Abstract
Although the prognostic role of neutrophil-lymphocyte ratio (NLR) has been confirmed in a variety of tumors, the prognostic role of NLR in pancreatic neuroendocrine tumors (PNETs) has not been examined. The present study was performed to assess the role of NLR as a prognostic factor in patients with PNETs. Clinical data were retrospectively retrieved from a single institution. The best cut-off value for baseline NLR levels was determined by the receiver operating characteristic (ROC) curve and area under the ROC curve. The primary event was overall survival and event times were assessed by the Kaplan-Meier method. Potential factors associated with the elevation of NLR in PNETs were examined. A total of 165 consecutive patients with pathologically confirmed PNETs were included in this study. The cutoff value of NLR was 2.4 by ROC curve (area under ROC curve, 0.70). NLR >2.4 was found to be a poor prognostic factor in the univariate and multivariate analyses. Patients with a NLR value >2.4 had a higher proportion of tumor size at >3 cm (P=0.001), TNM stage III or IV (P=0.019), and G2/G3 (P=0.003). We concluded that NLR is an independent predictor of overall survival for patients with PNETs. Aberrant elevation of NLR identifies high-risk patients with aggressive characteristics.
Keywords: pancreatic neuroendocrine tumor, prognosis, pancreatic neuroendocrine tumors, neutrophil-lymphocyte ratio
Introduction
Pancreatic neuroendocrine tumors (PNETs) are rare but clinical important tumors with an incidence of approximately 1 in 100,000 of the population, accounting for 1–2% of all pancreatic tumors in incidence and 10% in prevalence (1–4). They are broadly categorized as functioning or non-functioning tumors based on their clinical manifestation (5). Unlike functioning neoplasms, non-functioning PNETs are often detected at an advanced stage due to the lack of specific symptoms (6). PNETs are highly heterogeneous neoplasms presenting a spectrum of biologic behavior (7,8). Aggressive progression can even be observed in incidentally detected and small tumors (9). Potential prognostic factors, including mitoses, vascular invasion, metastasis, necrosis, Ki-67 expression, and nuclear grade, are mostly based on pathological examination (2,3,10,11). Therefore, circulating biomarkers are needed to predict their malignant behavior and prognosis.
Neutrophil-lymphocyte ratio (NLR) is derived from the absolute neutrophil count divided by the absolute lymphocyte count and is a routinely used, reliable, and convenient marker (12). It is an index of systemic inflammation, which is a common phenomenon and prognostic determinant of cancer (12). In recent years, increasing evidence has demonstrated the role of NLR in evaluating treatment response and predicting prognosis in various types of cancer (13–17). For example, in pancreatic adenocarcinoma, NLR may be used to assess survival in unselected cohorts, patients with advanced diseases treated with chemotherapy, and patients undergoing curative surgery (13–16). In addition, NLR was statistically significantly associated with tumor stage, differentiation, performance status, CA19-9, C-reactive protein, and albumin levels in pancreatic adenocarcinoma (16). However, the prognostic role of NLR in PNETs has not been evaluated.
The present study was performed to examine the role of blood NLR as a prognostic factor in a large cohort of patients with PNETs. Potential clinicopathological factors associated with the abnormal elevation of NLR in PNETs were also evaluated.
Materials and methods
Patients
The databases of Shanghai Cancer Center, Fudan University (Shanghai, China) were collected to identify potential patients with pathological confirmed PNETs between 2006 and 2015. Data were retrieved regarding patient demographics, symptoms, tumor size, location, functioning status, histologic grade, lymph node involvement, vessel invasion, nerve invasion, and TNM stages. Positive symptoms included abdominal and back pain, weight loss, nausea, vomiting, fatigue and jaundice. Patients with functioning PNETs or patients with non-functioning PNETs and without the above mentioned symptoms were viewed as incidental PNETs (18). The patients were staged based on the 7th edition of American Joint Committee on Cancer (AJCC) TNM staging system. Tumors were categorized as G1, G2, G3 according to the 2010 World Health Organization (WHO) classification (based on the Ki-67 index and the mitotic index) (19). Patients were followed up >18 months or until death. The laboratory data including neutrophil and lymphocyte were obtained before major treatments within 2 weeks. The NLR was calculated by the absolute neutrophil count divided by the absolute lymphocyte count. The receiver operating characteristic (ROC) curve and area under the ROC curve were applied to select the best cut-off values for baseline NLR. The current study was approved by the ethics committee of Shanghai Cancer Center, Fudan University.
Statistical analysis
Univariate and multivariate analyses based on a Cox proportional hazards model were used to analyze potential prognostic factors. Factors with a P<0.05 in the univariate analysis were further included in the multivariate analysis. The effect of the NLR and other factors on survival was estimated using the Kaplan-Meier method. Pearson's χ2 test or Fisher's exact test was used to analyze categorical data as appropriate. The analysis was performed using the STATA 12.0 statistical software package (StataCorp LP, College Station, TX, USA). A two-sided P<0.05 was considered to indicate a statistically significant difference.
Results
Patients data and survival analysis
A total of 165 consecutive patients with pathologically confirmed PNETs were included in the present study (Table I). The median age was 52, with 58.2% of patients having an age >50. More than 50% of patients were female, with a female-to-male ratio of 1.2:1. A total of 68 patients (41.2%) had tumors located at the head of the pancreas and 97 patients (58.8%) at the body, or tail of the pancreas, or whole pancreas. The median size was 4 cm, with >60% of cases having tumors >3 cm in diameter. More than 60% of patients had stage I or II tumors. Nearly 50% of the patients had G1 tumors (G1 46.3%, G2 42.6%, G3 11.0%) and 18 patients (10.9%) had functioning diseases. Nearly half of the patients (47.3%) had PNETs with symptoms.
Table I.
Demographics and clinical characteristics.
| Demographic or clinical characteristic | Parameter | No. (%) |
|---|---|---|
| Age | ≤50 | 69 (41.8) |
| >50 | 96 (58.2) | |
| Gender | Male | 76 (46.1) |
| Female | 89 (53.9) | |
| Location | Head | 68 (41.2) |
| Others | 97 (58.8) | |
| Size (cm) | ≤3 | 57 (34.5) |
| >3 | 108 (65.5) | |
| TNM stage | I, II | 107 (64.8) |
| III, IV | 58 (35.2) | |
| Gradea | G1 | 63 (46.3) |
| G2 | 58 (42.6) | |
| G3 | 15 (11.0) | |
| Functioning | Positive | 18 (10.9) |
| Negative | 147 (89.1) | |
| Symptom | Positive | 78 (47.3) |
| Negative | 87 (52.7) |
Only 136 cases had information for grade. TNM, tumor-node-metastasis.
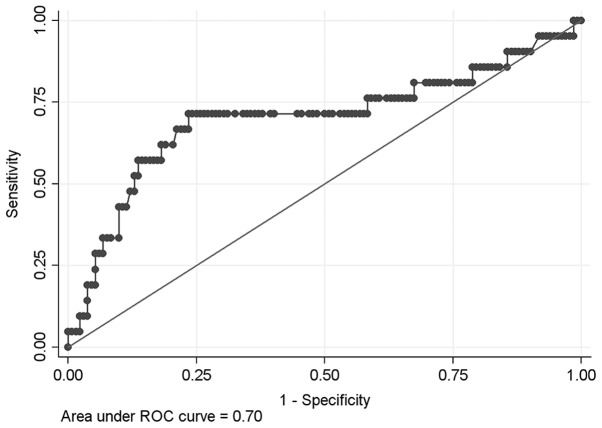
The cut-off value of NLR as a prognostic predictor of patients with PNETs was 2.4 by ROC curve (area under ROC curve, 0.70, sensitivity 71.4%, specificity 76.5%, Fig. 1), with 28.5% of patients having NLR levels higher than the selected cut-off value. Sixty-five patients were followed up <18 months and 1 patient had perioperative death and 10 patients were lost to follow-up (10/165, 6.1%), leaving 89 patients for the survival analysis. The univariate analysis, TNM stage III or IV (HR=14.33, P<0.01), NLR >2.4 (HR=7.15, P=0.003), G3 diseases (HR=17.82, P<0.01), and incidental PNETs (HR=0.27, P=0.006) were prognostic factors for patients' overall survival, whereas gender, age, tumor size, and location were not statistically significantly associated with overall survival (Table II; Fig. 2). In the multivariate analysis, TNM stage III or IV (HR=6.70, P=0.001), NLR >2.4 (HR=3.60, P=0.011), and G3 diseases (HR=6.31, P=0.004) were poor prognostic factors for overall survival (Table II).
Figure 1.
The receiver operating characteristic (ROC) curve and area under the ROC curve for the neutrophil-lymphocyte ratio as a prognostic factor.
Table II.
Univariate and multivariate analysis for overall survival of all patients using the Cox proportional hazards modela.
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | Parameter | No. | HR | 95% CI | P-value | HR | 95% CI | P-value |
| Age | ≤50 | 40 | 1 | – | – | |||
| >50 | 49 | 0.84 | 0.35–2.01 | 0.688 | ||||
| Gender | Male | 38 | 1 | – | – | |||
| Female | 51 | 0.56 | 0.23–1.36 | 0.201 | ||||
| Size (cm) | ≤3 | 35 | 1 | – | – | |||
| >3 | 54 | 2.06 | 0.75–5.68 | 0.140 | ||||
| Location | Head | 33 | 1 | – | – | |||
| Others | 56 | 0.87 | 0.36–2.13 | 0.761 | ||||
| TNM stage | I, II | 63 | 1 | – | – | 1 | – | – |
| III, IV | 26 | 14.33 | 4.75–41.20 | 0.001 | 6.70 | 2.12–21.15 | 0.001 | |
| NLR | ≤2.4 | 61 | 1 | – | – | 1 | – | – |
| >2.4 | 28 | 7.15 | 2.72–18.74 | 0.001 | 3.60 | 1.33–9.71 | 0.011 | |
| Grade | G1, G2 | 63 | 1 | – | – | 1 | – | – |
| G3 | 12 | 17.82 | 5.64–56.29 | 0.001 | 6.31 | 1.83–21.79 | 0.004 | |
| Unknown | 14 | 6.78 | 2.07–22.24 | 0.002 | 3.72 | 1.09–12.67 | 0.036 | |
| Symptom | Positive | 43 | 1 | – | – | 1 | – | – |
| Negative | 46 | 0.27 | 0.10–0.74 | 0.006 | 0.57 | 0.19–1.67 | 0.305 | |
Only 65 patients followed up <18 months and 1 patient had perioperative death and 10 patients were lost to follow-up, leaving 89 patients for the survival analysis. HR, hazard ratio; CI, confidence interval; NLR, neutrophil-lymphocyte ratio; TNM, tumor-node-metastasis.
Figure 2.
Kaplan-Meier survival curves according to neutrophil-lymphocyte ratio (NLR) ≤2.4 or NLR >2.4.
Parameters correlated with baseline NLR levels
The χ2 test was employed to analyze clinical and pathologic factors correlated with baseline NLR levels (NLR ≤2.4 and NLR >2.4, Table III). A NLR >2.4 was statistically significantly associated with tumor size (P=0.001), TNM stage III or IV (P=0.019), and tumor grade (P=0.003), but not with age, gender, location, symptoms, vessel invasion, and nerve invasion. Of note, NLR >2.4 was associated with positive lymph status (P=0.067) and functioning status (P=0.084).
Table III.
NLR, patient demographics and clinical characteristics.
| Demographic or clinical characteristic | Parameter | NLR ≤2.4 | NLR >2.4 | P-value |
|---|---|---|---|---|
| Age | ≤50 | 48 | 21 | 0.638 |
| >50 | 70 | 26 | ||
| Gender | Male | 56 | 20 | 0.568 |
| Female | 62 | 27 | ||
| Location | Head | 52 | 16 | 0.238 |
| Others | 66 | 31 | ||
| Size (cm) | ≤3 | 50 | 7 | 0.001 |
| >3 | 68 | 40 | ||
| TNM stage | I, II | 83 | 24 | 0.019 |
| III, IV | 35 | 23 | ||
| Grade | G1 | 54 | 9 | 0.003 |
| G2/G3 | 46 | 27 | ||
| Lymph statusa | Positive | 14 | 7 | 0.067 |
| Negative | 56 | 10 | ||
| Vessel invasiona | Positive | 15 | 6 | 0.340 |
| Negative | 48 | 11 | ||
| Nerve invasiona | Positive | 13 | 1 | 0.136 |
| Negative | 47 | 16 | ||
| Functioning | Positive | 16 | 2 | 0.084 |
| Negative | 102 | 45 | ||
| Symptom | Positive | 52 | 26 | 0.191 |
| Negative | 66 | 21 |
For cases that underwent curative resection only. NLR, neutrophil-lymphocyte ratio, TNM, tumor-node-metastasis.
Discussion
To the best of our knowledge, this study is the first to investigate the prognostic role of NLR in PNETs. Using ROC curve, the cut-off value of NLR as a prognostic predictor of patients with PNETs was 2.4. NLR >2.4 was found to be a poor prognostic factor in both univariate and multivariate analyses for patients with PNETs. We also showed that an NLR >2.4 was statistically significantly associated with tumor size >3 cm, TNM stage III or IV, and tumor grade. We demonstrated that NLR is a prognostic marker of patients with PNETs which may predict their clinical outcome and aggressive features.
NLR is more widely available and convenient compared with other biomarkers. Therefore, considering its prognostic role in PNETs, NLR may be used to stratify patients with high risk of therapeutic resistance, early recurrence, or metastasis. In addition, NLR has the potential to determine therapeutic strategy, monitor disease progression, and evaluate treatment response.
Mounting evidence has shown that cancer-associated inflammation is a key factor of prognosis in patients with cancer (12). In a study by Hochwald et al, tumor necrosis was correlated strongly with prognosis in patients with PNETs (10). Previous findings demonstrated that a higher NLR level was significantly correlated with a larger tumor size, histologic tumor necrosis, and tumor differentiation (20). In this study, we also showed that NLR was significantly correlated with tumor size, TNM stage, and tumor grade, which are all strongly associated with tumor necrosis.
A variety of markers of systemic inflammation have been evaluated over the past decade for therapeutic response and predicting survival, including NLR, Glasgow prognostic score and its modified version, prognostic index, platelet lymphocyte ratio, and prognostic nutrition index (21,22). Of these markers, NLR is a routinely available and promising marker that can be used to assess systemic inflammation and therapeutic responses (13,21,22). For example, Wang et al found that NLR was the only marker of inflammation for prognosis on multivariate analysis and elevated NLR was better than the modified Glasgow prognostic score, prognostic index, platelet lymphocyte ratio, and prognostic nutrition index for prognostication in patients with pancreatic cancer (22). Another study demonstrated that baseline NLR and NLR, which were used as potential prognostic markers in patients with advanced pancreatic cancer, were altered following treatment with chemotherapy (13). In the current study, we confirm the prognostic role of NLR in patients with PNETs by both univariate and multivariate analyses.
In addition to specific markers in functioning PNETs, there are general biomarkers used to diagnose and monitor functioning and non-functioning PNETs (23). Chromogranin A (CgA) is the most widely used biomarker and has been reported to be elevated in 50–80% of patients with PNETs. It is an ideal biomarker used to monitor disease progression for cases with CgA elevation (23). However, in a study conducted by Sherman et al, no correlation between CgA and survival was found in patients with PNETs in a multivariate analysis (24). Pancreatic polypeptide is a 36 amino acid protein secreted by endocrine cells located primarily in the pancreatic head and uncinate process, with a low sensitivity of 31% in PNETs (25). Neuron-specific enolase is a biomarker with a sensitivity of 33% and a specificity of 100% in patients with gastroenteropancreatic neuroendocrine tumors (26). It is also an independent predictor of survival for patients with advanced G1/2 PNETs undergoing peptide receptor radionuclide therapy (27). Pancreastatin is a post-translational fragment of CgA peptide and has great sensitivity and specificity for detecting PNETs and correlates with survival (24). However, although not varying with proton pump inhibitor as CgA, pancreastatin may be affected by insulin and glucose homeostasis (23). Therefore, NLR has the potential to serve as a supplemental prognostic predictor to these biomarkers.
The current study has several shortcomings. Firstly, despite a relatively large sample size, the retrospective feature of this study may limit its clinical application. Further prospective evidence with large sample size is needed. In addition, NLR is a non-specific marker that could be affected by several confounders, mainly including bacterial inflammation, immunologic response, and anticancer treatments (13). In addition, the predicting role of NLR in combination with other biomarkers including CgA, pancreatic polypeptide, and neuron-specific enolase was not demonstrated. Furthermore, the dynamics of NLR during treatment and follow-up were not presented in the current study.
In conclusion, baseline NLR is a strong independent predictor of overall survival for patients with PNETs. A high level of NLR has a significant correlation with large tumor size, advanced stage, and high grade. Aberrant elevation of NLR identifies high-risk patients who may require special treatment and close follow-up.
Acknowledgements
The present study was jointly funded by the National Science Foundation for Distinguished Young Scholars of China (no. 81625016), the National Natural Science Foundation of China (nos. 81372649, 81172276, 81370065 and 81372653), Shanghai Municipal Commission of Health and Family Planning scientific research (no. 20144Y0170), and basic research projects of the Science and Technology Commission of Shanghai Municipality (no. 15JC1401200).
References
- 1.Oberg K, Eriksson B. Endocrine tumours of the pancreas. Best Pract Res Clin Gastroenterol. 2005;19:753–781. doi: 10.1016/j.bpg.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 2.Luo G, Liu Z, Guo M, Jin K, Xiao Z, Liu L, Xu J, Zhang B, Liu C, Huang D, et al. 18F-FDG PET/CT can be used to detect non-functioning pancreatic neuroendocrine tumors. Int J Oncol. 2014;45:1531–1536. doi: 10.3892/ijo.2014.2570. [DOI] [PubMed] [Google Scholar]
- 3.O'Grady HL, Conlon KC. Pancreatic neuroendocrine tumours. Eur J Surg Oncol. 2008;34:324–332. doi: 10.1016/j.ejso.2007.07.209. [DOI] [PubMed] [Google Scholar]
- 4.Ricci C, Casadei R, Taffurelli G, D'Ambra M, Monari F, Campana D, Tomassetti P, Santini D, Minni F. WHO 2010 classification of pancreatic endocrine tumors. Is the new always better than the old? Pancreatology. 2014;14:539–541. doi: 10.1016/j.pan.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Luo G, Fu D, Jin C, Hao S, Yang F, Wang X, Yao L, Ni Q. Preoperative diagnosis of nonfunctioning pancreatic neuroendocrine tumors. Med Oncol. 2011;28:1027–1031. doi: 10.1007/s12032-010-9611-3. [DOI] [PubMed] [Google Scholar]
- 6.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, et al. One hundred years after ‘carcinoid’: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 7.Singh S, Dey C, Kennecke H, Kocha W, Maroun J, Metrakos P, Mukhtar T, Pasieka J, Rayson D, Rowsell C, et al. Consensus recommendations for the diagnosis and management of pancreatic neuroendocrine tumors: Guidelines from a Canadian national expert group. Ann Surg Oncol. 2015;22:2685–2699. doi: 10.1245/s10434-014-4145-0. [DOI] [PubMed] [Google Scholar]
- 8.Wiedenmann B, Pavel M, Kos-Kudla B. From targets to treatments: A review of molecular targets in pancreatic neuroendocrine tumors. Neuroendocrinology. 2011;94:177–190. doi: 10.1159/000329386. [DOI] [PubMed] [Google Scholar]
- 9.Haynes AB, Deshpande V, Ingkakul T, Vagefi PA, Szymonifka J, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: Short-term and long-term patient outcomes. Arch Surg. 2011;146:534–538. doi: 10.1001/archsurg.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochwald SN, Zee S, Conlon KC, Colleoni R, Louie O, Brennan MF, Klimstra DS. Prognostic factors in pancreatic endocrine neoplasms: An analysis of 136 cases with a proposal for low-grade and intermediate-grade groups. J Clin Oncol. 2002;20:2633–2642. doi: 10.1200/JCO.2002.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Cherenfant J, Talamonti MS, Hall CR, Thurow TA, Gage MK, Stocker SJ, Lapin B, Wang E, Silverstein JC, Mangold K, et al. Comparison of tumor markers for predicting outcomes after resection of nonfunctioning pancreatic neuroendocrine tumors. Surgery. 2014;156:1504–1510, discussion 1510–1511. doi: 10.1016/j.surg.2014.08.043. [DOI] [PubMed] [Google Scholar]
- 12.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: Experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 13.Luo G, Guo M, Liu Z, Xiao Z, Jin K, Long J, Liu L, Liu C, Xu J, Ni Q, et al. Blood neutrophil-lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol. 2015;22:670–676. doi: 10.1245/s10434-014-4021-y. [DOI] [PubMed] [Google Scholar]
- 14.Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: Neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200:197–203. doi: 10.1016/j.amjsurg.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Stotz M, Gerger A, Eisner F, Szkandera J, Loibner H, Ress AL, Kornprat P, AlZoughbi W, Seggewies FS, Lackner C, et al. Increased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109:416–421. doi: 10.1038/bjc.2013.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: A meta-analysis. World J Gastroenterol. 2015;21:2807–2815. doi: 10.3748/wjg.v21.i9.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paramanathan A, Saxena A, Morris DL. A systematic review and meta-analysis on the impact of pre-operative neutrophil lymphocyte ratio on long term outcomes after curative intent resection of solid tumours. Surg Oncol. 2014;23:31–39. doi: 10.1016/j.suronc.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Luo G, Liu Z, Guo M, Jin K, Xiao Z, Liu L, Liu C, Xu J, Ni Q, Long J, et al. A comprehensive comparison of clinicopathologic and imaging features of incidental/symptomatic non-functioning pancreatic neuroendocrine tumors: A retrospective study of a single center. Pancreatology. 2015;15:519–524. doi: 10.1016/j.pan.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 19.Mocci E, Milne RL, Méndez-Villamil EY, Hopper JL, John EM, Andrulis IL, Chung WK, Daly M, Buys SS, Malats N, et al. Risk of pancreatic cancer in breast cancer families from the breast cancer family registry. Cancer Epidemiol Biomarkers Prev. 2013;22:803–811. doi: 10.1158/1055-9965.EPI-12-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viers BR, Thompson R Houston, Boorjian SA, Lohse CM, Leibovich BC, Tollefson MK. Preoperative neutrophil- lymphocyte ratio predicts death among patients with localized clear cell renal carcinoma undergoing nephrectomy. Urol Oncol. 2014;32:1277–1284. doi: 10.1016/j.urolonc.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad J, Grimes N, Farid S, Morris-Stiff G. Inflammatory response related scoring systems in assessing the prognosis of patients with pancreatic ductal adenocarcinoma: A systematic review. Hepatobiliary Pancreat Dis Int. 2014;13:474–481. doi: 10.1016/S1499-3872(14)60284-8. [DOI] [PubMed] [Google Scholar]
- 22.Wang DS, Luo HY, Qiu MZ, Wang ZQ, Zhang DS, Wang FH, Li YH, Xu RH. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol. 2012;29:3092–3100. doi: 10.1007/s12032-012-0226-8. [DOI] [PubMed] [Google Scholar]
- 23.Landry CS, Cavaness K, Celinski S, Preskitt J. Biochemical prognostic indicators for pancreatic neuroendocrine tumors and small bowel neuroendocrine tumors. Gland Surg. 2014;3:215–218. doi: 10.3978/j.issn.2227-684X.2014.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sherman SK, Maxwell JE, O'Dorisio MS, O'Dorisio TM, Howe JR. Pancreastatin predicts survival in neuroendocrine tumors. Ann Surg Oncol. 2014;21:2971–2980. doi: 10.1245/s10434-014-3728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter T, Chardon L, Chopin-laly X, Raverot V, Caffin AG, Chayvialle JA, Scoazec JY, Lombard-Bohas C. Is the combination of chromogranin A and pancreatic polypeptide serum determinations of interest in the diagnosis and follow-up of gastro-entero-pancreatic neuroendocrine tumours? Eur J Cancer. 2012;48:1766–1773. doi: 10.1016/j.ejca.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E. Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol. 2001;12(Suppl 2):S69–S72. doi: 10.1023/A:1011128311785. [DOI] [PubMed] [Google Scholar]
- 27.Ezziddin S, Khalaf F, Vanezi M, Haslerud T, Mayer K, Al Zreiqat A, Willinek W, Biersack HJ, Sabet A. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:925–933. doi: 10.1007/s00259-013-2677-3. [DOI] [PubMed] [Google Scholar]