Abstract
A carcinoid tumor of the pancreas (CTP) is a rare pancreatic neoplasm, and usually presents with carcinoid syndrome (CS). CS consists of the classic symptom triad of cutaneous flushing, diarrhea and valvular disease, and occurs in the majority of patients with liver metastases. In the present study, the patient presented with symptoms of CS. A diagnosis of CTP with CS was suspected due to high levels of urine 5-hydroxyindolacetic acid, and this was confirmed by a fine-needle aspiration biopsy. Computed tomography showed extended lymphadenopathy, but no liver metastases. The patient was managed conservatively with octreotide long acting repeatable. To the best of our knowledge, this is the second literature case of CS associated with CTP without liver metastases.
Keywords: carcinoid tumor of the pancreas, carcinoid syndrome, liver metastases
Introduction
A carcinoid tumor of the pancreas (CTP) is a rare neuroendocrine tumor arising from the serotonin-secreting cells (enterochromaffin or Kultscitsky's cells) (1). Modlin et al (2) estimated an incidence of ~0.57% from a 5-decade survey of 13,715 carcinoid tumors located in the gastrointestinal tract, including the pancreas and rectum, and the bronchopulmonary system. In a review of 29 patients with CTP, Maurer et al (3) reported a female to male ratio of 1.5:1, with a mean age of 49.3 yeawrs (range, 22–78 years). When CTP metastasizes to the liver, the typical triad of carcinoid syndrome (CS) symptoms, namely, cutaneous flushing, diarrhea and valvular heart disease, may be observed (4). However, the absence of CS does not rule out the presence of a CTP, even when liver metastases have occurred (5).
Patients with CTP and without distant metastases may have a survival rate as high as 84% 1 year subsequent to diagnosis and tumor resection (3). However, the prognosis of patients with CTP associated with CS (i.e. CTP with distant metastases) is poor, with an overall 5-year survival rate of 37.5% (2).
The current study presents the case of a patient with CTP without hepatic metastases, presenting initially with CS and managed conservatively due to advanced disease at the time of diagnosis.
Case report
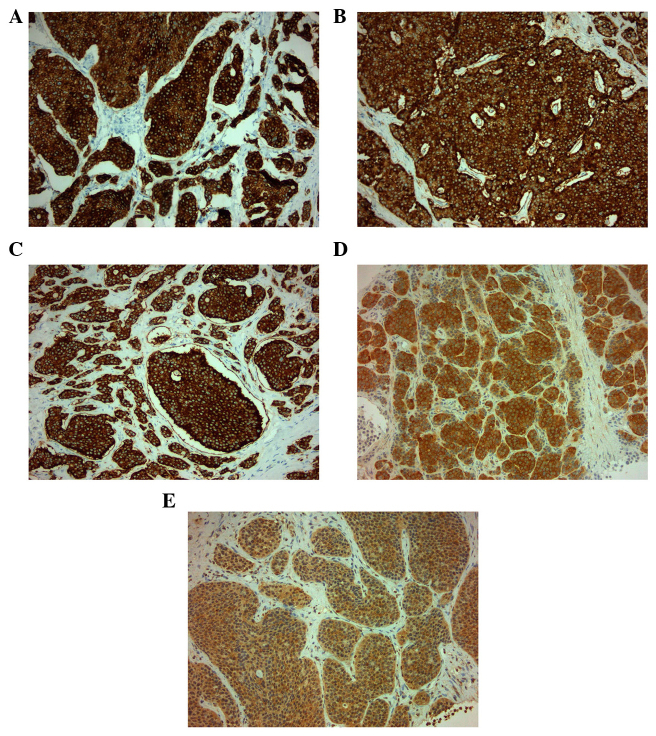
On October 15, 2009, a 73-year-old male was referred to the Third Department of Surgery, University Hospital ‘ATTIKON’ (Athens, Greece) with a 9-month history of extensive watery diarrhea (up to 1 l/day) occurring once or twice a week, weight loss of 10 kg, and periodic flushing of the face, neck, upper chest, and upper and lower limbs. The patient's medical history revealed mild heart failure due to tricuspid regurgitation and pulmonary stenosis. The physical examination of the abdomen was unremarkable. A chest X-ray was normal. Hematological and liver function tests were within normal ranges, and thyroid function tests were within normal limits. Serum serotonin levels were 820 mg/ml (normal range, 101–283 ng/ml). Urine 5-hydroxyindolacetic acid (5-HIAA) was 95 mg/24 h (normal range, 0.7–8.2 mg/24 h). The serum α-fetoprotein level was 2.3 ng/ml (normal range, 0.0–3.4 ng/ml), the carbohydrate antigen (CA)19-9 level was 19.84 U/ml (normal range, 0.0–27 U/ml) and the CA125 level was 28.39 U/ml (normal range, 0.0–35 U/ml). A contrast-enhanced computed tomography (CT) scan showed a well-circumscribed mass of 2.2 cm in diameter, located at the junction of the neck and body of the pancreas, which was slightly hypodense relative to the pancreatic parenchyma and was causing dilatation of the distal pancreatic duct (Fig. 1). Numerous enlarged regional and distal lymph nodes (splenic, hepatic, mesenteric and aortic) were present, but there were no signs of metastatic lesions in the liver and the rest of the peritoneal cavity. Fine-needle aspiration biopsy (FNAB) of the pancreatic mass was performed under CT guidance, and revealed a CTP. Histologically, archived fine-needle aspiration biopsy information showed a tumor composed of relatively uniform cells, with round or oval nuclei mainly arranged in a solid pattern. Occasional pseudorosettes were noted, and lymphatic invasion was evident focally. Immunohistochemical analysis was performed at the time of diagnosis on 2-µm formalin-fixed paraffin-embedded tissues. Antibodes from Dako (Glostrup, Denamrk) were used according to the manufacturer's protocol. The endocrine nature of the tumor was confirmed by staining for cytokeratin AE1/AE3 (Fig. 2A), chromogranin A (Fig. 2B), synaptophysin (Fig. 2C) and neuron-specific enolase (NSE) (Fig. 2D). Notably, tumor cells exhibited diffuse cytoplasmic immunoreactivity to somatostatin (Fig. 2E). Staining for S100 protein was negative. Mitosis was evident, and in the highest areas of mitotic activity the mitotic rate, expressed as the number of mitoses/10 high-power fields, was 5, whereas the Ki-67 index was estimated to be ~8% (Fig. 3). These findings were conclusive of a diagnosis of a well-differentiated grade 2 endocrine tumor. Given the clinical setting, the diagnosis was consistent with a serotonin-secreting endocrine tumor. Indium-111 (111In)-pentetreotide scintigraphy (Octreoscan™, Mallinckrodt Medical BV, Petten, Netherlands) showed selective binding in somatostatin receptors in the region of the tumor at 24 and 48 h post-injection, and excluded the possibility of metastases (Fig. 4). The patient was managed conservatively due to extended lymphadenopathy at the time of diagnosis. Octreotide long acting repeatable (LAR) therapy was initiated with a subcutaneous dose of 20 mg/month for 6 months, and reaching a final dose of 30 mg/month for 12 months. The associated symptoms of CS were relieved for an 18-month period prior to the patient succumbing to severe heart failure.
Figure 1.
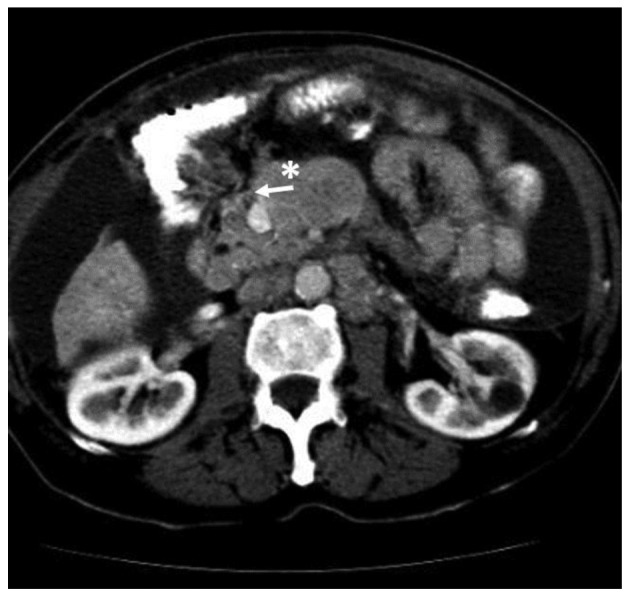
Slightly hypervascularized damage in the region of the neck of the pancreas (asterisk) causing compression and flattening of the upper mesenteric vein (white arrow). The damage is separated from the upper mesenteric artery by a thin fat layer.
Figure 2.
Positive immunohistochemical staining for (A) cytokeratin AE1/AE3, (B) chromogranin A, (C) synaptophysin. (D) neuron-specific enolase and (E) somatostatin. Magnification, ×20.
Figure 3.
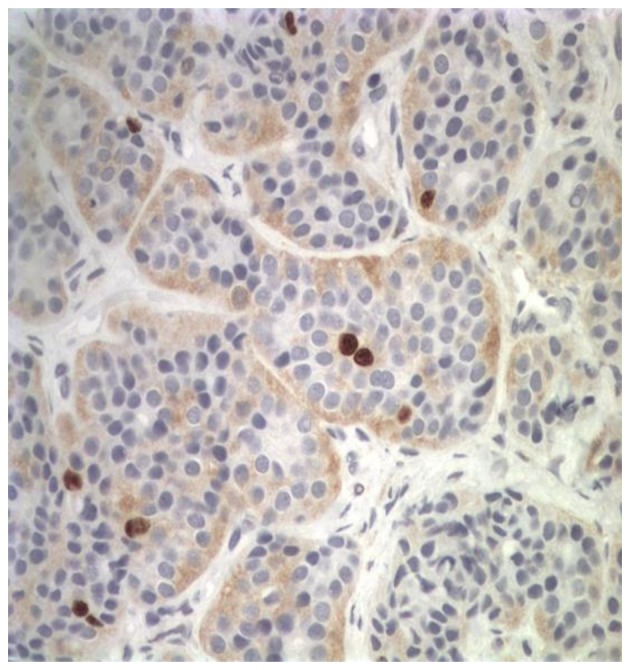
Tissue obtained from fine needle biopsy. Ki-67 index=8%. Magnification, ×20.
Figure 4.
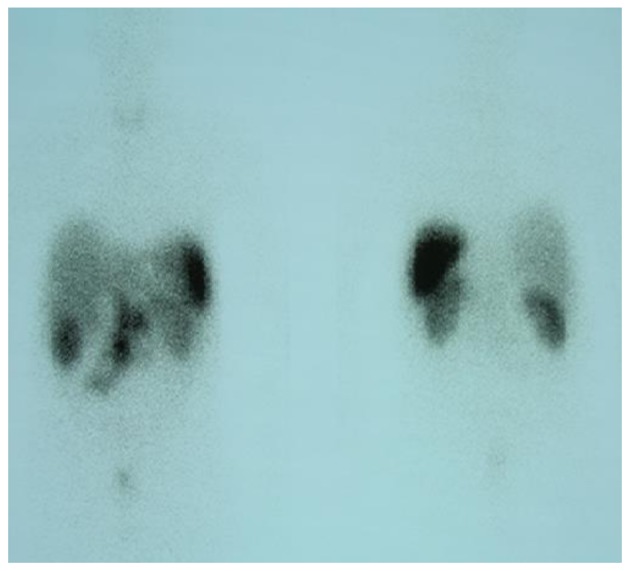
Indium-111 pentetreotide scintigraphy showed a selective binding in somatostatin receptors in the region of the tumor and excluded metastases.
Discussion
The present study described an extremely rare case of a CTP with CS without liver metastases. A search in Pubmed (http://www.ncbi.nlm.nih.gov/pubmed) and Scopus (http://www.scopus.com/) using the terms ‘carcinoid syndrome’, ‘carcinoid syndrome without liver metastasis’, ‘gastrointestinal carcinoid tumor’ and ‘carcinoid tumor of the pancreas’ revealed only one previous study concerning a CTP with CS without liver metastases (6). In all other cases with CS without liver metastases, the carcinoid tumor location was either the intestine (7–10) or was not stated (11,12).
Lubarsch first described the unique characteristics of carcinoid tumors in 1888 (13). However, the term ‘carcinoid’ was introduced by Oberndorfer (14) in 1907 to describe presumably endocrine tumors arising from the digestive system, lungs, thymus, ovaries and testes (15). Currently, the term carcinoid is believed to be inappropriate and has been replaced by the term neuroendocrine tumor (15). In the case of a CTP, according to the WHO classification, the term carcinoid is used for the serotonin-secreting neoplasms associated with the CS (15).
The location and size of carcinoid tumors are the most significant prognostic factors of possible metastases (4). Carcinoid tumors >2 cm in size tend to be associated with metastases in >80% of cases, while tumors <1 cm in size are associated with metastases in <2% of cases (4). The regional lymph nodes and liver are the most common sites of metastasis (5). Less common sites include the brain, breasts and bones (5).
CTP was first described by Pataky et al (16) in 1959, and since then, ~140 cases have been reported in the English literature (17). An analysis of 156 cases of CTP showed that the tumor is located mostly in the head of the pancreas (44.9%), followed by the tail (21%), the body-tail (14.5%) and the body (12.3%) (18). Although CTP is derived from enterochromaffin cells, it appears that it is not a true islet cell tumor (19), but a tumor derived from a multipotential precursor (20). Comparing CTPs to carcinoids located in other anatomical sites, studies have shown that CTPs have the lowest incidence rate and the highest incidence of metastases, and that they tend to be the largest tumors, with the worst prognosis (19).
The association of CTP and CS occurs in 20–65% of cases (2,16), and in the majority of patients with liver metastases (4). In the case of CTP and CS without liver metastases, as in the present study, two possible mechanisms have been proposed as causes: Either serotonin secreted by the CTP enters into the systemic circulation via the lymph nodes, or the liver does not degrade it (6). However, neither of these hypotheses have been proven. In the present case, a possible explanation could be that the liver could not degrade the total elevated amount of circulated serotonin in the serum.
The diagnosis of a CTP can be established by the elevated levels of serotonin in the blood, and/or 5-HIAA in the urine (20), and by immunohistochemical staining (21). Mao et al (18) stated that the detection of high levels of 5-HIAA in the urine is an accurate tool for the diagnosis of CTP in 84% of patients. However, there is no minimal threshold of 5-HIAA for the accurate diagnosis of a CTP. In addition, high levels of serotonin in the serum may be a diagnostic criterion in the case of low or unmeasured 5-HIAA (18). In the present case, the high levels of serotonin in the serum and its metabolite, 5-HIAA, in the urine were strong factors for the diagnosis of the CTP. Positive immunohistochemical staining for chromogranin A, synaptophysin, NSE, cytokeratins AE1/AE3, somatostatin and S100 protein in tissues obtained either by FNAB under CT guidance, as in the present case, or by endoscopic ultrasound (EUS) guidance (22), may further confirm the diagnosis of a CTP.
Imaging studies for the detection of a primary CTP may require a variety of radiological procedures, including CT scan, magnetic resonance imaging, EUS and somatostatin receptor scintigraphy, for the accurate diagnosis of a CTP (22). CT scan findings may reveal hypodense areas with intratumoral calcifications (23), and profound enhancement of the tumor following administration of the intravenous contrast element. Furthermore, dilatation of the main pancreatic duct distal to the tumor, suggesting tumor invasion, may be observed (24). Similarly, in the present case, a slight hypodense mass with dilatation of the distal pancreatic duct was observed, but without calcifications. However, CT cannot differentiate a functioning CTP from a non-functioning tumor, or a CTP from other islet cell tumors of the pancreas (23). The recognition that neuroendocrine tumors of the gastroenteropancreatic tract express somatostatin receptors led to the development of radiolabeled somatostatin for the diagnosis of various types of neuroendocrine tumors (24). The reported sensitivity of this technique ranges from 61 to 96% (24). In the case of a CTP, Octreoscan with 111In may be used in order to identify not only the localizing tumor but also as a method of screening for distal metastases (3,24).
An early diagnosis and surgical resection is probably the only curative therapy for CTPs, due to the questionable effectiveness of chemotherapy (21), while the extent of surgery depends on the tumor size and location, and the presence of lymph node infiltration (25). Octreotide LAR, in a dose ranging from 10 to 30 mg/month, has been found to have tumorostatic and clinical control of the long-term symptoms of CS (26). Although long-term data from the impact of octreotide LAR on the overall survival of patients with CTPs are lacking, it appears that the use of octreotide LAR has a stabilizing effect on patients with neuroendocrine tumors in terms of reducing CS and therefore, morbidity and mortality (27).
In conclusion, the present study reported a rare case of a 73-year old male who was diagnosed with CTP associated with CS without liver metastases. Although, an accurate diagnosis was based on serological, urine and CT scan findings, confirmation of the disease was made via pathological examination. The treatment options available are restricted in these cases and supporting therapy is mandatory to relieve the symptoms of CS and offer an improved quality of life.
Glossary
Abbreviations
- CTP
carcinoid tumor of the pancreas
- CS
carcinoid syndrome
- 5-HIAA
5-hydroxyindolacetic acid
- CA
carbohydrate antigen
- CT
computed tomography
- FNAB
fine-needle aspiration biopsy
- NSE
neuron-specific enolase
- LAR
long-acting repeatable
- EUS
endoscopic ultrasound
References
- 1.Waisberg J, de Matos LL, Dos Santos HV, Dos Santos AB, Reis GC, Capelozzi VL. Pancreatic carcinoid: A rare case of diarrhogenic syndrome. Clinics (Sao Paolo) 2006;61:175–178. doi: 10.1590/s1807-59322006000200015. [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. doi: 10.1002/cncr.11105. [DOI] [PubMed] [Google Scholar]
- 3.Maurer CA, Baer HU, Dyong TH, Mueller-Garamvoelgyi E, Friess H, Ruchti C, Reubi JC, Büchler MW. Carcinoid of the pancreas: Clinical characteristics and morphological features. Eur J Cancer 32A. 1996:1109–1116. doi: 10.1016/0959-8049(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 4.Prasad S, Patankar T, Joshi A, Desmukh H. Pancreatic carcinoid: An unusual tumour in an uncommon locations. J Postgrad Med. 1998;44:97–98. [PubMed] [Google Scholar]
- 5.Hiller N, Berlowitz D, Fisher D, Blinder G, Hadas-Halperin I. Primary carcinoid of the pancreas. Abdom Imaging. 1998;23:188–190. doi: 10.1007/s002619900319. [DOI] [PubMed] [Google Scholar]
- 6.Haq AU, Yook CR, Hiremath V, Kasimis BS. Carcinoid syndrome in the absence of liver metastasis: A case report and review of literature. Med Pediatr Oncol. 1992;20:221–223. doi: 10.1002/mpo.2950200307. [DOI] [PubMed] [Google Scholar]
- 7.Moertel CG, Sauer WG, Dockerty MB, Baggenstos AH. Life history of the carcinoid tumor of the small intestine. Cancer. 1961;14:901–912. doi: 10.1002/1097-0142(196109/10)14:5<901::AID-CNCR2820140502>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 8.Wareing TH, Sawyers JL. Carcinoids and the carcinoid syndrome. Am J Surg. 1983;145:769–772. doi: 10.1016/0002-9610(83)90136-8. [DOI] [PubMed] [Google Scholar]
- 9.Feldman JM, Jones RS. Carcinoid syndrome from the gastrointestinal carcinoid without liver metastasis. Ann Surg. 1982;196:33–37. doi: 10.1097/00000658-198207000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossain J, al-Mofleh I, Tandon R, Dhahada NM. Carcinoid syndrome without liver metastasis. Postgrad Med J. 1989;65:597–599. doi: 10.1136/pgmj.65.766.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen S Collatz, Stage JG, Henriksen FW. Angiography in the diagnosis of carcinoid syndrome. Scand J Gastroenterol Suppl. 1979;53:111–114. [PubMed] [Google Scholar]
- 12.Jaffe BM. Prostaglandins and serotonine. Nonpeptide diarreogenic hormones. World J Surg. 1979;3:565–578. doi: 10.1007/BF01654761. [DOI] [PubMed] [Google Scholar]
- 13.Lubarsch O. On primary cancer of the ileum and remarks on the concurrence of cancer and tuberculosis. Virchows Arch Pathol. Anat Physiol Klin Med. 1888;111:280–317. (In German) [Google Scholar]
- 14.Oberndorfer S. Carcinoid tumors of the small intestine. Frankurter Zeitschrift Fur Pathologie. 1907;1:1426–1429. (In German) [Google Scholar]
- 15.La Rosa S, Franzi F, Albarello L, Schmitt A, Bernasconi B, Tibiletti MG, Finzi G, Placidi C, Perren A, Capella C. Serotonin-producing enterochromaffin cell tumors of the pancreas: Clinicopathologic study of 15 cases and comparison with intestinal enterochromaffin cell tumors. Pancreas. 2011;40:883–895. doi: 10.1097/MPA.0b013e31822041a9. [DOI] [PubMed] [Google Scholar]
- 16.Pataky Z, Nagy L, Popik E. A study on a tumour infestation of primary argentaffin cells originating from the head of pancreas. Zbl Allg Pathol. 1959;99:442–444. (In German) [PubMed] [Google Scholar]
- 17.Modlin IM, Shapiro MD, Kidd M. An analysis of rare carcinoid tumors: Clarifying these clinical conundrums. World J Surg. 2005;29:92–101. doi: 10.1007/s00268-004-7443-z. [DOI] [PubMed] [Google Scholar]
- 18.Mao C, el Attar A, Domenico DR, Kim K, Howard JM. Carcinoid tumors of the pancreas. Status report based on two cases and review of the world's literature. Int J Pancreatol. 1998;23:153–164. doi: 10.1385/IJGC:23:2:153. [DOI] [PubMed] [Google Scholar]
- 19.Soga J. Carcinoids of the pancreas: An analysis of 156 cases. Cancer. 2005;104:1180–1187. doi: 10.1002/cncr.21291. [DOI] [PubMed] [Google Scholar]
- 20.Pearce AGE. The APUD concept: Embryology, cytochemistry and ultra structure of the diffuse neuroendocrine system. In: Friesen SR, editor. Surgical Endocrinology: Clinical Syndromes. Lippincott; Philadelphia: 1978. pp. 18–34. [Google Scholar]
- 21.Liu FH, Wang C, Xing YL, Wu JH, Tang Y. Clinical characteristics and prognosis of primary pancreatic carcinoid tumors: A report of 13 cases from a single institution. Oncol Lett. 2015;9:780–784. doi: 10.3892/ol.2014.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramage JK, Ahmed A, Ardill J, Bax N, Breen DJ, Caplin ME, Corrie P, Davar J, Davies AH, Lewington V, et al. Guidelines for the management of gastroenteropancreatic neuroendocrine (including pancreatic) tumours (NETs) Gut. 2012;61:6–32. doi: 10.1136/gutjnl-2011-300831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villanueva A, Pérez C, Llauger J, Traid C, Parellada JA, Puig P. Carcinoid tumors of the pancreas: CT findings. Abdom Imaging. 1994;19:221–224. doi: 10.1007/BF00203511. [DOI] [PubMed] [Google Scholar]
- 24.Takaji R, Mashumot S, Mori H, Yamada Y, Hongo N, Tanue S, Ota M, Kitano S, Fukuzawa K. Carcinoid tumors of the pancreas: Dynamic CT and MRI features with pathological correlations. Abdom Imaging. 2009;34:753–758. doi: 10.1007/s00261-008-9470-y. [DOI] [PubMed] [Google Scholar]
- 25.He XW, Wu XJ, He XS, Zou YF, Ke J, Wang JP, Lan P. Clinicopathologic analysis of eight cases of pancreatic carcinoid tumors. Chin Med J (Engl) 2009;122:1591–1594. [PubMed] [Google Scholar]
- 26.Woltering EA, Mamikunian PM, Zietz S, Krutzik SR, Go VL, Vinik AI, Vinik E, O'Dorisio TM, Mamikunian G. Effect of octreotide LAR dose and weight on octreotide blood levels in patients with neuroendocrine tumors. Pancreas. 2005;31:392–400. doi: 10.1097/01.mpa.0000186249.89081.0d. [DOI] [PubMed] [Google Scholar]
- 27.Schran HF, Hager DF. Comments on ‘Clinical value of monitoring plasma octreotide levels during chronic octreotide Long-acting repeatable therapy in carcinoid patients’. Pancreas. 2008;37:334–335. doi: 10.1097/MPA.0b013e31818adf6e. [DOI] [PubMed] [Google Scholar]