Abstract
Immunotherapy with dendritic cells (DCs) is a great promise for the treatment of neoplasms. However, the obtainment and protocol of differentiation of these cells may depend on extrinsic factors such as the tumor itself. The aim of the present study was to verify the influence of cervical neoplasia on different protocols of differentiation of monocyte-derived DCs resulting in an increased maturation phenotype. A total of 83 women were included in the study. The patients were grouped in low-grade squamous intraepithelial lesion (LSIL) (n=30), high-grade squamous intraepithelial lesion (HSIL) (n=22), cervical cancer (n=10) and healthy patients (n=21) groups. The mononuclear cells of patients were subjected to three differentiation protocols. In protocol I (pI), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-4 and tumor necrosis factor (TNF)-α were used for the differentiation of mature DCs (pIDCs). In protocol II (pII), monocytes were stimulated with GM-CSF, IL-4, TNF-α and activated lymphocytes in the absence of non-adherent cells (pIIDCs). In protocol III (pIII), monocytes were stimulated with GM-CSF, IL-4, TNF-α and activated lymphocytes in the presence of non-adherent cells (pIIIDCs). These cells were evaluated by flow cytometry for the expression of maturation markers such as cluster of differentiation (CD)11c, CD86 and human leukocyte antigen-antigen D related (HLA-DR). The main cytokines secreted (IL-4, IL-12 and transforming growth factor-β) were measured by ELISA. Our results indicate a significantly lower mature profile of pIIDCs and a significant increase in CD11c+ pIIIDCs able to produce IL-12 (P=0.0007). Furthermore, a significant reduction in cervical cancer HLA-DR+ pIDCs (P=0.0113) was also observed. HSIL patients exhibited a higher percentage of HLA-DR+ pIIDCs (P=0.0113), while LSIL patients had a lower percentage of CD11c+ pIIIDCs (P=0.0411). These findings suggest that the extent of cervical lesions affects the process of differentiation of DCs. Furthermore, activated lymphocytes may induce a better maturation of monocyte-derived DCs, and the presence of mononuclear cells appears to contribute to the DC differentiation process.
Keywords: cervical cancer, squamous intraepithelial lesion, dendritic cell, immunotherapy
Introduction
Cervical cancer is the fourth most common cancer among women worldwide and is considered an important public health problem (1). According to the World Health Organization, this type of tumor is the second most common among women in less developed countries (1). In 2012, it was estimated the diagnosis of 528,000 new cases and 270,000 mortalities of women by this type of tumor in the world (1).
Cervical cancer develops from premalignant precursor lesions known as cervical intraepithelial neoplasia (CIN). According to the cytological degree of commitment, CINs are classified as low-grade squamous intraepithelial lesions (LSILs) or high-grade squamous intraepithelial lesions (HSILs) (2). Studies have shown that the immune system serves a critical role in the elimination of these lesions, and defects in the function of this system appear to be associated with the mechanism of tumor escape from immune control (3,4).
Evidence suggests that dysfunction can be reversed by stimulating the immune system with antigen-presenting cells and cytokines such as interleukin (IL)-2 (5,6). Dendritic cells (DCs) are known as professional antigen-presenting cells and are part of the innate immune system. These cells can be detected in the majority of peripheral tissues, where they act on the initiation and modulation of the immune response during infections by pathogens and on the development of antitumor immune responses (7,8).
DCs are differentiated from pluripotent precursors located in the bone marrow. They have two distinct pathways of differentiation: i) The myeloid pathway, which generates myeloid DCs, including Langerhans cells, dermal DCs and interstitial DCs, which are characterized by the expression of the myeloid marker cluster of differentiation (CD)11c; and ii) the lymphoid pathway, which generates plasmacytoid DCs, which are characterized by the expression of the cell marker CD123 (7,9,10).
These cells are transported from the bloodstream to peripheral tissues in an immature form known as immature DCs (iDCs), which are characterized by little or no expression of co-stimulatory molecules such as CD40, CD80 and CD86, which are important for the T lymphocyte activation process (11). In this stage of differentiation, these immature cells have a reduced ability to activate the immune system (11,12).
The recognition and processing of antigens results in phenotypic and functional changes of iDCs, which leads to the maturation of these cells. During the maturation process, iDCs lose the molecules associated with the recognition of antigens and start to present on their surface molecules of the major histocompatibility complex, adhesion and co-stimulatory molecules, including CD209, CD80 and CD86 (13). Additionally, these cells become able to synthesize cytokines such as IL-1, IL-12, IL-18 and IL-23, which are important in the T lymphocyte activation process (13–15).
Thus, depending on the activating stimulus and the extent of maturation, DCs can modulate the differentiation of T helper (Th) lymphocytes (CD4+) in distinct subpopulations (16,17). Th1 lymphocytes are characterized by the synthesis of inflammatory cytokines such as IL-2 and interferon (IFN)-γ, which activate the cellular immune response (18). The main function of IL-2 is the self-regulation of the proliferation of Th1 lymphocytes (19). In addition, both IL-2 and IFN-γ regulate the activation of other leucocytes, including cytotoxic T lymphocytes (CD8+), natural killer (NK) cells and macrophages, which are key cells involved in the elimination of tumor cells (18,19).
By contrast, Th2 and regulatory T (Treg) cells are associated with inhibition of the differentiation of CD8+ T lymphocytes, NK cells and macrophages. This occurs by inducing an immune condition that favors tumor growth via the production of cytokines such as TGF-β by Treg cells and IL-4 by Th2 lymphocytes (20,21).
Based on the ability of DCs to initiate an specific and intense antitumor immune response through the induction of T-cell clones, Th1, CD8+ and NK cells, the study of new antitumor therapies has been supported by the use of this cell type as a therapeutic tool (22). Previous studies have focused on the development of immunotherapies with DCs using new differentiation and maturation protocols for the induction of an effective antitumor immune response (23,24).
Therefore, the purpose of the present study is to aid the development of new therapeutic strategies based on the differentiation and maturation of DCs in order to increase the effectiveness of antitumor vaccines. However, since it is a customized process, as it is performed with the patient's own cells, the degree of maturation reached by DCs following the same stimuli may be different from one patient to another. The present study intends to verify if there is any influence of the degree of malignancy on the maturation process and to evaluate the efficacy of different protocols. Accordingly, it was determined whether the type of cervical injury influences the differentiation of DCs in vitro and whether the use of other protocols of differentiation could result into mature DCs, which could be used as vaccines to induce an antitumor response.
Materials and methods
Patients and controls
Eighty-three patients aged 36.5±11.5 years were recruited from the Clinical Hospital of the Federal University of Triângulo Mineiro (Uberaba, MG, Brazil) between 2013 and 2015. Patients were selected through histopathological and cytological diagnosis, and were grouped in LSIL (n=30), HSIL (n=22), cervical cancer (n=10) and healthy patients (control, n=21) groups. The number of cervical cancer patients was not sufficient for the statistical analysis of the differentiation of DCs using protocols II and III. Patients with immunosuppressive diseases or autoimmune diseases, and those who were using immunosuppressive or antitumor drugs or were pregnant, were excluded from the study.
All the patients and healthy controls involved in the present study were counseled regarding its aims, and provided written consent for participation. The study was approved by the Ethics Committee of the Federal University of the Triângulo Mineiro of Uberaba (Uberaba, MG, Brazil; approval no. 683-2006).
Generation of mature DCs and alternatively activated DCs
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation of peripheral blood using Ficoll-Paque™ PLUS solution (GE Healthcare Life Sciences, Chalfont, UK) at 600 × g for 30 min at 18°C. These cells were subjected to three different protocols of differentiation and activation. Mature DCs [protocol I (pI), pIDCs] were generated by culture of PBMCs (5×106 cells) in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 (0.5 ng/ml each; BD Pharmingen, San Diego, CA, USA) followed by the addition on day 6 of tumor necrosis factor (TNF)-α (0.5 ng/ml; BD Pharmingen) for 48 h. Alternatively, activated DCs were generated by culture of PBMCs (5×106 cells) in the presence of GM-CSF and IL-4 (0.5 ng/ml each; BD Pharmingen) for 5 days in the absence [protocol II (pII), pIIDCs] or presence [protocol III (pIII), pIIIDCs] of non-adherent cells to obtain iDCs. These cells were matured by the addition of TNF-α (0.5 ng/ml; BD Pharmingen) on day 6 and of supernatant derived from lymphocytes stimulated with lipopolysaccharide (LPS) (10 mg/ml for 48 h; Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) on day 7 for 24 h. DCs were cultured in Iscove's modified Dulbecco's medium (Sigma-Aldrich; Merck Millipore), while lymphocytes were cultured in RPMI-1640 medium (Sigma-Aldrich; Merck Millipore) supplemented with 40 mg/ml gentamycin, 200 mM L-glutamine and 10% fetal bovine serum (Sigma-Aldrich; Merck Millipore) at 37°C with 5% CO2.
Flow cytometry
On day 8 of culture, mature DCs and alternatively activated DCs were incubated with the corresponding antibodies to examine the expression of maturation markers. Antibodies used for flow cytometry included anti-CD11c-allophycocyanin, anti-human leukocyte antigen-antigen D related (HLA-DR)-peridinin chlorophyll protein, anti-CD86-phycoerythrin and isotypic controls (BD Pharmigen). Data were acquired in the flow cytometer FACSCalibur™ (BD Biosciences, Franklin Lakes, NJ, USA).
ELISA
Supernatants were harvested from DCs culture on day 8 and stored at −80°C. The procedure was performed according to the manufacturer's protocol. Cytokines, including IL-12, p40, IL-4 and TGF-β, were measured by sandwich ELISA (BD OptEIA™; BD Biosciences).
Statistical analysis
Statistical analysis was performed with GraphPad Prism 5.00 (GraphPad Software, Inc., La Jolla, CA, USA). The data for each variable were tested to assess whether they were normally distributed. For comparisons of non-normally distributed data, Mann-Whitney test was used for comparison of paired groups, and Kruskal-Wallis and post hoc Dunn's tests were used for comparisons among three or more groups. The results are expressed as the median and range. P<0.05 was considered to indicate a statistically significant difference.
Results
pI results in a semi-mature phenotype of DCs from cervical cancer patients
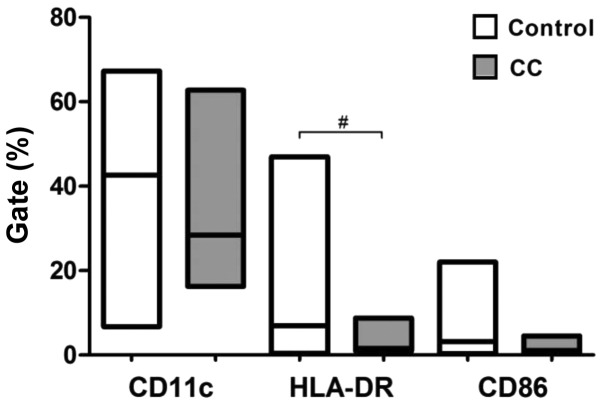
The expression of co-stimulatory molecules and surface markers (CD11c, CD86 and HLA-DR) was evaluated by flow cytometry to examine the maturity status of DCs differentiated according to pI from healthy subjects and cervical cancer patients. Compared with normal pIDCs, cervical cancer pIDCs expressed lower levels of the surface marker CD11c and the co-stimulatory molecule CD86, and significantly lower levels of the antigen-presenting molecule HLA-DR (P=0.0113) (Fig. 1). These results suggest that cervical cancer monocyte-derived DCs matured according to pI exhibit a lower maturation phenotype. There were no significant changes for the other groups using this maturation protocol (Table I).
Figure 1.
Expression of co-stimulatory molecules and surface markers on mature DCs obtained with protocol I. Peripheral blood mononuclear cells were stimulated with granulocyte-macrophage colony-stimulating factor, interleukin-4 and tumor necrosis factor-α to generate mature DCs. Data are presented as the median and range (Mann-Whitney test). #P<0.113. Control, healthy patients. CC, cervical cancer; CD, cluster of differentiation; DC, dendritic cell; HLA-DR, human leukocyte antigen-antigen D related.
Table I.
Comparison between the different protocols used for the activation profile and cytokine production of monocyte-derived dendritic cells derived from cervical intraepithelial lesions patients.
| A, Gate (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Protocol I | Protocol II | Protocol III | ||||||||
| Cytokine | Control | LSIL | HSIL | CC | Control | LSIL | HSIL | Control | LSIL | HSIL |
| CD11c | 42.65 | 30.91 | 39.54 | 33.87 | 2.19a | 1.57a | 7.26b,c | 30.17 | 46.36b,c | 32.76 |
| HLA-DR | 6.96 | 5.75 | 5.65 | 1.59c | 0.70a | 0.62b | 1.76 | 6.60 | 6.95 | 4.23 |
| CD86 | 3.25 | 2.05 | 2.56 | 1.12 | 0.57b | 0.67b | 1.11 | 1.91 | 1.59 | 3.44 |
| B, Cytokine production (pg/ml) | ||||||||||
| Protocol I | Protocol II | Protocol III | ||||||||
| Cytokine | Control | LSIL | HSIL | CC | Control | LSIL | HSIL | Control | LSIL | HSIL |
| IL-4 | 26.68 | 34.97 | 29.95 | 31.30 | 330.60b | 47.31b | 39.16 | 19.43 | 13.02b | 11.34b |
| IL-12 | 272.80 | 271.50 | 277.20 | 244.60 | 42.77a | 105.00 | 84.21 | 544.00b | 1,059.00b | 641.00 |
| TGF-β | 260.20 | 334.50 | 526.50 | 479.30 | 688.30b | 721.90b | 626.10 | 1,602.00a | 4,525.00a | 3,254.00b |
P<0.01
P<0.05 (comparison between protocols)
P<0.05 (comparison between patient groups). Results are expressed as the median (Mann-Whitney test for comparison of paired groups; Kruskal-Wallis and post hoc Dunn's tests for comparisons among three or more groups). CD, cluster of differentiation; HLA-DR, human leukocyte antigen-antigen D related; IL, interleukin; TGF, transforming growth factor; LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; CC, cervical cancer.
pII induces a significantly lower maturation profile of DCs
The expression of co-stimulatory molecules and surface markers (CD11c, CD86 and HLA-DR) was evaluated by flow cytometry to determine if the use of different protocols of differentiation could result into DCs with increased maturation profile. Compared with pIDCs and pIIIDCs, pIIDCs exhibited significantly lower expression of CD11c (P<0.0001), CD86 (P=0.0006) and HLA-DR (P<0.0001) (Fig. 2A-C). Furthermore, these cells displayed a significant reduction in IL-12 (P<0.0001) production and a significant increase in IL-4 production (P<0.0001) (Fig. 3A and B). These findings suggest that, in the absence of non-adherent cells, monocyte-derived DCs show a reduction in their differentiation and maturation processes.
Figure 2.
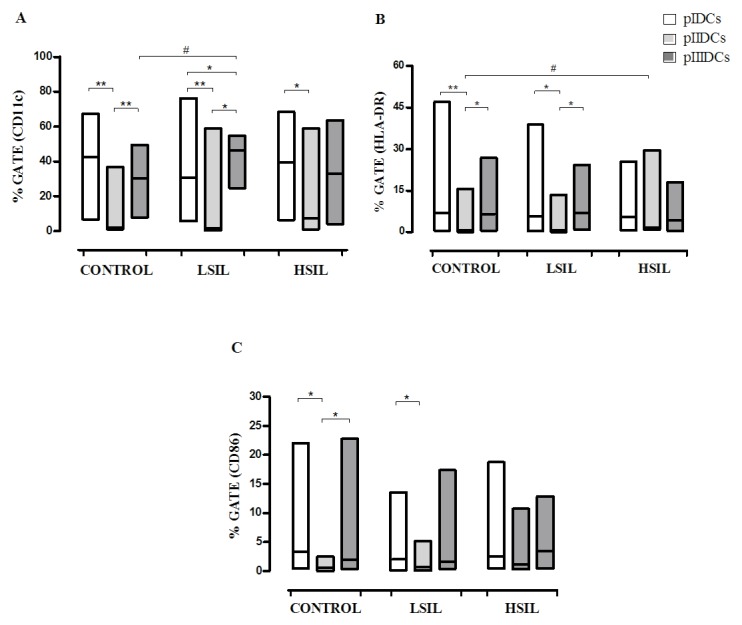
Phenotypic profiles of monocyte-derived pIDCs, pIIIDCs and pIIDCs. PBMCs were stimulated with GM-CSF, IL-4 and TNF-α to generate pIDCs. The expression of co-stimulatory molecules and surface markers (A) CD11c, (B) HLA-DR and (C) CD86 and was evaluated by flow cytometry to evaluate the effect of different protocols of differentiation on the maturation profile. PBMCs were stimulated with GM-CSF, IL-4, TNF-α and activated lymphocytes in the absence of non-adherent cells to generate pIIDCs. PBMCs were stimulated with GM-CSF, IL-4, TNF-α and activated lymphocytes in the presence of non-adherent cells to generate pIIIDCs. Data are presented as the median and range (Mann-Whitney and Kruskal-Wallis tests). #P<0.05 (comparison between patient groups); *P<0.05, **P<0.01 (comparison between protocols). Control, healthy patients. LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; PBMC, peripheral blood mononuclear cell; pI–III, protocol I–III; CD, cluster of differentiation; DC, dendritic cell; HLA-DR, human leukocyte antigen-antigen D related; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; TNF, tumor necrosis factor.
Figure 3.
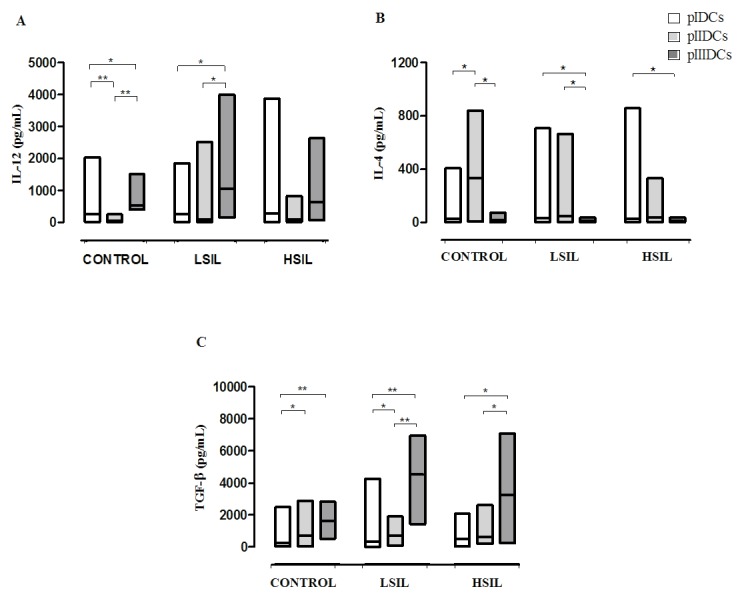
Cytokine profile of monocyte-derived pIDCs, pIIIDCs and pIIDCs. PBMCs were stimulated with GM-CSF, IL-4 and TNF-α to generate pIDCs. PBMCs were stimulated with GM-CSF, IL-4, TNF-α and activated lymphocytes in the absence of non-adherent cells to generate pIIDCs. PBMCs were stimulated with GM-CSF, IL-4, TNF-α and activated lymphocytes in the presence of non-adherent cells to generate pIIIDCs. LSIL pIIIDCs exhibited (A) a significant increase in IL-12 production and (B) a significant decrease in IL-4 production. (C) LSIL and HSIL pIDCs, pIIDCs and pIIIDCs exhibited a significantly increased TGF-β production. Data are presented as the median and range (Kruskal-Wallis test). *P<0.05, **P<0.01. Control, healthy patients. LSIL, low-grade squamous intraepithelial lesion; HSIL, high-grade squamous intraepithelial lesion; PBMC, peripheral blood mononuclear cell; pI–III, protocol I–III; DC, dendritic cell; TGF, transforming growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; TNF, tumor necrosis factor.
pII induces increased HLA-DR expression in HSIL DCs
Compared with normal DCs, HSIL DCs differentiated according to pII exhibited significantly increased expression of HLA-DR (P=0.0113) (Fig. 2B). These findings suggest that the presence of factors produced by non-adherent cells can inhibit the expression of this molecule in monocyte-derived DCs.
pIII induces high CD11c+ IL-12-producing LSIL DCs
Compared with control pIIIDCs (P=0.0058), LSIL pIDCs (P=0.0411) and LSIL pIIDCs (P=0.0003), LSIL pIIIDCs expressed significantly increased levels of the surface marker CD11c (Fig. 2A). Furthermore, LSIL pIIIDCs also show a significant increase in IL-12 production (P=0.0007) and a significant decrease in IL-4 production (P=0.0038) (Fig. 3A and B and Table I). Together, these findings suggest that, in the presence of non-adherent cells, monocyte-derived DCs stimulated with activated lymphocytes may be more effective in modulating the antitumor response during immunotherapy for patients with low-grade cervical lesions.
pIIDCs and pIIIDCs show increased TGF-β production
Compared with control, LSIL and HSIL pIDCs, pIIDCs (P=0.0189) and pIIIDCs (P=0.0002) showed a significantly increased TGF-β production (Fig. 3C). These findings suggest that stimulation of non-adherent cells with LPS may induce an increased production of this cytokine by Treg cells.
Discussion
The identification of specific immune responses in cancer patients stimulated the development of numerous immunotherapies for tumors (25). In 1994, Sallusto and Lanzavecchia (26) developed a technique capable of inducing the differentiation of mature DCs from peripheral blood monocytes using a combination of the cytokines IL-4, GM-CSF and TNF-α. Since then, several protocols have been developed in order to improve the function of these cells, since their ability to activate naive T lymphocytes and to initiate a specific immune response depends on their state of maturity (26–28).
Among the protocols used, it is worth citing stimulation with TNF-α, IL-1β, bacterial products such as LPS, IFNs and prostaglandins (29–31). Conventional maturation protocols use a combination of the cytokines IL-1β, IL-6, TNF-α and prostaglandin E2, which are capable of inducing the differentiation of mature DCs with great co-stimulatory capacity and migration ability, but with reduced capacity of production of IL-12 (32,33).
The present study verified the differentiation profile of DCs from patients with cervical lesions, and assessed whether different protocols of maturation could result in DCs in a better state of maturity. Studies have shown that cancer patients have alterations in the DCs differentiation and maturation processes, which can be associated with the mechanism of escaping immune surveillance (34). No studies were identified in the literature correlating the influence of the degree of malignancy of cervical intraepithelial lesions or the influence of non-adherent cells with the maturation of DCs.
In the present study, a reduction in the maturity of monocyte-derived DCs from cervical cancer patients was observed. Gabrilovich et al (4) observed that monocyte-derived DCs from patients with breast cancer exhibited a reduction in antigen presentation, which is consistent with the findings of our study.
When exposed to lymphocytes activated with LPS, a significant increase in CD11c+ pIIIDCs was observed when comparing the LSIL group with the control group. Our results also indicate a significant reduction in CD11c+ pIIDCs compared with pIIIDCs and pIDCs in the LSIL and control groups. In addition, a significant increase in CD11c+ pIIIDCs compared with pIDCs was observed. These results suggest that the presence of non-adherent cells in the supernatant of monocyte-derived DCs culture may assist in their differentiation.
In 2003, Wolf et al demonstrated an increased number of Treg lymphocytes in peripheral blood from patients with different types of cancer (35). These cells can synthesize TGF-β, a cytokine able to assist in tumor development (36). Luttmann et al reported that this cytokine is able to inhibit HLA-DR expression in peripheral blood cells (37). The significant increase in HLA-DR+ pIIDCs in the HSIL group compared with the control group observed in the present study may be associated with the absence of Treg lymphocytes in the culture of monocyte-derived pIIDCs.
When evaluating HLA-DR expression across the different protocols used, lower HLA-DR+ pIIDCs were observed in all the groups of patients. This reduction was statistically significant when comparing pIIDCs with pIDCs and pIIIDCs in the LSIL and control groups, suggesting that DCs maturation is also influenced by the presence of non-adherent cells in the culture supernatant.
Orsini et al observed that colorectal cancer patients had deficiency in monocyte-derived DCs differentiation, with reduced expression of co-stimulatory molecules and reduced IL-12 and TNF-α synthesis (38). In our study, no significant changes in the expression of the co-stimulatory molecule CD86 were observed, suggesting that the degree of the cervical lesion was not able to interfere on the expression of this molecule.
Previous studies have shown that lymphocytes of the innate immune response, including NK, NKT and γδ T cells, are able to induce DCs maturation, which may be evidenced by increased CD86 expression and IL-12 production (39–41). In the present study, it was noticed a lower expression of CD86 in pIIDCs compared with DCs derived from the other protocols. This reduction was significant when comparing CD86+ pIIDCs with CD86+ pIDCs and pIIIDCs in the LSIL and control groups, which suggested that lymphocytes present in the culture supernatant not only stimulate HLA-DR expression, but also induce CD86 expression in monocyte-derived DCs.
Human monocyte-derived CD11c+ DCs induce predominantly the differentiation of naive T lymphocytes in Th1 cells by IL-12 production, whereas CD11c− cells induce prevalently Th2 responses (13,42). Furthermore, IL-12 produced by DCs is capable of stimulating cytotoxicity and production of IFN-γ by CD8+ T lymphocytes and NK cells (43,44).
In our study, no statistically significant differences were observed for the production of this cytokine among the LSIL, HSIL, cervical cancer or control groups. By contrast, when comparing the different protocols used for differentiation, lower IL-12 production was noticed in pIIDCs compared with pIDCs and pIIIDCs. This reduction in IL-12 synthesis may be due to the absence of non-adherent cells in the supernatants of DCs culture, since these cells appear to be directly associated with the maturation of DCs and the synthesis of IL-12, as described above. In addition, increased IL-12 production and a significant reduction in IL-4 production by pIIIDCs was observed, suggesting that it could be more effective in inducing antitumor responses during immunotherapy with DCs since IL-12 cytokine induces the differentiation of Th1 lymphocytes, which is important in the antitumor response.
In normal cells, TGF-β acts as a tumor suppressor because it can inhibit cell proliferation and promote cell differentiation and apoptosis (36). However, in the early stages of tumorigenesis, cells lose the inhibition of growth mediated by TGF-β as a result of mutation or loss of expression of genes associated with the TGF-β signaling pathway (36). Once they have become resistant against growth inhibition by TGF-β, tumor cells and stromal cells present in the tumor increase the production of this cytokine, which stimulates angiogenesis, cell motility and suppression of the immune system, thus promoting the invasion and metastasis of tumor cells (36).
pIIIDCs have shown increased TGF-β production in patients with SILs in the present study. When comparing the different protocols used, it was observed that there was an increase in TGF-β production in pIIDCs and pIIIDCs compared with pIDCs for all the groups of patients evaluated. This increase was significant when comparing pIIIDCs with pIDCs in the control group, and when comparing pIIIDCs with pIDCs and pIIDCs in patients with LSIL and HSIL. This may be associated with the presence of Treg lymphocytes in the culture supernatant, which, according to Caramalho et al, can be activated by stimulation with LPS (45).
From these results, it can be concluded that the cell-to-cell contact of activated lymphocytes is able to induce a better differentiation of monocyte-derived DCs. Furthermore, it was also observed that activated non-adherent cells were capable of inducing increased CD86 and HLA-DR expression, and probably capable of inducing Th1 responses as well, which are notably important for the antitumor response. This could be observed by the increase in IL-12 production characteristic of this profile and by the reduction in IL-4 synthesis, which is a cytokine produced by Th2 cells. The presence of non-adherent cells in the culture of DCs appears to contribute to their differentiation process, since their removal induced a significant reduction in the expression of the surface markers CD11c, CD86 and HLA-DR, and induced a significant reduction in IL-12 synthesis. In addition, it was also observed that the extent of the cervical lesion can influence the differentiation process of DCs, since a significant reduction in HLA-DR+ DCs differentiated according to pI was obtained from patients with cervical cancer. Therefore, future studies are required to understand the effects of non-adherent cells on the differentiation and maturation of DCs.
Acknowledgements
The authors acknowledge the funding received from the Brazilian National Council for Scientific and Technological Development (grant no. 302011/2015-3), the Uberaba Foundation for Teaching and Research (grant no. 255/2012) and the Foundation for Research Assistance of the State of Minas Gerais (grant no. Rede 11/14).
References
- 1.Stewart BW, Wild CP, editors. World Cancer Report 2014. World Health Organization, IARC; Lyon: 2016. [Google Scholar]
- 2.Apgar BS, Zoschnick L, Wright TC., Jr The 2001 bethesda system terminology. Am Fam Phisician. 2003;68:1992–1998. [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine. 2006;24:11–25. doi: 10.1016/j.vaccine.2006.05.111. (Suppl 3) [DOI] [PubMed] [Google Scholar]
- 4.Gabrilovich DI, Corak J, Ciernik IF, Kavanaugh D, Carbone DP. Decreased antigen presentation by dendritic cells in patients with breast cancer. Clin Cancer Res. 1997;3:483–490. [PubMed] [Google Scholar]
- 5.Vopenkova K, Mollova K, Buresova I, Michalek J. Complex evaluation of human monocyte-derived dendritic cells for cancer immunotherapy. J Cell Mol Med. 2012;16:2827–2837. doi: 10.1111/j.1582-4934.2012.01614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrantini M, Capone I, Belardelli F. Dendritic cells and cytokines in immune rejection of cancer. Cytokine Growth Factor Rev. 2008;19:93–107. doi: 10.1016/j.cytogfr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 8.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 9.van Nierop K, de Groot C. Human follicular dendritic cells: Function, origin and development. Semin Immunol. 2002;14:251–257. doi: 10.1016/S1044-5323(02)00057-X. [DOI] [PubMed] [Google Scholar]
- 10.Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelleher P, Knight SC. IL-12 increases CD80 expression and the stimulatory capacity of bone marrow-derived dendritic cells. Int Immunol. 1998;10:749–755. doi: 10.1093/intimm/10.6.749. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science. 1990;248:1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 14.Randolph GJ, Sanchez-Schmitz G, Angeli V. Factors and signals that govern the migration of dendritic cells via lymphatics: Recent advances. Springer Semin Immunopathol. 2005;26:273–287. doi: 10.1007/s00281-004-0168-0. [DOI] [PubMed] [Google Scholar]
- 15.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu YJ. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell. 2001;106:259–262. doi: 10.1016/S0092-8674(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 17.Bradley LM. Migration and T-lymphocyte effector function. Curr Opin Immunol. 2003;15:343–348. doi: 10.1016/S0952-7915(03)00043-8. [DOI] [PubMed] [Google Scholar]
- 18.Corthay A, Skovseth DK, Lundin KU, Røsjø E, Omholt H, Hofgaard PO, Haraldsen G, Bogen B. Primary antitumor immune response mediated by CD4+ T cells. Immunity. 2005;22:371–383. doi: 10.1016/j.immuni.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Pardoll DM, Topaliant SL. The role of CD4+ T cell responses in antitumor immunity. Courrent Opin Immunol. 1998;10:588–594. doi: 10.1016/S0952-7915(98)80228-8. [DOI] [PubMed] [Google Scholar]
- 20.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065X.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 21.Fukaura H, Kent SC, Pietrusewicz MJ, Khoury SJ, Weiner HL, Hafler DA. Induction of circulating myelin basic protein and proteolipid protein-specific transforming growth factor- beta 1-secreting Th3 T cells by oral administration of myelin in multiple sclerosis patients. J Clin Invest. 1996;98:70–77. doi: 10.1172/JCI118779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koido S, Homma S, Takahara A, Namiki Y, Tsukinaga S, Mitobe J, Odahara S, Yukawa T, Matsudaira H, Nagatsuma K, et al. Current immunotherapeutic approaches in pancreatic cancer. Clin Dev Immunol. 2011;2011:267539. doi: 10.1155/2011/267539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodrigues CM, Matias BF, Murta EF, Michelin MA. The role of T lymphocytes in cancer patients undergoing immunotherapy with autologous dendritic cells. Clin Med Insights Oncol. 2011;5:107–115. doi: 10.4137/CMO.S6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matias BF, de Oliveira TM, Rodrigues CM, Abdalla DR, Montes L, Murta EF, Michelin MA. Influence of immunotherapy with autologous dendritic cells on innate and adaptive immune response in cancer. Clin Med Insights Oncol. 2013;7:165–172. doi: 10.4137/CMO.S12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baxevanis CN, Perez SA, Papamichail M. Combinatorial treatments including vaccines, chemotherapy and monoclonal antibodies for cancer therapy. Cancer Immunol Immunother. 2009;58:317–324. doi: 10.1007/s00262-008-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sallusto BF, Lanzavecchia A. Efficient presentation of solube antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Apostolopoulos V, Pietersz GA, Tsibanis A, Tsikkinis A, Stojanovska L, McKenzie IF, Vassilaros S. Dendritic cell immunotherapy: Clinical outcomes. Clin Transl Immunol. 2014;3:e21. doi: 10.1038/cti.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: Mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 29.Han TH, Jin P, Ren J, Slezak S, Marincola FM, Stroncek DF. Evaluation of 3 clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-gamma. J Immunother. 2009;32:399–407. doi: 10.1097/CJI.0b013e31819e1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, van Ham SM. The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization. Vaccine. 2007;25:7145–7152. doi: 10.1016/j.vaccine.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 31.Snijders A, Kalinski P, Hilkens CM, Kapsenberg ML. High-level IL-12 production by human dendritic cells requires two signals. Int Immunol. 1998;10:1593–1598. doi: 10.1093/intimm/10.11.1593. [DOI] [PubMed] [Google Scholar]
- 32.Jonuleit H, Kühn U, Müller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk AH. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 33.Nicolette CA, Healey D, Tcherepanova I, Whelton P, Monesmith T, Coombs L, Finke LH, Whiteside T, Miesowicz F. Dendritic cells for active immunotherapy: Optimizing design and manufacture in order to develop commercially and clinically viable products. Vaccine. 2007;25:B47–B60. doi: 10.1016/j.vaccine.2007.06.006. (Suppl 2) [DOI] [PubMed] [Google Scholar]
- 34.Pinzon-Charry A, Maxwell T, López JA. Dendritic cell dysfunction in cancer: A mechanism for immunosuppression. Immunol Cell Biol. 2005;83:451–461. doi: 10.1111/j.1440-1711.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 35.Wolf AM, Wolf D, Steurer M, Gastl G, Gunsilius E, Grubeck-loebenstein B. Increase of regulatory T Cells in the peripheral blood of cancer patients. Clin Cancer Res. 2003;9:606–612. [PubMed] [Google Scholar]
- 36.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342:1350–1308. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 37.Luttmann W, Franz P, Schmidt S, Barth J, Matthys H, Virchow JC., Jr Inhibition of HLA-DR expression on activated human blood eosinophils by transforming growth factor-beta1. Scand J Immunol. 1998;48:667–671. doi: 10.1046/j.1365-3083.1998.00446.x. [DOI] [PubMed] [Google Scholar]
- 38.Orsini G, Legitimo A, Failli A, Ferrari P, Nicolini A, Spisni R, Miccoli P, Consolini R. Defective generation and maturation of dendritic cells from monocytes in colorectal cancer patients during the course of disease. Int J Mol Sci. 2013;14:22022–22041. doi: 10.3390/ijms141122022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mocikat R, Braumüller H, Gumy A, Egeter O, Ziegler H, Reusch U, Bubeck A, Louis J, Mailhammer R, Riethmüller G, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity. 2003;19:561–569. doi: 10.1016/S1074-7613(03)00264-4. [DOI] [PubMed] [Google Scholar]
- 40.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, Schmidt R, Harris AL, Old L, Cerundolo V. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–5147. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 41.Leslie DS, Vincent MS, Spada FM, Das H, Sugita M, Morita CT, Brenner MB. CD1-mediated gamma/delta T cell maturation of dendritic cells. J Exp Med. 2002;196:1575–1584. doi: 10.1084/jem.20021515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maldonado-López R, De Smedt T, Michel P, Godfroid J, Pajak B, Heirman C, Thielemans K, Leo O, Urbain J, Moser M. CD8alpha+ and CD8alpha- subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J Exp Med. 1999;189:587–592. doi: 10.1084/jem.189.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorman SE, Holland SM. Interferon-gamama and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev. 2000;11:321–333. doi: 10.1016/S1359-6101(00)00010-1. [DOI] [PubMed] [Google Scholar]
- 44.Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD, Dahm P, Niedzwiecki D, Gilboa E, Vieweg J. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–417. doi: 10.1172/JCI0214364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caramalho I, Lopes-Carvalho T, Ostler D, Zelenay S, Haury M, Demengeot J. Regulatory T cells selectively express toll-like receptors and are activated by lipopolysaccharide. J Exp Med. 2003;197:403–411. doi: 10.1084/jem.20021633. [DOI] [PMC free article] [PubMed] [Google Scholar]