
Unconditional latent growth model of self-reported cannabis use frequency from 14 to 17 years (a) centered at 14 years and (b) the average cannabis use frequency across adolescence. As depicted by the growth factor (slope) loadings (0, 1, 2, 2.4), frequency of cannabis use increased steadily (linearly) from 14 to 16 years with the increase becoming flatter from 16 to 17 years. Can, Cannabis; r, residual variance.
Is cognitive function in early adolescence (13–14 years) associated with CU onset intercept and later CU frequency slope?
To examine the links between NCF and CU onset and later CU frequency, we included cognitive variables in early adolescence (13 or 14 years), together with family risk, as predictors of later CU frequency (intercept and slope factors) and CU onset. The results in Table 3 show that poor performance on the PAL and SOP tasks, tapping into short-term and working memory, were associated with earlier onset of CU. In contrast, higher verbal IQ was associated with both earlier onset and a steeper increase in CU frequency.
Table 3.
Cognitive function at 13–14 years and cannabis use age of onset and frequency of use
| Cannabis Use Onset Age
|
Cannabis Frequency Intercept (14 years)
|
Slope (14–17 years)
|
||||
|---|---|---|---|---|---|---|
| β | p | β | p | β | p | |
| Age 13 | ||||||
| Verbal IQ | −0.16 | .03 | 0.05 | .63 | 0.20 | .04 |
| Number random | 0.11 | .13 | −0.08 | .28 | −0.07 | .42 |
| DS Forward | −0.10 | .18 | 0.06 | .42 | 0.10 | .27 |
| DS back | −0.01 | .84 | 0.10 | .25 | 0.08 | .39 |
| Age 14 | ||||||
| SOP | 0.14 | .04 | −0.02 | .79 | −0.05 | .56 |
| PAL | 0.21 | .02 | −0.20 | .11 | −0.19 | .12 |
| Conditional | −0.01 | .87 | −0.05 | .62 | 0.11 | .25 |
| CAPT at 13 | −0.07 | .27 | 0.10 | .26 | −0.03 | .64 |
Note: The sign for the variables that code for errors was changed so that higher scores on any measure indicate better ability. Model fit: χ2 (25) = 64.33, comparative fit index = 0.94, root mean square error of approximation = 0.07, standardized root mean square residual = 0.03. Bold values are significant at p < .05. DS, Digit span; SOP, self-ordered pointing; PAL, paired associates learning; CAPT, card playing task.
Are age of onset and frequency of adolescent CU associated with NCF decline in early adulthood?
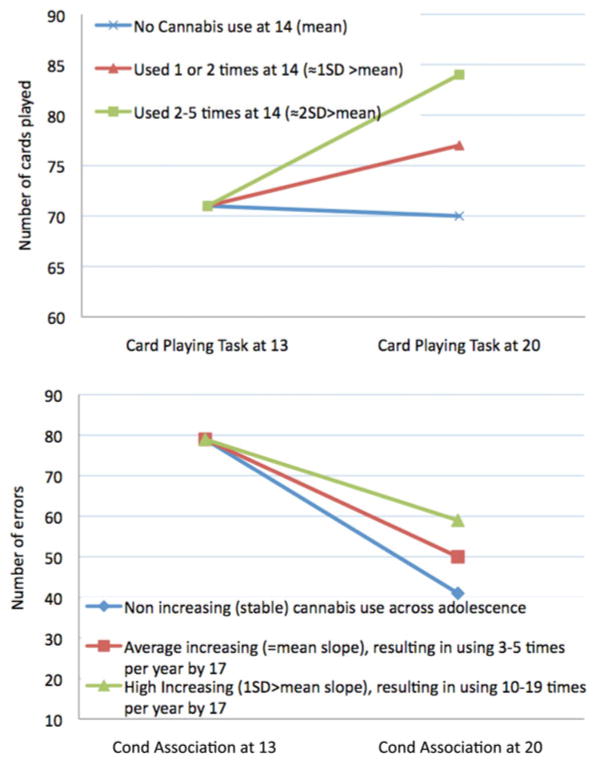
Significant associations between covariates and cognitive function in early adolescence and at 20 years were found (see Table S.2 in the supplementary material). Of note, high family risk, externalizing problems, and poor academic achievement were associated with poor performance on most, if not all, cognitive tasks. In addition, as shown in Table 4 (Model 1), cognitive performance on verbal IQ, PAL, and SOP tasks in early adolescence, as well as SU at 20 years were significantly associated with change in performance on many of the cognitive tasks. The results presented in Table 4 also show that although early age of CU onset was generally associated with a decline in cognitive function on the vocabulary, SOP, and CAT (Model 1), this was no longer the case when intercept and slope factors of CU were taken into account (Model 3, which used the residualized score of age of onset). In contrast, initial levels of CU frequency at 14 years (intercept factor) remained significantly associated with a decline in performance on the CAPT task (β = –0.27, p < .001), and a steeper increase in CU frequency across adolescence (slope factor) remained associated with a decline in performance on the vocabulary (β = –0.17, p < .01) and conditional association (β = –0.28, p < .001) tasks after controlling for multiple testing (Model 3). Once we adjusted for adolescent alcohol and tobacco use frequency, high school graduation, and other externalizing behaviors in Model 4, the significant associations between initial levels of CU frequency at 14 years and the CAPT (β = –0.30, p < .001) and between growth in CU frequency and the CAT remained (β = –0.21, p < .001; see Figure 2 for a graphical representation of these results). However, the association between growth in CU frequency and change in vocabulary was no longer significant; only high school graduation was significantly associated with change in vocabulary (β = 0.20, p < .001).
Table 4.
Change in cognitive function (from 13–14 to 20 years) and cannabis use age of onset, frequency at 14 years (intercept), and growth from 14 to 17 years
| Vocabularya
|
Number Randomization
|
DS Forward
|
DS Backward
|
SOP
|
PAL
|
Conditional Association
|
CAPT
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | β | p | β | p | β | p | β | p | β | p | |
| Model 1 | ||||||||||||||||
| Age of onset | 0.11 | .034 | −0.02 | .78 | −0.05 | .43 | 0.01 | .92 | 0.12 | .04 | 0.07 | .21 | 0.17 | .010 | −0.01 | .93 |
| Verbal IQ at 13 | 0.26 | <.001 | −0.15 | .025 | −0.12 | .09 | −0.15 | .014 | 0.21 | <.001 | −0.07 | .34 | 0.02 | .76 | −0.05 | .49 |
| Age at adult testing | 0.06 | .20 | 0.12 | .041 | 0.05 | .41 | 0.01 | .85 | −0.10 | .056 | 0.03 | .52 | 0.14 | .012 | −0.24 | <.001 |
| Family risk | −0.35 | <.001 | −0.02 | .73 | 0.03 | .60 | −0.05 | .43 | −0.11 | .052 | 0.04 | .56 | 0.22 | <.001 | 0.04 | .55 |
| Cannabis use 20 | −0.01 | .90 | −0.03 | .72 | −0.39 | <.001 | −0.05 | .52 | −0.22 | .001 | 0.08 | .18 | 0.09 | .24 | 0.00 | .97 |
| Tobacco use 20 | −0.10 | .08 | 0.00 | .97 | 0.15 | .010 | −0.01 | .87 | 0.04 | .43 | −0.03 | .61 | 0.04 | .51 | 0.15 | .023 |
| Other drug use 20 | 0.19 | .001 | 0.00 | .97 | 0.29 | <.001 | 0.02 | .79 | 0.17 | .006 | 0.02 | .65 | −0.05 | .52 | −0.13 | .08 |
| PAL 14 | 0.03 | .70 | 0.14 | .033 | 0.09 | .28 | 0.28 | <.001 | 0.19 | .007 | −0.54 | <.001 | 0.23 | .010 | −0.05 | .61 |
| SOP 14 | 0.16 | .002 | 0.12 | .063 | 0.20 | .001 | −0.01 | .95 | −0.47 | <.001 | 0.00 | .99 | −0.17 | .012 | −0.17 | .025 |
| Academic achieve 13–15 | −0.02 | .76 | −0.04 | .61 | −0.05 | .43 | 0.13 | .10 | 0.00 | .99 | −0.01 | .93 | 0.00 | .99 | 0.00 | .97 |
| R2 | 0.35 | <.001 | 0.04 | .08 | 0.12 | .001 | 0.11 | .001 | 0.39 | <.001 | 0.37 | <.001 | 0.12 | .001 | 0.12 | .004 |
| Model 2 | ||||||||||||||||
| Cannabis intercept | 0.02 | .82 | 0.16 | .19 | 0.20 | .064 | 0.04 | .70 | 0.01 | .92 | −0.02 | .81 | −0.15 | .10 | −0.29 | .002 |
| Cannabis slope | −0.17 | .042 | −0.19 | .09 | −0.02 | .84 | −0.02 | .78 | −0.12 | .16 | 0.02 | .86 | −0.28 | .004 | 0.08 | .47 |
| R2 | 0.36 | <.001 | 0.08 | .06 | 0.14 | <.001 | 0.11 | <.001 | 0.39 | <.001 | 0.37 | <.001 | 0.18 | <.001 | 0.18 | <.001 |
| Model 3 | ||||||||||||||||
| Cannabis intercept | 0.01 | .85 | 0.16 | .18 | 0.19 | .07 | 0.03 | .71 | −0.01 | .94 | −0.02 | .80 | −0.15 | .18 | −0.28 | .002 |
| Cannabis slope | −0.17 | .016 | −0.19 | .09 | −0.02 | .83 | −0.03 | .77 | −0.12 | .15 | 0.01 | .88 | −0.28 | .003 | 0.08 | .45 |
| Age onset (residual) | 0.04 | .39 | −0.03 | .67 | 0.06 | .36 | 0.00 | .99 | 0.04 | .52 | 0.05 | .22 | −0.06 | .33 | −0.12 | .062 |
| R2 | 0.36 | <.001 | 0.08 | .06 | 0.15 | <.001 | 0.12 | .001 | 0.40 | <.001 | 0.37 | <.001 | 0.18 | <.001 | 0.19 | <.001 |
| Model 4 | ||||||||||||||||
| Cannabis intercept | 0.05 | .51 | 0.23 | .20 | 0.21 | .17 | 0.08 | .57 | 0.14 | .31 | −0.02 | .87 | −0.07 | .65 | −0.37 | .003 |
| Cannabis slope | −0.11 | .16 | −0.19 | .12 | −0.01 | .91 | −0.01 | .94 | −0.07 | .43 | 0.05 | .52 | −0.21 | .028 | 0.04 | .74 |
| Age onset (residual) | 0.02 | .77 | −0.05 | .44 | 0.05 | .43 | −0.01 | .93 | 0.00 | .98 | 0.04 | .36 | −0.08 | .15 | −0.11 | .11 |
| High-school graduation | 0.21 | <.001 | 0.08 | .36 | −0.06 | .43 | −0.03 | .69 | 0.07 | .33 | 0.11 | .08 | 0.02 | .76 | 0.05 | .53 |
| ADHD 13–15 years | −0.11 | .07 | 0.18 | .043 | 0.07 | .38 | −0.09 | .27 | −0.04 | .57 | 0.08 | .26 | 0.10 | .19 | −0.02 | .82 |
| CD 13–15 years | −0.09 | .12 | −0.05 | .49 | 0.11 | .21 | 0.03 | .63 | −0.11 | .18 | −0.10 | .17 | −0.05 | .49 | 0.09 | .23 |
| Alcohol use 13–17 years | −0.03 | .67 | 0.05 | .41 | 0.01 | .89 | 0.04 | .62 | −0.02 | .71 | −0.17 | .024 | −0.16 | .025 | 0.04 | .63 |
| Tobacco use 13–17 years | 0.01 | .90 | −0.20 | .17 | −0.15 | .24 | −0.11 | .32 | −0.13 | .23 | 0.16 | .13 | −0.07 | .59 | 0.10 | .52 |
| R2 | 0.41 | <.001 | 0.11 | .033 | 0.17 | <.001 | 0.12 | .001 | 0.42 | <.001 | 0.40 | <.001 | 0.20 | <.001 | 0.20 | <.001 |
Note: The sign for the variables that code for errors was changed so that higher scores on any measure indicates better ability. The results using change scores are presented, but analyses using neurocognitive residualized scores and baseline scores as covariates were also conducted, with equivalent results. Values in bold indicate that they remain significant after controlling for multiple testing. DS, Digit span; SOP, self-ordered pointing; PAL, paired associates learning; CAPT, Card Playing Task. Model fit for Model 1: χ2 (2) = 1.83, root mean square error of approximation (RMSEA) = 0.00, comparative fit index (CFI) = 1.00, standardized root mean square residual (SRMR) = 0.01; Model 2: χ2 (39) = 94.46, RMSEA = 0.07, CFI = 0.95, SRMR = 0.03; Model 3: χ2 (41) = 101.28, RMSEA = 0.07, CFI = 0.95, SRMR = 0.03; Model fit for Model 4: χ2 (51) = 101.43, RMSEA =0.06, CFI =0.96, SRMR =0.02. Although only presented under Model 1, standardized coefficients are adjusted for age at testing in adulthood; family risk; average academic achievement 13–15; verbal IQ at 13; SOP and PAL at 14; and concurrent cannabis, alcohol, stimulant, and tobacco use frequency at 20 years in all models.
The verbal IQ change score was calculated by standardizing the sentence completion task (verbal IQ) at 13 years (z scores) and subtracting it from standardized (z-scored) vocabulary scores at 20 years. Analyses using change scores computed using the vocabulary subtest at 15 years as baseline scores were equivalent to those reported here (differing only by a few decimals), with analyses yielding equivalent results (see Data Preparation and Statistical Analysis section in the online supplementary material).
Figure 2.
Change in response perseveration (card playing task) and working memory, planning, and trial and error learning (conditional association task) from early adolescence to early adulthood by adolescent cannabis use scores. In the card playing task, playing more than 75 cards is considered response perseveration (Séguin et al., 2002). Cond Association, conditional association task.
Because high school graduation was significantly and negatively associated with growth in CU frequency (slope factor): B = –0.12, SE = 0.057, OR = 0.54, 95% CI [0.30, 0.97], and age of onset, B = 0.06, SE = 0.025, OR = 1.34, 95% CI [1.02, 1.73], but not CU frequency at 14 years (intercept factor): B = –0.07, SE = 0.039, OR = 0.83, 95% CI [0.51, 1.34], the significance of indirect effects from CU frequency slope to change in vocabulary through high school graduation was tested using the Rmediation program (Tofighi & MacKinnon, 2011). The indirect effect from growth in CU frequency from 14–17 to change in verbal IQ through high school graduation was significant (slope): ab = –0.163, 95% CI [–0.334, –0.032].
Is adolescent CU frequency associated with declines in functioning on the CAPT and the CAT in early adulthood in those that had abstained from CU over 12 months prior to adult cognitive testing?
In order to investigate whether the effects of initial levels of CU frequency and change across adolescence (intercept and slope factors) on declines in cognitive function could be considered long-term residual effects (as opposed to acute effects), analyses were repeated on the sample of participants that reported no CU over the last 12 months at age 20 (N = 143). Forty adolescents of this subsample had used cannabis across adolescence, but none with onset before 15 years. Consequently, in the absence of adolescents who used cannabis at 14 years among this sample of abstainers, we were unable to confirm whether the effect of CU frequency at 14 years on the CAPT remained once adolescents abstained from CU in adulthood. However, in those who had abstained from using cannabis 12 months prior to cannabis testing at 20 years, the increase in CU across adolescence (slope) was still significantly associated with a decline in conditional association from preadolescence to early adulthood (B = –0.20, p = .017), suggesting that this association was not only due to concurrent or continued active CU into adulthood.
Discussion
This study was conducted to examine bidirectional effects between CU and cognitive function and to determine the extent to which age of onset or frequency of CU across adolescence were linked to neurocognitive decline between early adolescence pre-CU onset and early adulthood, after adjusting for key confounds. First, findings suggest that the relationship between neurocognition and CU may be bidirectional. In early adolescence poor short-term and working memory, but high verbal IQ, were associated with an earlier age of onset of CU. In turn, an earlier age of onset and more frequent use at 14 years and across adolescence were associated with neurocognitive decline by early adulthood. More specifically, adolescent CU frequency and earlier age of onset were associated not only with lower rates of high school graduation, controlling for early adolescent academic achievement, but also with neurocognitive decline in early adulthood, independently from high school graduation, concurrent cannabis and other SU, adolescent alcohol, and tobacco use, and externalizing behaviors. Second, links were not observed globally across all neurocognitive tasks, but only for verbal IQ and tasks tapping trial and error learning, an executive function process indexed by the conditional association learning task, and response perseveration on the CAPT (which also assesses reward processing). Third, although the results indicate that earlier age of onset was associated with a decline in a few NCFs, these effects were not independent of frequency of use. Despite an attempt to control for the high correlation between age of onset and frequency of CU by creating a residual age of onset score, it may not be possible to examine the true role age of onset plays in cognitive decline given this high correlation with frequency of use. However, the results suggest that CU during adolescence was associated with declines in specific cognitive functions through two different pathways or mechanisms: one more “social” mechanism related to school engagement, and another more “biological” mechanism related to potential neurotoxic effects on the adolescent brain.
More specifically, adolescent CU frequency was associated with declines in verbal IQ, but these effects were accounted for by high school graduation. Accordingly, our findings are consistent with studies showing that childhood and adolescent-onset mental health and CU (and other SU) problems are linked with failure to graduate from high school (Breslau et al., 2008; Lee et al., 2009), a key functional impairment, which in the present study helped explain the association between CU frequency and a decline in vocabulary by early adulthood. These results provide some support for the hypothesis that the link between CU and declines in cognitive function could be at least partially because adolescent cannabis users are less likely to complete school (e.g., Silins et al., 2014), which in turn has an impact on their cognitive development (Brinch & Galloway, 2012). Thus, it could be that the link between CU and declines in verbal IQ operates partly through truancy or low levels of school engagement (Mokrysz et al., 2016).
Nonetheless, the findings showed that adolescent CU was significantly associated with declines in performance on two cognitive tasks, the conditional association and CAPT, even after controlling for high school graduation and a number of other important confounders, suggesting that declines in performance could potentially be a direct result of CU on the developing brain. Note that a decline in performance was observed on the only two tasks in our battery that included a learning component, in this case, learning inductively through trial and error, a complex cognitive function related to executive function and the prefrontal cortices of the brain. It is also notable that, although it may be hard to disentangle the effects of age of onset and frequency of use in this sample, the age of onset of CU was associated with declines in the CAT (Table 3, Model 1), a finding that was confirmed by group-based analyses (see supplementary results and Tables S.3 and S.4 in the supplementary material) showing that participants who used cannabis before the age of 16 reported larger declines in performance on this task and differed significantly from nonusers. These findings, taken together with the finding that CU at 14 years (that remained constant across adolescence) was associated with declines in performance on the CAPT, suggest that age of onset does matter, providing support for the hypothesis that CU may have more detrimental neurotoxic effects on the developing brain of adolescents compared to the those on the adult brain. CU during adolescence is hypothesized to lead to alterations in synaptic pruning and a disruption of the development of the PFCs, which have a high density of cannabinoid receptors and continue to develop during adolescence (Fuhrmann, Knoll, & Blakemore, 201l; Lubman et al., 2015; Rubino & Parolaro, 2016). This in turn is hypothesized to impact on cognitive function related to prefrontal cortices more than those related to other brain areas. Indirect support for this comes from studies showing that early adolescent CU is associated with poor white matter integrity in the PFC (Becker, Wagner, Gouzoulis-Mayfrank, Spuentrup, & Daumann, 2010a; Gruber, Dahlgren, Sagar, Gonenc, & Lukas, 2014), decreased cortical thickness in the superior PFC and frontal cortices (Churchwell, Lopez-Larson, & Yurgelun-Todd, 2010; Lopez-Larson et al., 2011), and with abnormal brain activation in the PFC and parietal brain regions (Becker, Wagner, Gouzoulis-Mayfrank, Spuentrup, & Daumann, 2010b; Cheng et al., 2014; Jager, Block, Luijten, & Ramsey, 2010).
Further, effects of CU frequency at 14 years that remained stable across adolescence on CAPT remained after controlling for change in the CAT (see the Results section in the supplementary material). This suggests that early frequent CU may also have effects on reward and punishment processing, possibly leading to a failure to modulate behavior in light of motivationally significant cues. This is consistent with findings showing that (a) chronic CU is associated to poor decision making through a decreased sensitivity to loss and greater sensitivity to gains (Fridberg et al., 2010), and alterations in neural activity related to the processing of motivation-ally relevant stimuli and errors (Fridberg, Skosnik, Hetrick, & O’Donnell, 2013); and (b) CU is associated with reduced gray matter in the OFC (particularly the right medial OFC), a brain region involved in reward processing and motivation (Churchwell et al., 2010; Filbey et al., 2014).
However, we were unable to confirm whether effects of CU on the CAPT were still observable in a sample of abstinent adults as all adolescents who had a CU onset at 14 years (n = 27) continued to use cannabis at 20 years. Thus, it was not possible to confirm whether the effects of CU frequency at 14 years on decline in performance on the CAPT task were due to frequent use (using on average 6 or more times per year) at this age that continued across adolescence or to frequent early adolescent use combined with continued active use into adulthood. The effects of CU frequency across adolescence on the CAT, however, remained even in adults who abstained from CU over the 12 months prior to adult cognitive testing, confirming that these effects are not only attributable to concurrent or continued active use. This contrasts with conclusions from meta-analyses suggesting that cognitive deficits may be recoverable after a month of abstinence (Schreiner & Dunn, 2012; Schulte et al., 2014), but is consistent with findings by Meier et al. (2012), which showed that persistent moderate- to high-frequency CU from adolescence onward was associated with declining NCF in middle-aged adults. However, current results differ from Meier et al. (2012), though, in that they do not support that adolescent CU is associated with global neurocognitive decline in early adulthood. Instead, they suggest that residual long-term effects (as opposed to acute, short-term effects that recover with abstinence) of early-onset and frequent adolescent CU, as defined in this sample as going beyond experimentation or using 6 times or more over a year, may be limited to NCFs involved in trial and error learning. Overall, these results suggest that, in addition to academic failure, fundamental life skills necessary for problem solving and daily adaptation, namely, the capacity to learn from trial and error and altering an initially rewarded course of action in motivationally significant situations, may be affected by early cannabis exposure.
The current findings also highlight the importance of assessing baseline individual differences in pre-CU cognitive functions and bidirectional associations between cannabis and cognitive function. For example, we found that poor working memory capacity, which has been shown to be associated with change in CU frequency and severity (Cousijn et al., 2014; Squeglia et al., 2014) and other cognitive functions (Carlson, Zelazo, & Faja, 2013), significantly predicted earlier onset of CU in this sample of boys. This latter methodological point is possibly important for future research. For example, in this sample, higher IQ, not lower, was shown to be associated with early onset of CU and a steeper increase in CU frequency across adolescence. This is not the first study to show that higher IQ is associated with frequent SU in adolescence (Johnson, Hicks, McGue, & Iacono, 2009; White & Batty, 2012). In another study, which included a different subsample of the MLES study, higher verbal IQ was also found to predict alcohol use frequency in early adolescence (Castellanos-Ryan et al., 2013) and is consistent with the hypothesis that some cognitive abilities may facilitate reward-oriented behaviors, including SU (Hyman, Malenka, & Nestler, 2006). Taken together, these findings highlight the importance of assessing NCFs more broadly before the onset of CU, and of conducting longitudinal studies to help clarify bidirectional effects between CU and neurocognitive functioning.
Still, some limitations of the current study should be noted. First, measures of CU quantity were unavailable. Future studies should include more sensitive measures of quantity and frequency over time to better establish dose–response associations between CU and NCF. Second, no biological measures of drug use were obtained, and CU frequency was gathered through self-report, which is susceptible to bias and may limit the validity of the data. However, studies have shown that self-reports are reliable when assessing SU in adolescence (Clark & Winters, 2002) and hence are useful for clinical practice and research. Together with guaranteed confidentiality to participants, this should contribute to the reliability and validity of these data. Third, although the current study adjusted for effects of alcohol use, concurrent CU, and externalizing problems (Tamm et al., 2014), it did not adjust for childhood trauma or neglect and other mental health problems that may be associated to CU and may be linked to neurocognitive development, such as schizophrenia (Meier et al., 2012, did control for this), depression, mania, anxiety, and suicidality (Tamm et al., 2014). Fourth, adolescent CU in the current study was assessed during the early 1990s (1991–1995), before the observed trend over the last 20 years of increases in the potency of cannabis products (ElSohly et al., 2016; Hall & Degenhardt, 2009). Thus, it is possible that more potent cannabis products used today, which have a higher content of Δ-9-tetrahydrocannabinol, may have greater neurotoxic effects on the brain. Fifth and finally, the current sample was made up entirely of French-speaking boys of European American origin living in low-SES neighborhoods in Montreal. Thus, replication of these findings is needed with cannabis available today, including girls, and more diverse populations.
There have been many recent commentaries highlighting some limitations of the literature on the association between CU and neurocognition, as well as highlighting the need for trials and longitudinal studies that investigate the effects of CU on the developing brain (e.g., Fuhrmann et al., 2015; Volkow et al., 2016). Although the current study cannot address all limitations, it does provide further clarity on the potential effects of early frequent CU on NCF and their specificity, though it does not demonstrate causality. Nonetheless, it provides an additional basis for improving methodology. In the absence of randomized-controlled trials, the findings from this prospective longitudinal study provide a clear evidence base for directing prevention efforts toward delaying the onset and reducing the frequency of CU, particularly in light of the increasing trend of adolescents viewing CU favorably and less harmful than other drugs. A number of recent studies have shown that prevention approaches that target early risk factors for SU, such as childhood disruptive behaviors and impulsivity, have been effective in delaying the onset and reducing the frequency of alcohol and drug use during adolescence (Castellanos-Ryan et al., 2013; Conrod, Castellanos-Ryan, & Strang, 2010).
Supplementary Material
Acknowledgments
This research was made possible by a fellowship (to N.C.-R.) from the Ministère de l’Éducation, du Loisir et du Sport du Québec (No. 149169) and the Fonds de Recherche en Santé du Québec (No. 22530); and grants from the Canadian Institutes of Health Research (MOP-97910), the Social Science and Humanities Research Council of Canada (412-2000-1003), the National Health Research and Development Program, the Fonds Québécois de Recherche sur la Société et la Culture (2002-RS-79238 and 2009-RG-124779), the Fonds Québécois de Recherche en Santé, the American National Science Foundation (SES-9911370), and the National Consortium on Violence Research (supported under Grant SBR-9513040 from the National Science Foundation). These funding agencies had no role in the study design, collection, analysis, or interpretation of the data; writing the manuscript; or the decision to submit the paper for publication. The authors thank the boys, their families, and teachers for their long-term commitment to this project.
Footnotes
To view the supplementary material for this article, please visit https://doi.org/10.1017/S0954579416001280.
References
- Barker ED, Tremblay RE, van Lier PA, Vitaro F, Nagin DS, Assaad JM, Séguin JR. The neurocognition of conduct disorder behaviors: Specificity to physical aggression and theft after controlling for ADHD symptoms. Aggressive Behavior. 2011;37:63–72. doi: 10.1002/ab.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. Altered parahippocampal functioning in cannabis users is related to the frequency of use. Psychopharmacology (Berlin) 2010a;209:361–374. [Google Scholar]
- Becker B, Wagner D, Gouzoulis-Mayfrank E, Spuentrup E, Daumann J. The impact of early-onset cannabis use on functional brain correlates of working memory. Progress in Neuropsychopharmacology and Biological Psychiatry. 2010b;34:837–845. doi: 10.1016/j.pnpbp.2010.03.032. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Boisjoli R, Vitaro F, Lacourse Â, Barker ED, Tremblay RE. Impact and clinical significance of a preventive intervention for disruptive boys: 15-year follow-up. British Journal of Psychiatry. 2007;191:415–419. doi: 10.1192/bjp.bp.106.030007. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Breslau J, Michael L, Nancy SB, Kessler RC. Mental disorders and subsequent educational attainment in a US national sample. Journal of Psychiatric Research. 2008;42:708–716. doi: 10.1016/j.jpsychires.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinch CN, Galloway TA. Schooling in adolescence raises IQ scores. Proceedings of the National Academy of Sciences. 2012;109:425–430. doi: 10.1073/pnas.1106077109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation modeling. Thousand Oaks, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, Yucel M, Solowij N. Acute and chronic effects of cannabinoids on human cognition—A systematic review. Biological Psychiatry. 2016;79:557–567. doi: 10.1016/j.biopsych.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, Eng WS, … Hargreaves RJ. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proceedings of the National Academy of Sciences. 2007;104:9800–9805. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SM, Zelazo PD, Faja S. Executive function. In: Zelazo PD, editor. The Oxford handbook of developmental psychology: Vol. 1. Body and mind. New York: Oxford University Press; 2013. pp. 706–743. [Google Scholar]
- Castellanos-Ryan N, Séguin JR, Vitaro F, Parent S, Tremblay RE. A multimodal intervention for disruptive kindergarten children reduces substance-use across adolescence: A randomized control trial. British Journal of Psychiatry. 2013;203:188–195. doi: 10.1192/bjp.bp.112.123182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Skosnik PD, Pruce BJ, Brumbaugh MS, Vollmer JM, Fridberg DJ, … Newman SD. Resting state functional magnetic resonance imaging reveals distinct brain activity in heavy cannabis users—A multi-voxel pattern analysis. Journal of Psychopharmacology. 2014;28:1030–1040. doi: 10.1177/0269881114550354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier JM. Épreuve Individuelle d’Habileté Mentale [Individual tasks of mental ability] Montréal: Institut de Recherches Psychologiques; 1989. [Google Scholar]
- Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Frontiers in Psychology. 2010;1:225. doi: 10.3389/fpsyg.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DB, Winters KC. Measuring risks and outcomes in substance use disorders prevention research. Journal of Consulting and Clinical Psychology. 2002;70:1207–1223. doi: 10.1037//0022-006x.70.6.1207. [DOI] [PubMed] [Google Scholar]
- Colom R, Karama S, Jung RE, Haier RJ. Human intelligence and brain networks. Dialogues in Clinical Neuroscience. 2010;12:489–501. doi: 10.31887/DCNS.2010.12.4/rcolom. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrod PJ, Castellanos-Ryan N, Strang J. Brief, personality-targeted coping skills interventions and survival as a non-drug user over a 2-year period during adolescence. Archives of General Psychiatry. 2010;67:85–93. doi: 10.1001/archgenpsychiatry.2009.173. [DOI] [PubMed] [Google Scholar]
- Cousijn J, Wiers RW, Ridderinkhof KR, van den Brink W, Veltman DJ, Goudriaan AE. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: A prospective fMRI study. Human Brain Mapping. 2014;35:2470–2482. doi: 10.1002/hbm.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence-based review of acute and long-term effects of cannabis use on executive cognitive functions. Journal of Addiction Medicine. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Stockings E, Patton G, Hall WD, Lynskey M. The increasing global health priority of substance use in young people. Lancet Psychiatry. 2016;3:251–264. doi: 10.1016/S2215-0366(15)00508-8. [DOI] [PubMed] [Google Scholar]
- ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): Analysis of current data in the United States. Biological Psychiatry. 2016;79:613–619. doi: 10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Psychosocial sequelae of cannabis use and implications for policy: Findings from the Christchurch Health and Development Study. Social Psychiatry and Psychiatric Epidemiology. 2015;50:1317–1326. doi: 10.1007/s00127-015-1070-x. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Beautrais AL. Cannabis and educational achievement. Addiction. 2003;98:1681–1692. doi: 10.1111/j.1360-0443.2003.00573.x. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Asian S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, Segall J. Long-term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences. 2014;111:16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flory K, Lynam D, Milich R, Leukefeld C, Clayton R. Early adolescent through young adult alcohol and marijuana use trajectories: Early predictors, young adult outcomes, and predictive utility. Development and Psychopathology. 2004;16:193–213. doi: 10.1017/s0954579404044475. [DOI] [PubMed] [Google Scholar]
- Fridberg DJ, Queller S, Ahn WY, Kim W, Bishara AJ, Busemeyer JR, … Stout JC. Cognitive mechanisms underlying risky decision-making in chronic cannabis users. Journal of Mathematical Psychology. 2010;54:28–38. doi: 10.1016/j.jmp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberg DJ, Skosnik PD, Hetrick WP, O’Donnell BF. Neural correlates of performance monitoring in chronic cannabis users and cannabis-naive controls. Journal of Psychopharmacology. 2013;27:515–525. doi: 10.1177/0269881113477745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Gray R. Neurocognitive consequences of marihuana—A comparison with pre-drug performance. Neurotoxicology and Teratology. 2005;27:231–239. doi: 10.1016/j.ntt.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a sensitive period of brain development. Trends in Cognitive Science. 2015;19:558–566. doi: 10.1016/j.tics.2015.07.008. [DOI] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Schreiber L, Odlaug BL. Neuropsychological deficits associated with cannabis use in young adults. Drug and Alcohol Dependence. 2012;121:159–162. doi: 10.1016/j.drugalcdep.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gonenc A, Lukas SE. Worth the wait: Effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology. 2014;231:1455–1465. doi: 10.1007/s00213-013-3326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychology of Addictive Behaviors. 2012;26:496–506. doi: 10.1037/a0026269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall WD, Degenhardt L. Adverse health effects of non-medical cannabis use. Lancet. 2009;374:1383–1391. doi: 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Hall WD, Patton G, Stockings E, Weier M, Lynskey M, Morley KI, Degenhardt L. Why young people’s substance use matters for global health. Lancet Psychiatry. 2016;3:265–279. doi: 10.1016/S2215-0366(16)00013-4. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addictive Behaviors. 2010;35:970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Sellman JD, Porter RJ, Frampton CM. The relationship between non-acute adolescent cannabis use and cognition. Drug and Alcohol Review. 2007;26:309–319. doi: 10.1080/09595230701247772. [DOI] [PubMed] [Google Scholar]
- Hawkins JD, Graham JW, Maguin E, Abbott R, Hill KG, Catalano RF. Exploring the effects of age of alcohol use initiation and psychosocial risk factors on subsequent alcohol misuse. Journal of Studies on Alcohol and Drugs. 1997;58:280–290. doi: 10.15288/jsa.1997.58.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatbakhsh MR, Najman JM, Jamrozik K, Mamun AA, Alati R. Do parents’ marital circumstances predict young adults’ DSM-IV cannabis use disorders? A prospective study. Addiction. 2006;101:1778–1786. doi: 10.1111/j.1360-0443.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- Heron J, Barker ED, Joinson C, Lewis G, Hickman M, Munafo M, Macleod J. Childhood conduct disorder trajectories, prior risk factors and cannabis use at age 16: Birth cohort study. Addiction. 2013;108:2129–2138. doi: 10.1111/add.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibell B, Guttormsson U, Ahlström S, Balakireva O, Bjarnason T, Kokkevi A, Kraus L. The 2011 ESPAD Report: Substance use among students in 36 European countries. Stockholm: Swedish Council for Information on Alcohol and Other Drugs; 2012. [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: The role of reward-related learning and memory. Annual Review of Neuroscience. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jager G, Block RI, Luijten M, Ramsey NF. Cannabis use and memory brain function in adolescent boys: A cross-sectional multi-center functional magnetic resonance imaging study. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49:561–572. doi: 10.1016/j.jaac.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W, Hicks BM, McGue M, Iacono WG. How intelligence and education contribute to substance use: Hints from the Minnesota Twin Family Study. Intelligence. 2009;37:613–624. doi: 10.1016/j.intell.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Monitoring the future: National survey results on drug use 1975–2012: Vol. 1. Secondary school students. Ann Arbor, MI: University of Michigan, Institute for Social Research; 2013. [Google Scholar]
- Lee S, Tsang A, Breslau J, Aguilar-Gaxiola S, Angermeyer M, Borges G, … Kessler RC. Mental disorders and termination of education in high-income and low- and middle-income countries: Epidemiological study. British Journal of Psychiatry. 2009;194:411–417. doi: 10.1192/bjp.bp.108.054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisdahl KM, Price JS. Increased marijuana use and gender predict poorer cognitive functioning in adolescents and emerging adults. Journal of the International Neuropsychological Society. 2012;18:678–688. doi: 10.1017/S1355617712000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Bogorodzki P, Rogowska J, McGlade E, King JB, Terry J, Yurgelun-Todd D. Altered prefrontal and insular cortical thickness in adolescent marijuana users. Behavioural Brain Research. 2011;220:164–172. doi: 10.1016/j.bbr.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovallo WR, Farag NH, Sorocco KH, Acheson A, Cohoon AJ, Vincent AS. Early life adversity contributes to impaired cognition and impulsive behavior: Studies from the Oklahoma Family Health Patterns Project. Alcoholism: Clinical and Experimental Research. 2013;37:616–623. doi: 10.1111/acer.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubman DI, Cheetham A, Yucel M. Cannabis and adolescent brain development. Pharmacology and Therapeutics. 2015;148:1–16. doi: 10.1016/j.pharmthera.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Coffey C, Degenhardt L, Carlin JB, Patton G. A longitudinal study of the effects of adolescent cannabis use on high school completion. Addiction. 2003;98:685–692. doi: 10.1046/j.1360-0443.2003.00356.x. [DOI] [PubMed] [Google Scholar]
- Lynskey MT, Hall W. The effects of adolescent cannabis use on educational attainment: A review. Addiction. 2000;95:1621–1630. doi: 10.1046/j.1360-0443.2000.951116213.x. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Bar JL, Panizzon MS, Toomey R, Eisen S, Xian H, Tsuang MT. Neuropsychological consequences of regular marijuana use: A twin study. Psychological Medicine. 2004;34:1239–1250. doi: 10.1017/s0033291704002260. [DOI] [PubMed] [Google Scholar]
- Mathias CW, Blumenthal TD, Dawes MA, Liguori A, Richard DM, Bray B, … Dougherty DM. Failure to sustain prepulse inhibition in adolescent marijuana users. Drug and Alcohol Dependence. 2011;116:110–116. doi: 10.1016/j.drugalcdep.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey DF, Pacula RL, Han B, Ellickson P. Marijuana use and high school dropout: The influence of unobservables. Health Economics. 2010;19:1281–1299. doi: 10.1002/hec.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Hanson KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Neuropsychological functioning in adolescent marijuana users: Subtle deficits detectable after a month of abstinence. Journal of the International Neuropsychological Society. 2007;13:807–820. doi: 10.1017/S1355617707071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RSE, … Moffitt TE. Persistent cannabis users show neuro-psychological decline from childhood to midlife. Proceedings of the National Academy of Sciences. 2012;109:E2657–E2664. doi: 10.1073/pnas.1206820109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Meier MH, Caspi A, Poulton R. Reply to Rogeberg and Daly: No evidence that socioeconomic status or personality differences confound the association between cannabis use and IQ decline. Proceedings of the National Academy of Sciences. 2013;110:E983. doi: 10.1073/pnas.1300618110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokrysz C, Landy R, Gage SH, Munafo MR, Roiser JP, Curran HV. Are IQ and educational outcomes in teenagers related to their cannabis use? A prospective cohort study. Journal of Psychopharmacology. 2016;30:159–168. doi: 10.1177/0269881115622241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. Los Angeles: Author; 1998–2009. [Google Scholar]
- Newman JP, Patterson CM, Kosson DS. Response perseveration in psychopaths. Journal of Abnormal Psychology. 1987;96:145–148. doi: 10.1037//0021-843x.96.2.145. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Paus T. Maturation of structural and functional connectivity in the human brain. In: Jirsa VK, McIntosh AR, editors. Understanding complex systems. Vol. 2007. 2007. pp. 463–475. [Google Scholar]
- Petrides M. Nonspatial conditional learning impaired in patients with unilateral frontal but not unilateral temporal lobe excisions. Neuropsychologia. 1990;28:137–149. doi: 10.1016/0028-3932(90)90096-7. [DOI] [PubMed] [Google Scholar]
- Petrides M, Alivisatos B, Evans AC, Meyer E. Dissociation of human middorsolateral from posterior dorsolateral frontal cortex in memory processing. Proceedings of the National Academy of Sciences. 1993;90:873–877. doi: 10.1073/pnas.90.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacological Research. 2009;60:132–138. doi: 10.1016/j.phrs.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Rhemtulla M, Brosseau-Liard PE, Savalei V. When can categorical variables be treated as continuous? A comparison of robust continuous and categorical SEM estimation methods under suboptimal conditions. Psychological Methods. 2012;17:354–373. doi: 10.1037/a0029315. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. The impact of exposure to cannabinoids in adolescence: Insights from animal models. Biological Psychiatry. 2016;79:578–585. doi: 10.1016/j.biopsych.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M, … Parolaro D. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiology of Diseases. 2015;73:60–69. doi: 10.1016/j.nbd.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, … Parolaro D. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: A meta-analysis. Experimental and Clinical Psychopharmacology. 2012;20:420–429. doi: 10.1037/a0029117. [DOI] [PubMed] [Google Scholar]
- Schulte MH, Cousijn J, den Uyl TE, Goudriaan AE, van den Brink W, Veltman DJ, … Wiers RW. Recovery of neurocognitive functions following sustained abstinence after substance dependence and implications for treatment. Clinical Psychology Review. 2014;34:531–550. doi: 10.1016/j.cpr.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Brown SA, Tapert SF. The influence of marijuana use on neurocognitive functioning in adolescents. Current Drug Abuse Review. 2008;1:99–111. doi: 10.2174/1874473710801010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguin JR, Arseneault L, Boulerice B, Harden PW, Tremblay RE. Response perseveration in adolescent boys with stable and unstable histories of physical aggression: The role of underlying processes. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2002;43:481–494. doi: 10.1111/1469-7610.00039. [DOI] [PubMed] [Google Scholar]
- Séguin JR, Arseneault L, Tremblay RE. The contribution of “cool” and “hot” components of decision-making in adolescence: Implications for developmental psychopathology. Cognitive Development. 2007;22:530–543. [Google Scholar]
- Séguin JR, Boulerice B, Harden PW, Tremblay RE, Pihl RO. Executive functions and physical aggression after controlling for attention deficit hyperactivity disorder, general memory, and IQ. Journal of Child Psychology and Psychiatry. 1999;40:1197–1208. [PubMed] [Google Scholar]
- Séguin JR, Nagin D, Assaad JM, Tremblay RE. Cognitive-neuropsychological function in chronic physical aggression and hyperactivity. Journal of Abnormal Psychology. 2004;113:603–613. doi: 10.1037/0021-843X.113.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Séguin JR, Pihl RO, Harden PW, Tremblay RE, Boulerice B. Cognitive and neuropsychological characteristics of physically aggressive boys. Journal of Abnormal Psychology. 1995;104:614–624. doi: 10.1037//0021-843x.104.4.614. [DOI] [PubMed] [Google Scholar]
- Silins E, Horwood LJ, Patton GC, Fergusson DM, Olsson CA, Hutchinson DM … Cannabis Cohorts Research Committee. Young adult sequelae of adolescent cannabis use: An integrative analysis. Lancet Psychiatry. 2014;1:286–293. doi: 10.1016/S2215-0366(14)70307-4. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Nguyen-Louie TT, Tapert SF. Inhibition during early adolescence predicts alcohol and marijuana use by late adolescence. Neuropsychology. 2014;28:782–790. doi: 10.1037/neu0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiby AI, Hickman M, Munafo MR, Heron J, Yip VL, Macleod J. Adolescent cannabis and tobacco use and educational outcomes at age 16: Birth cohort study. Addiction. 2015;110:658–668. doi: 10.1111/add.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Epstein JN, Lisdahl KM, Molina B, Tapert S, Hinshaw SP … MTA Neuroimaging Group. Impact of ADHD and cannabis use on executive functioning in young adults. Drug and Alcohol Dependence. 2014;133:607–614. doi: 10.1016/j.drugalcdep.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Granholm E, Leedy NG, Brown SA. Substance use and withdrawal: Neuropsychological functioning over 8 years in youth. Journal of the International Neuropsychological Society. 2002;8:873–883. doi: 10.1017/s1355617702870011. [DOI] [PubMed] [Google Scholar]
- Thissen D, Steinberg L, Kuang D. Quick and easy implementation of the Benjamini–Hochberg procedure for controlling the false positive rate in multiple comparisons. Journal of Educational and Behavioral Statistics. 2002;27:77–83. [Google Scholar]
- Tofighi D, MacKinnon DP. RMediation: An R package for mediation analysis confidence intervals. Behavior Research Methods. 2011;43:692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay RE, Loeber R, Gagnon C, Charlebois P, Larivee S, Le-Blanc M. Disruptive boys with stable and unstable high fighting behavior patterns during junior elementary school. Journal of Abnormal Child Psychology. 1991;19:285–300. doi: 10.1007/BF00911232. [DOI] [PubMed] [Google Scholar]
- Tremblay RE, Pihl RO, Vitaro F, Dobkin PL. Predicting early onset of male antisocial behavior from preschool behavior. Archives of General Psychiatry. 1994;51:732–739. doi: 10.1001/archpsyc.1994.03950090064009. [DOI] [PubMed] [Google Scholar]
- Veroff J, McClelland L, Marquis K. Measuring intelligence and achievement motivation in surveys: Final report to the U.S. Department of Health, Education, and Welfare. New York: Office of Economic Opportunity; 1971. [Google Scholar]
- Verrico CD, Gu H, Peterson ML, Sampson AR, Lewis DA. Repeated D9-tetrahydrocannabinol exposure in adolescent monkeys: Persistent effects selective for spatial working memory. American Journal of Psychiatry. 2014;171:416–425. doi: 10.1176/appi.ajp.2013.13030335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitaro F, Brendgen M, Pagani L, Tremblay RE, McDuff P. Disruptive behavior, peer association, and conduct disorder: Testing the developmental links through early intervention. Development and Psychopathology. 1999;11:287–304. doi: 10.1017/s0954579499002060. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, … Baler R. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: A review. JAMA Psychiatry. 2016;73:292–297. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- von Sydow K, Lieb R, Pfister H, Hofler M, Wittchen HU. What predicts incident use of cannabis and progression to abuse and dependence? A 4-year prospective examination of risk factors in a community sample of adolescents and young adults. Drug and Alcohol Dependence. 2002;68:49–64. doi: 10.1016/s0376-8716(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Revised. New York: Psychological Corporation; 1987. [Google Scholar]
- White J, Batty GD. Intelligence across childhood in relation to illegal drug use in adulthood: 1970 British Cohort Study. Journal of Epidemiology and Community Health. 2012;66:767–774. doi: 10.1136/jech-2011-200252. [DOI] [PubMed] [Google Scholar]
- Windle M, Wiesner M. Trajectories of marijuana use from adolescence to young adulthood: Predictors and outcomes. Development and Psychopathology. 2004;16:1007–1027. doi: 10.1017/s0954579404040118. [DOI] [PubMed] [Google Scholar]
- Winters KC. Development of an adolescent alcohol and other drug abuse screening scale: Personal Experience Screening Questionnaire. Addictive Behaviors. 1992;17:479–490. doi: 10.1016/0306-4603(92)90008-j. [DOI] [PubMed] [Google Scholar]
- Winters KC. Assessment of alcohol and other drug use behaviors among adolescents. In: Allen JP, Wilson VB, editors. Assessing alcohol problems: A guide for clinicians and researchers. 2. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.