Abstract
Endoplasmic reticulum (ER) is an essential site of cellular homeostasis regulation. ER stress (ERS) may induce autophagy in tumor cells that escape from apoptosis. The present study examined the effects and mechanism of ERS on cisplatin (DDP) sensitivity in ovarian carcinoma. SKOV3 tumor cells treated with Saquinavir were subjected to western blot and reverse transcription-quantitative polymerase chain reaction analysis to determine protein and messenger RNA (mRNA) expression levels of mechanistic target of rapamycin (mTOR) and Beclin 1. MTT assay was used to analyze the influence of Saquinavir on DDP resistance in SKOV3 cells. Saquinavir induced glucose-regulated protein 78 expression, which is a marker of ERS. Following treatment with various doses of Saquinavir, the sensitivity of ovarian cancer cells to DDP decreased significantly. Protein and mRNA expression levels of mTOR and Beclin 1 in SKOV3 cells were increased when the cells were exposed to Saquinavir or DDP for 24 h. Moreover, mTOR and Beclin 1 expression levels were highest in the Saquinavir + DDP group (0.684±0.072 and 0.6467±0.0468, respectively). SKOV3 tumor cells were also exposed to the autophagy inhibitor, 3-methyladenine (3-MA), and different concentrations of Saquinavir. Analysis of half maximal inhibitory concentration (IC50) values of DDP after this treatment demonstrated that IC50 values were significantly decreased compared with Saquinavir alone (P<0.001), suggesting that the sensitivity to DDP was improved in ovarian cancer cells after 3-MA exposure. These findings demonstrated that Saquinavir is able to induce ERS in SKOV3 cells effectively, and ER-induced stress may decrease the sensitivity of DDP in SKOV3 cells. Furthermore, ERS may regulate cell autophagy through the mTOR and Beclin 1 pathways, leading to a reduction in the sensitivity of DDP in SKOV3 cells. ERS in tumor cells and autophagy may be a potential target to improve the therapeutic effect of chemotherapy and reduce drug resistance in tumors.
Keywords: ovarian carcinoma, cisplatin, drug resistance, endoplasmic reticulum stress, autophagy
Introduction
Ovarian cancer is one of the most dangerous female malignant tumors (1). There are several types of ovarian carcinoma, but epithelial ovarian carcinoma (EOC) is the most frequent one, representing >90% of all ovarian cancers (2). Historically, treatment of ovarian cancer has involved surgery combined with platinum-based chemotherapy (3). The main factors affecting the prognosis of patients with ovarian cancer are advanced stage at diagnosis and primary or secondary chemotherapy drug resistance, especially for those persistent or recurrent ovarian carcinoma. The five-year survival rate for ovarian cancer patients is currently 30% (4). The molecular alterations of cisplatin (DDP)-resistant cancer cells have previously been researched, however, the underlying mechanisms promoting DDP resistance in ovarian cancer cells remain to be elucidated (5). Therefore, research on the mechanisms of DDP resistance and reversing drug resistance in EOC are particularly urgent for the patients with current and refractory EOC.
The endoplasmic reticulum (ER) is an essential cellular compartment for protein synthesis and maturation. It also has other functions, including calcium storage and maintenance in Ca2+ homeostasis, steroid synthesis, and lipid and glycogen synthesis (6). Various physiological and pathological conditions may affect ER homeostasis, ultimately causing ER stress (ERS) (4–9). Accumulation of misfolded proteins and alterations in Ca2+ homeostasis in ER results in ERS and activation of autophagy in carcinoma cells. Typically, autophagy activates pro-survival mechanisms, as well as cell death programs, particularly if autophagy activated following ERS is a pro-survival response to restore the ER homeostasis by clearing the unfolded aggregates (10). Several studies have reported that ERS induces autophagy in mammalian cancer cell lines and mouse embryonic fibroblasts (11,12). Heat shock proteins function as molecular chaperones that regulate cellular homeostasis and promote cell survival responses. Inhibition of autophagy and molecular chaperones may be a suitable pharmacological target to promote apoptosis in tumor cells. A previous study revealed that Hsp27 was involved in DDP- and ERS induced autophagy activation in HCC cells (13). It has also been suggested that autophagy may be more active in DDP-resistant ovarian cancer cells (14). The 78-kDa glucose-regulated protein 78 (GRP78), also known as BiP or HSPA5, is predominantly localized in the ER as a molecular chaperone, and is associated with regulating the ERS pathway. Induction of GRP78 has been recognized as a marker for ERS and the onset of the unfolded protein response (15). Previous studies have indicated that the mechanistic target of rapamycin (mTOR) and Beclin 1 may have important roles in regulating cell autophagy (16). The present study aimed to determine the ERS effect on the DDP sensitivity of SKOV3 ovarian cancer cells. On this basis, the mechanism of the drug resistance of ovarian cancer cells to DDP was studied, which may contribute to the reversal of DDP resistance in ovarian cancer cells.
Materials and methods
Cell lines and culture conditions
SKOV3 human ovarian cancer cells were obtained from the Chinese Academy of Medical Sciences (Beijing, China) and Peking Union Medical College (Beijing, China). Cell lines were cultured at 37°C in RPMI-1640 culture medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (Invitrogen; Thermo Fisher Scientific, Inc.) in an atmosphere containing 5% CO2 and 90% air.
Reagents
Human immunodeficiency virus (HIV) proteinase inhibitor, Saquinavir, was purchased from R&D Systems, Inc., (Minneapolis, MN, USA). 3-Methyladenine (3-MA), an autophagy inhibitor, was purchased from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Protein kinase inhibitor, LY294002, was obtained from Promega Corp. (Madison, WI, USA). GRP78, mTOR, Beclin 1 and β-actin antibodies were obtained from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA). First Strand cDNA Synthesis kit was purchased from Beijing ComWin Biotech Co., Ltd (Beijing, China). BCA Protein Assay kit was purchased from Beyotime Institute of Biotechnology (Wuhan, China). Primers for mTOR, Beclin 1 and β-actin were acquired from Sangon Biotech Co., Ltd., (Shanghai, China)
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and complementary DNA (cDNA) was synthesized using a First Strand cDNA Synthesis kit according to the manufacturer's instructions. Subsequently, 1 µl synthesized cDNA was used for each PCR with each primer pair. qPCR was performed with 2X EasyTaq® PCR SuperMix kit (Beijing Transgen Biotech Co., Ltd., Beijing, China), and 25 µl of the reaction mixture was used in a qPCR program. The 25-µl reaction mixture contained 1 µl each primer, 12.5 µl 2X PCR Mix, 1 µl template cDNA and ≤9.5 µl double-distilled water (catalog no. KGDN4500; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) The primers were synthesized by Sangon Biotech Co., Ltd., as follows: mTOR, forward 5′-TTGAGGTTGCTATGACCAGAGAGAA-3′ and reverse 5′-TTACCAGAAAGGACACCAGCCAATG-3′, 566 bp; Beclin 1, forward 5′-CAGTTTGGCACAATCAATAAC-3′ and reverse 5′-CATCCATCCTGTAGGGAAGAC-3′, 352 bp; β-actin, forward 5′-TGTTTGAGACCTTCAACACCC-3′ and reverse 5′-AGCACTGTGTTGGCGTACAG-3′, 340 bp. Protocol parameters were as follows: Initial incubation at 95°C for 10 min, followed by 30 cycles of denaturation at 95°C for 30 sec, annealing (55°C) for 30 sec, elongation at 72°C for 1 min and a final extension at 72°C for 8 min. The experiment was repeated three times, and the efficiency of cDNA synthesis from each sample was estimated using β-actin primers, which served as an endogenous control. Relative gene expression levels were calculated and analyzed using Quantity One analysis software (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Data were analyzed using the 2−ΔΔCq method (17).
Western blot analysis
Cells were harvested and lysed in radioimmunoprecipitation assay buffer. Protein concentration was determined using a BCA Protein Assay kit. Denatured proteins (50 µg) were separated by 12% SDS-PAGE and transferred to polyvinylidene difluoride membranes using the Bio-Rad electro-transfer system (Bio-Rad Laboratories, Inc.). The membranes were incubated with antibodies to visualize the proteins. Following blocking with 5% w/v non-fat dried milk for 1 h, the membranes were incubated overnight with primary antibodies against GRP78 (1:500; bs-1219R), mTOR (1:500; bs-1992R) and Beclin 1 (1:500; bs-1353R) (all from Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China), rinsed with TBS-Tween 20, and subsequently incubated at 37°C for 2 h with anti-rabbit immunoglobulin G secondary antibody conjugated to horseradish peroxidase (sc-2004; Santa Cruz Biotechnology, Inc.) at a dilution of 1:1,000. After applying Enhanced Chemiluminescent Plus detection reagents (EMD Millipore, Billerica, MA, USA), protein bands were visualized using an X-ray film (Fujifilm, Tokyo, Japan). β-actin was used as an internal control for protein loading and analysis. Quantitation of band intensity was performed by densitometry using Quantity One 1-D analysis software version 4.6.9 (Bio-Rad Laboratories, Inc.). The experiment was conducted three times.
MTT assay
MTT assays were used to assess the number of viable cells. After cells (180-µl solution containing 1.0×105 cells/ml) were cultured for 24 h in 96-well plates (1.8×104 cells/well), culture medium was removed and replaced by 200 µl complete culture medium containing DDP. Final concentrations of DDP were 100, 50, 25, 12.5, 6.25, 3.125, 1.56 and 0.78 µg/ml, respectively. Each treatment was repeated in four wells. The control group received the same volume of culture medium with cells, without drugs, whereas blank wells contained the same volume of culture medium without cells and drugs. Cells were cultured for 72 h. Cell viability was assessed using the MTT colorimetric assay. Briefly, 20 µl MTT was added and incubated for 4 h. Subsequently, 150 µl dimethyl sulfoxide was added to dissolve the formazan crystals. Following shaking for 10 min, the absorbance values were measured at a wavelength of 570 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). The experiment was repeated three times. The DDP half maximal inhibitory concentration (IC50) in SKOV3 cells was calculated.
Statistical analysis
All values were shown as the mean ± standard deviation from at least three independent experiments. Data were analyzed by Student's t-test (for comparing two intergroup results) or one-way analysis of variance with SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). IC50 was analyzed with the linear regression. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of GRP78 in SKOV3 tumor cells
As an ER chaperone, GRP78 is commonly used as a marker of ERS. Western blotting analysis demonstrated that GRP78 protein expression levels were increased when tumor cells SKOV3 were treated with Saquinavir for 24 h. Moreover, as shown in Table I and Fig. 1, the expression levels of GRP78 significantly increased following treatment with Saquinavir in a dose-dependent manner (Ρ<0.05). These results were significantly different when compared with each other (P<0.05). These findings indicated that Saquinavir may lead to ERS, and that the level of ERS may be associated with the concentration of Saquinavir.
Table I.
Relative protein expression levels of GRP78 following treatment with Saquinavir.
| Groups | GRP78 |
|---|---|
| Control | 0.361±0.009 |
| Saquinavir 10 µmol/l | 0.446±0.010 |
| Saquinavir 20 µmol/l | 0.489±0.105 |
| Saquinavir 40 µmol/l | 0.511±0.010 |
Data are presented as the mean ± standard deviation. GRP78, glucose-related protein 78.
Figure 1.
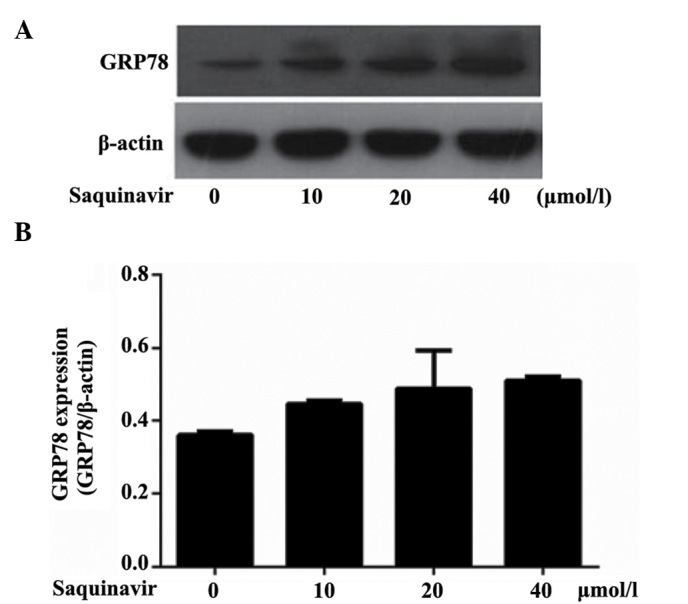
GRP78 expression levels in SKOV3 ovarian cancer tumor cells. (A) GRP78 protein expression levels following treatment with 0, 10, 20 and 40 µmol/l Saquinavir in SKOV3 cells, as determined by western blotting. (B) Subsequent quantitative analysis of GRP78 protein expression levels. GRP78, glucose-related protein 78.
Effect of Saquinavir-induced ERS on the sensitivity of ovarian cancer cells to DDP
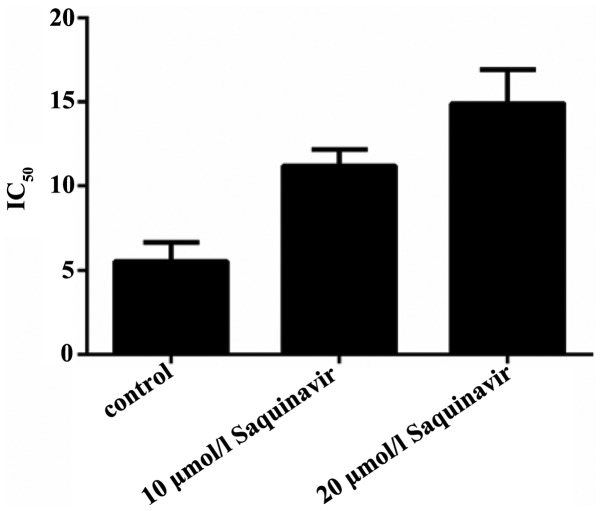
To confirm the effect of ERS on the sensitivity of SKOV3 ovarian cancer cells to DDP, the cells were treated with different concentrations of Saquinavir for 24 h. The IC50 of the SKOV3 cells was 5.490±1.148 µg/l. Following exposure to 10 and 20 µmol/l Saquinavir, the IC50 of SKOV3 cells significantly increased to 11.199±0.984 and 14.906±2.015 µg/l, respectively (P=0.003 and P<0.001); however, no statistically significant difference was detected between the cells treated with 10 and 20 µmol/l Saquinavir (P=0.21; Fig. 2). These findings suggest that Saquinavir resulted in ERS in SKOV3 tumor cells, which reduced the sensitivity of these ovarian cancer cells to DDP.
Figure 2.
Sensitivity of SKOV3 human ovarian cancer cells to cisplatin decreased after treatment with 10 and 20 µmol/l Saquinavir. IC50, half maximal inhibitory concentration.
Effect of Saquinavir on protein and messenger RNA (mRNA) expression levels of mTOR and Beclin 1 in SKOV3 cells
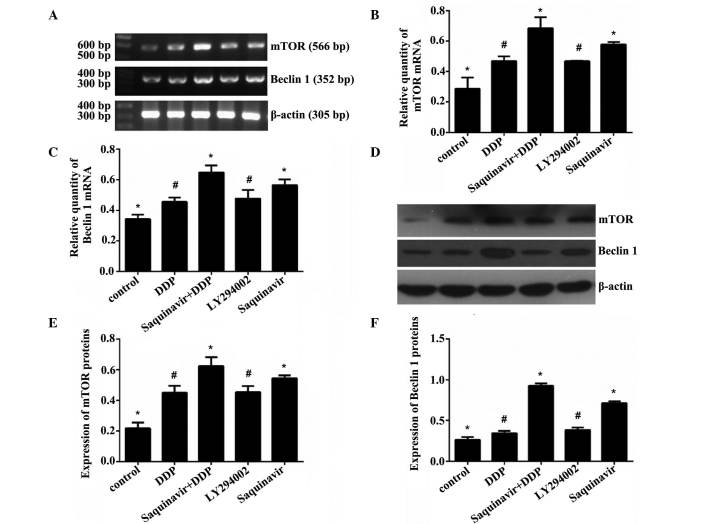
Saquinavir, LY294002 (PI3K inhibitor) and DDP were added to the different cell groups, respectively, to final concentrations of 20, 20 and 5 µg/ml, respectively. The expression levels of autophagy-related genes mTOR and Beclin 1 were assessed in SKOV3 ovarian cancer cells by RT-qPCR and western blotting. As shown in Tables II and III and Fig. 3, when the cells were exposed to Saquinavir for 24 h, the protein and mRNA expression levels of mTOR and Beclin 1 in SKOV3 cells were increased. The results demonstrated that the protein and mRNA expression levels of mTOR and Beclin 1 in the Saquinavir + DDP group were significantly increased in comparison with the other groups. The Saquinavir group exhibited the next highest expression levels. Moreover, the protein and mRNA expression levels of mTOR and Beclin 1 in SKOV3 cells treated with Saquinavir were higher than those exhibited by cells in the LY294002 group. These results suggested that the PI3K inhibitor, LY294002, did not inhibit the mTOR expression completely through the PI3K/AKT/mTOR pathway. The protein and mRNA expression levels of mTOR and Beclin 1 among the groups of experiment were demonstrated to be significantly different (F=23.140, P<0.001 and F=24.389, P<0.001, respectively). Protein expression levels of mTOR and Beclin 1 among the groups were also significantly different (F=39.345, P<0.001 and F=261.877, P<0.001, respectively). However, further comparison indicated that the protein and mRNA expression levels of mTOR and Beclin 1 were significantly different between any two groups (control vs. DDP; control vs. Saquinavir + DDP; control vs. LY294002; control vs. Saquinavir; DDP vs. Saquinavir + DDP; DDP vs. Saquinavir; Saquinavir + DDP vs. LY294002; Saquinavir + DDP vs. Saquinavir; and LY294002 vs. Saquinavir), with the exception of the LY294002 and DDP groups.
Table II.
Expression of mTOR and Beclin 1 messenger RNA in SKOV3 human ovarian cancer cells.
| Groups | mTOR | Beclin 1 |
|---|---|---|
| Control | 0.287±0.073 | 0.342±0.029 |
| DDP | 0.468±0.031 | 0.456±0.028 |
| Saquinavir + DDP | 0.684±0.072 | 0.647±0.047 |
| LY294002 | 0.467±0.005 | 0.477±0.056 |
| Saquinavir | 0.5772±0.016 | 0.565±0.037 |
Data are presented as the mean ± standard deviation. DPP, cisplatin; mTOR, mechanistic target of rapamycin.
Table III.
Expression of mTOR and Beclin 1 proteins in SKOV3 human ovarian cancer cells.
| Groups | mTOR | Beclin 1 |
|---|---|---|
| Control | 0.218±0.038 | 0.265±0.033 |
| DDP | 0.450±0.045 | 0.345±0.029 |
| Saquinavir + DDP | 0.624±0.058 | 0.924±0.033 |
| LY294002 | 0.453±0.041 | 0.384±0.030 |
| Saquinavir | 0.544±0.019 | 0.712±0.024 |
Data are presented as the mean ± standard deviation. DPP, cisplatin; mTOR, mechanistic target of rapamycin.
Figure 3.
Effect of Saquinavir on the (A-C) mRNA and (D-F) protein expression levels of mTOR and Beclin 1 in SKOV3 cells. (A) mRNA expression of mTOR and Beclin 1 in SKOV3 cells. (B) Bar chart of mTOR mRNA expression in SKOV3 cells. (C) Bar chart of Beclin 1 mRNA expression in SKOV3 cells. (D) Protein expression levels of mTOR and Beclin 1 in SKOV3 cells. (E) Bar chart of mTOR protein expression of in SKOV3 cells. (F) Bar chart of Beclin 1 protein expression in SKOV3 cells. Data are presented as the mean + standard deviation. *P<0.001 vs. all other groups. #P<0.001 vs.control, Saquinavir + DDP and Saquinavir groups. mTOR, mechanistic target of rapamycin; DDP, cisplatin; mRNA, messenger RNA.
Effect of autophagy suppression on the sensitivity of ovarian cancer cells to DDP
The present study confirmed that Saquinavir resulted in ERS in SKOV3 cells, which reduced the sensitivity of ovarian cancer cells to DDP. Furthermore, it demonstrated that the sensitivity of ovarian cancer cells to DDP was associated with the level of ERS. In the present study, SKOV3 cells were exposed to the autophagy inhibitor, 3-MA, and various concentrations of Saquinavir. IC50 values in the control, Saquinavir 10 µmol/l, Saquinavir 10 µmol/l + 3-MA, Saquinavir 20 µmol/l and Saquinavir 20 µmol/l + 3-MA groups were 5.490±1.148, 11.199±0.984, 6.624±0.218, 14.906±2.015 and 7.888±0.086 µg/ml, respectively (Fig. 4). IC50 of DDP was decreased in SKOV3 cells after 3-MA combined with Saquinavir treatment compared with Saquinavir treatment alone. This indicated that the autophagic response induced by Saquinavir treatment was inhibited by 3-MA. These results suggest that the sensitivity to DDP was significantly improved in SKOV3 cells after 3-MA exposure (F=31.898, P<0.001).
Figure 4.
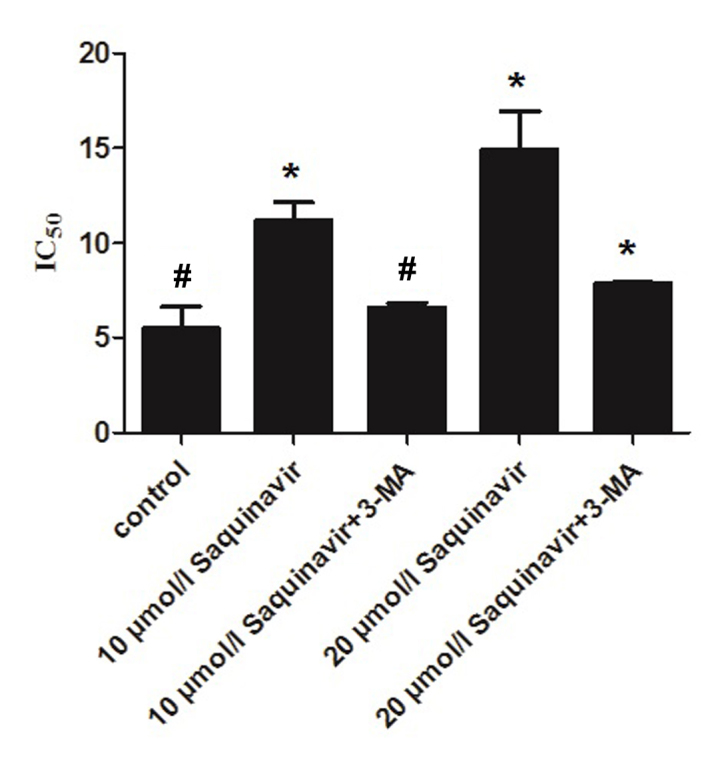
Effect of autophagy suppression on the sensitivity of SKOV3 ovarian cancer cells to cisplatin. *P<0.001 vs. all other groups. #P<0.001 vs. 10 µmol/l Saquinavir, 20 µmol/l Saquinavir and 20 µmol/l Saquinavir + 3-MA. 3-MA, 3-methyladenine; IC50, half maximal inhibitory concentration.
Discussion
ER is an essential intracellular organelle with multiple roles including the synthesis, folding, assembly and maturation of nascent proteins, Ca2+ storage, glycosylation, and the trafficking of newly-synthesized membrane and secretory proteins (18). Perturbations of these processes have been demonstrated to interfere with the proper functioning of ER, thus leading to a condition defined as ERS (8,9). Previous studies have shown that DDP resistance in cancer cells was associated with ERS (19,20). DDP results in the accumulation of unfolded and misfolded proteins in the lumen of the ER. This ERS subsequently induces autophagy. The majority of accumulated proteins and soluble proteins were cleared by autophagy pathways and tumor cells are able to survive (21,22). It has been demonstrated that tunicamycin, an ERS inducer, augmented DDP cytotoxicity by upregulating ERS-mediated apoptosis, indicating that autophagy has an important role in preventing DDP-induced apoptosis in HeLa cells (23). Saquinavir, which is a HIV proteinase inhibitor, is able to induce ERS in various tumors, including NC160 cells (24), non-small cell lung cancer (25) and melanocytoma (26). McLean et al (27) have previously shown that Saquinavir leads to ERS and cell autophagy.
Altering the tumor microenvironment and growth patterns could activate ERS. The survival of cells upon undergoing ERS induces better adaptation to various physiological and pathological conditions, which is one of the important mechanisms in tumor cells remaining malignant and promoting drug resistance (28). GRP78 has been well-established as an ER chaperone and is widely used as a marker for ERS (29). Our study confirmed that Saquinavir leads to ERS in SKOV3 tumor cells, which reduces the sensitivity of ovarian cancer cells to DDP. Furthermore, the sensitivity of ovarian cancer cells to DDP appears to be associated with the level of ERS. 3-MA is a specific inhibitor of the autophagic pathway. After SKOV3 tumor cells are exposed to the autophagy inhibitor 3-MA, sensitivity of ovarian cancer cells to DDP could be effectively reversed. Therefore, it was speculated that ERS may induce DDP resistance through enhanced autophagy in SKOV3 cells. This demonstrated that an increase in the level of ERS had an important role in DDP resistance in ovarian cancer, particularly secondary DDP resistance associated with continuous ERS and an increase in the level of autophagy in ovarian cancer exposed to DDP, a chemotherapeutic agent, periodically.
Accumulating evidence has indicated that ERS is associated with tumor cell survival, tumor progression and chemotherapy resistance (29–31); however, its precise mechanism remains unclear. Previous studies have shown that the PI3K/Akt/mTOR pathway is involved in ERS-triggered apoptosis, and is also associated with the regulation of autophagy (32,33); however, PI3K inhibitor did not inhibit the expression of mTOR completely. This suggested other signaling pathways may exist, requiring further investigation (34).
Autophagy is a ‘self-eating’ process by which a cell digests damaged organelles or misfolded proteins by sequestering the target cargo in a double membrane and fusing to lysosomes for degradation, thereby supplementing intermediate metabolism with the products of digestion (35,36). mTOR has a critical role in the initiation of the autophagic process. mTOR activation is able to inhibit autophagy. Beclin 1, which is a mammalian autophagy gene, was the first protein that was demonstrated to induce autophagy (37). Studies have identified that cell autophagy has a critical role in the occurrence and development of tumor cells, and it has been suggested that autophagy may have a role in cancer cell chemoresistance (38,39). A previous study researched co-treatment of DDP with trifluoperazine, an inducer of autophagy, which sensitized H460/cis DDP-resistant lung carcinoma cells to DDP, suggesting that the decreased levels of autophagy may promote DDP resistance in lung cancer (40). Another previous study suggested that ERS induced apoptosis and led to DDP resistance in human ovarian cancer cells, but provided limited information on the role of autophagy in DDP resistance (41). ERS may regulate autophagy through mTOR and Beclin 1 pathways. The present study further explored that when ERS was induced by Saquinavir in tumor cells SKOV3, the expression levels of mTOR and Beclin 1 were upregulated, decreasing the sensitivity of ovarian cancer cells to DDP. We hypothesize that ovarian cancer cells experienced chemotherapy or radiotherapy, and numerous organelles or proteins were destroyed in ovarian cancer cells. Enhanced cell autophagy would aid the clearance of these harmful substances to maintain stabilization of homoeostasis and the cells could prevent from death. Cells benefit from moderate ERS to alleviate damage, whereas sustained ERS induces cell death (42). Continuous autophagy in cells leads to the breakdown of important organelles and proteins and eventual cell death via autophagy. To prevent this type of cell death, the PI3K/AKT/mTOR pathway is activated in ovarian cancer cells to appropriately suppress autophagy. In the present study, it was also demonstrated that the expression levels of mTOR were upreguated, which suggested autophagy was inhibited. Our findings have identified that downregulating autophagy via 3-MA may prevent the effect of Saquinavir-induced ERS on the sensitivity of ovarian cancer cells to DDP. Therefore, we hypothesize that an increase in the level of autophagy had a dominant role in the ERS activation of ovarian cancer cells and decreased the drug sensitivity of ovarian cancer to DDP.
In conclusion, mTOR and Beclin 1 may be important in the regulation of cell autophagy. ERS acts like a double-edged sword, since it can induce apoptosis and promote cell survival. The present findings demonstrated that ERS was able to promote cell survival through regulation of the level of autophagy and have a role in protecting cell from being destroyed. It was speculated that ERS and enhanced cell autophagy were important mechanisms which resulted in DDP resistance in ovarian cancer. Targeting ERS or inhibiting autophagy may be an encouraging technique to overcome chemotherapeutic resistance.
Acknowledgements
The authors would like to thank Tianjin Medical University (Heping, China) and all the teachers in our lab, particularly Professor Hao Zhang and Professor Zheng Su, for their assistance during the experiment.
References
- 1.Khanra K, Panda K, Mitra AK, Sarkar R, Bhattacharya C, Bhattacharya N. Exon 8–9 mutations of DNA polymerase β in ovarian carcinoma patients from Haldia, India. Asian Pac J Cancer Prev. 2012;13:4183–4186. doi: 10.7314/APJCP.2012.13.8.4183. [DOI] [PubMed] [Google Scholar]
- 2.Ho CM, Chanq SF, Hsiao CC, Chien TY, Shih DT. Isolation and characterization of stromal progenitor cells from ascites of patients with epithelial ovarian adenocarcinoma. J Biomed Sci. 2012;19:23. doi: 10.1186/1423-0127-19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romanidis K, Nagorni EA, Halkia E, Pitiakoudis M. The role of cytoreductive surgery in advanced ovarian cancer: The general surgeon's perspective. J BUON. 2014;19:598–604. [PubMed] [Google Scholar]
- 4.Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 5.Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–1883. doi: 10.1038/onc.2011.384. [DOI] [PubMed] [Google Scholar]
- 6.Pang XL, He G, Liu YB, Wang Y, Zhang B. Endoplasmic reticulum stress sensitizes human esophageal cancer cell to radiation. World J Gastroenterol. 2013;19:1736–1748. doi: 10.3748/wjg.v19.i11.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haeri M, Knox BE. Endoplasmic reticulum stress and unfolded protein response pathways: Potential for treating age-related retinal degeneration. J Ophthalmic Vis Res. 2012;7:45–59. [PMC free article] [PubMed] [Google Scholar]
- 8.Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 9.Tabas I, Ron D. Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol. 2011;13:184–190. doi: 10.1038/ncb0311-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verfaillie T, Salazar M, Velasco G, Agostinis P. Linking ER stress to autophagy: Potential implications for cancer therapy. Int J Cell Biol. 2010;2010:930509. doi: 10.1155/2010/930509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kang JH, Chang YC, Maurizi MR. 4-O-carboxymethyl ascochlorin causes ER stress and induced autophagy in human hepatocellular carcinoma cells. J Biol Chem. 2012;287:15661–15671. doi: 10.1074/jbc.M112.358473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu D, Yang Y, Liu Q, Wang J. Inhibition of autophagy by 3-MA potentiates cisplatin-induced apoptosis in esophageal squamous cell carcinoma cells. Med Oncol. 2011;28:105–111. doi: 10.1007/s12032-009-9397-3. [DOI] [PubMed] [Google Scholar]
- 13.Chen R, Dai RY, Duan CY, Liu YP, Chen SK, Yan DM, Chen CN, Wei M, Li H. Unfolded protein response suppresses cisplatin-induced apoptosis via autophagy regulation in human hepatocellular carcinoma cells. Folia Biol (Praha) 2011;57:87–95. [PubMed] [Google Scholar]
- 14.Bao LJ, Jaramillo MC, Zhang Z, Zheng Y, Yao M, Zhang DD, Yi X. Induction of autophagy contributes to cisplatin resistance in human ovarian cancer cells. Mol Med Rep. 2015;11:91–98. doi: 10.3892/mmr.2014.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee AS. GRP78 induction in cancer: Therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 16.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittqen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Method. 2001;4:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Kato H, Nishitoh H. Sress responses from the endoplasmic reticulum in cancer. Front Oncol. 2015;5:93. doi: 10.3389/fonc.2015.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi S, Xu H, Li C, et al. Endoplasmic reticulum stress and tumor. Medical Recapitulate. 2009;15:525–527. [Google Scholar]
- 20.Yuan YF. Endoplasmic reticulum stress and apoptosis of tumor cells. J Mol Diagn Ther. 2010;2:128–134. [Google Scholar]
- 21.Liu Y, Ye Y. Roles of p97-associated deubiquitinases in protein quality control at the endoplasmic reticulum. Curr Protein Pept Sci. 2012;13:436–446. doi: 10.2174/138920312802430608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White MC, Johnson GG, Zhang W, Hobrath JV, Piazza GA, Grimaldi M. Sulindac sulfide inhibits sarcoendoplasmic reticulum Ca2+ ATPase, induces endoplasmic reticulum stress response and exerts toxicity in glioma cells: Relevant similarities to and important differences from celecoxib. J Neurosci Res. 2013;91:393–406. doi: 10.1002/jnr.23169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Y, Yu H, Qin H, Kang J, Yu C, Zhong J, Su J, Li H, Sun L. Inhibition of autophagy enhances cisplatin cytotoxicity through endoplasmic reticulum stress in human cervical cancer cells. Cancer Lett. 2012;314:232–243. doi: 10.1016/j.canlet.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, Warfel NA, Gardner ER, Danish M, et al. Nelfinavir, a lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007;13:5183–5194. doi: 10.1158/1078-0432.CCR-07-0161. [DOI] [PubMed] [Google Scholar]
- 25.Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, Warfel NA, Gardner ER, Danish M, et al. Nelfinavir, a lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007;13:5183–5194. doi: 10.1158/1078-0432.CCR-07-0161. [DOI] [PubMed] [Google Scholar]
- 26.Jiang W, Mikochik PJ, Ra JH, Lei H, Flaherty KT, Winkler JD, Spitz FR. HIV protease inhibitor nelfinavir inhibits growth of human melanoma cells by induction of cell cycle arrest. Cancer Res. 2007;67:1221–1227. doi: 10.1158/0008-5472.CAN-06-3377. [DOI] [PubMed] [Google Scholar]
- 27.McLean K, VanDeVen NA, Sorenson DR, Daudi S, Liu JR. The HIV protease inhibitor saquinavir induces endoplasmic reticulum stress, autophagy, and apoptosis in ovarian cancer cells. Gynecol Oncol. 2009;112:623–630. doi: 10.1016/j.ygyno.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Feldman DE, Chauhan V, Koong Ac. The unfolded protein response: A novel component of the hypoxic stress response in tumors. Mol Cancer Res. 2005;3:597–605. doi: 10.1158/1541-7786.MCR-05-0221. [DOI] [PubMed] [Google Scholar]
- 29.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–3651. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin Y, Wang Z, Liu L, Chen L. Akt is the downstream target of GRP78 in mediating cisplatin resistance in ER stress-tolerant human lung cancer cells. Lung Cancer. 2011;71:291–297. doi: 10.1016/j.lungcan.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Jiang CC, Mao ZG, Avery-Kiejda KA, Wade M, Hersey P, Zhang XD. Glucose regulatedprotein78 antagonizes cisplatin and adriamycin in human melanoma cells. Carcinogenesis. 2009;30:197–204. doi: 10.1093/carcin/bgn220. [DOI] [PubMed] [Google Scholar]
- 32.Kato H, Nakajima S, Saito Y, Takahashi S, Katoh R, Kitamura M. mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1-JNK pathway. Cell Death Differ. 2012;19:310–320. doi: 10.1038/cdd.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oh SH, Lim SC. Endoplasmic reticulum stress- mediated autophagy/apoptosis induced by capsaicin (8-methyl-N-vanillyl-6-nonenamide) and dihydrocapsaicin is regulated by the extent of c-Jun NH2-terminal kinase/extracellular signal-regulated kinase activation in WI38 lung epithelial fibroblast cells. J Pharmacol Exp Ther. 2009;329:112–122. doi: 10.1124/jpet.108.144113. [DOI] [PubMed] [Google Scholar]
- 34.Kadowaki H, Nishitoh H, Ichijo H. Survival and apoptosis signals in ER stress: The role of protein kinases. J Chen Neuroanat. 2004;28:93–100. doi: 10.1016/j.jchemneu.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Clarke R, Cook KL, Hu R, Facey CO, Tavassoly I, Schwartz JL, Baumann WT, Tyson JJ, Xuan J, Wang Y, et al. Endoplasmic reticulum stress, the unfolded protein response, autophagy, and the integrated regulation of breast cancer cell fate. Cancer Res. 2012;72:1321–1331. doi: 10.1158/0008-5472.CAN-11-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Ann Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, Liang C, Jung JU, Cheng JQ, Mulé JJ, et al. Bif-1 interacts with Beclin1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol. 2007;9:1142–1151. doi: 10.1038/ncb1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carew JS, Nawrocki ST, Cleveland JL. Modulating autophagy for therapeutic benefit. Autophagy. 2007;3:464–467. doi: 10.4161/auto.4311. [DOI] [PubMed] [Google Scholar]
- 39.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sirichanchuen B, Pengsuparp T, Chanvorachote P. Long-term cisplatin exposure impairs autophagy and causes cisplatin resistance in human lung cancer cells. Mol Cell Biochem. 2012;364:11–18. doi: 10.1007/s11010-011-1199-1. [DOI] [PubMed] [Google Scholar]
- 41.Yu H, Su J, Xu Y, Kang J, Li H, Zhang L, Yi H, Xiang X, Liu F, Sun L. p62/SQSTM1 involved in cisplatin resistance in human ovarian cancer cells by clearing ubiquitinated proteins. Eur J Cancer. 2011;47:1585–1594. doi: 10.1016/j.ejca.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Schönthal AH. Pharmacological targeting of endoplasmic reticulum stress signaling in cancer. Biochem Pharmacol. 2013;85:653–666. doi: 10.1016/j.bcp.2012.09.012. [DOI] [PubMed] [Google Scholar]