Abstract
Testicular germ cell tumors (TGCTs) represent a highly curable malignancy, however a small proportion of patients fails to be cured with cisplatin-based chemotherapy. Carbonic anhydrase IX (CA IX) is upregulated by hypoxia in several cancer types and correlates with a poor prognosis. The present translational study evaluated expression and prognostic value of CA IX in TGCTs. Surgical specimens from 228 patients with TGCTs were processed by the tissue microarray method and subjected to immunohistochemistry with the M75 monoclonal antibody. CA IX expression was evaluated in tumors vs. adjacent normal testicular tissues and correlated with clinicopathological characteristics and clinical outcome. CA IX expression was detected in 62 (30.2%) of TGCTs compared to 0 (0%) of normal tissue adjacent to testicular tumor (P<0.001). The highest frequency of the CA IX expression was detected in teratoma (39.0%), followed by seminoma (22.7%), yolk sac tumor (22.2%), embryonal carcinoma (11.9%) and choriocarcinoma (7.7%). None of germ cell neoplasias in situ (GCNIS) exhibited CA IX expression. Patients without the CA IX tumor expression showed significantly better progression-free survival, but not overall survival, compared to patients with the CA IX expression [hazard ratio (HR), 0.57; 95% CI, 0.32–1.02; P=0.037 and HR, 0.58; 95% CI, 0.29–1.16; P=0.088, respectively]. There was no significant correlation between the CA IX expression and clinicopathological variables. The intratumoral CA IX expression can serve as a prognostic marker in the TGCT patients. These results suggest that activation of the hypoxia-induced pathways may be important in the treatment failure in TGCTs patients.
Keywords: carbonic anhydrase IX, testicular germ cell tumors, prognostic marker
Introduction
Testicular germ cell tumors (TGCTs) belong to the most common malignancies among men aged between 20–40 years (1,2). TGCTs are highly curable malignancies and even the majority of metastatic patients may expect to be cured with the first-line cisplatin-based chemotherapy (3,4). Despite the high curative rate, there are ~20–30% of patients who fail to achieve a durable complete remission (3). Salvage chemotherapy based on the standard cisplatin dose and previously non-utilized chemotherapeutic agents or high-dose salvage chemotherapy with autologous stem cell transplantation can induce durable remission in 20–60% of patients with relapsed disease (3,5,6). Current treatment strategies in cisplatin-refractory and relapsed TGCTs patients are insufficient. Thus, novel treatment strategies, including drugs with antitumor activity, as well as novel biomarkers as effective tools for better stratification of patients are required.
The process of tumor development is characterized by rapid proliferation of cancer cells and the expansion of tumor tissue associated with hypoxia in the tumor microenvironment (7). Carbonic anhydrase IX (CA IX) is a zinc metalloenzyme, which catalyzes a reversible hydration of carbon dioxide to bicarbonate and a proton, and participates in pH regulation, in addition to cell adhesion-migration-invasion (8). Thus, CA IX, localized in the plasma membrane, enables tumor cells to maintain a normal intracellular pH under hypoxic conditions, while concurrently acidifying the extracellular microenvironment. The increased expression of CA IX promotes invasion and metastasis and is associated with treatment resistance in several types of cancer (9–12). Hypoxia and CA IX have been also linked with cancer stem cell properties (13–15). Furthermore, inhibition of angiogenesis is able to generate a hypoxic tumor microenvironment, thereby increasing the population of breast cancer stem cells (16). Thus, an increased proportion of circulating tumor cells derived from tumors that grow under hypoxic conditions may contribute to a poor treatment outcome and increased resistance to chemotherapy (17). Since cancer stem cells in TGCTs resemble embryonic stem cells (9,18), we propose that CA IX, as a mediator of tumor responses to hypoxia, may be involved in the pathogenesis of TGCTs.
The aim of the present translational study was to investigate the CA IX protein expression in TGCTs and to evaluate its potential prognostic role in these patients. We examined the CA IX expression in all histological subtypes of TGCTs, as well as in GCNIS (germ cell neoplasia in situ) specimens and in the normal testicular tissue adjacent to testicular tumor.
Patients and methods
Patients
The present translational study (Protocol IZLO1, Chair: M. Mego) involved 228 patients with TGCTs treated from January 2000 to September 2013 in the National Cancer Institute of Slovakia, Bratislava, Slovakia and St. Elisabeth Cancer Institute, Bratislava, Slovakia, with available paraffin embedded tumor tissue specimen and sufficient follow-up clinical data. Patients with a concurrent malignancy other than non-melanoma skin cancer in the previous 5 years were excluded from the study. In all patients, data regarding age, tumor histological subtype, clinical stage and type and number of metastatic lesions were recorded and compared with the CA IX expression. The Institutional Review Board of the National Cancer Institute, Bratislava, Slovakia approved this retrospective study and a waiver of patient consent was granted.
Tumor pathology
Pathological review was conducted at the Department of Pathology, Faculty of Medicine, Comenius University, Bratislava, Slovakia by two pathologists (Z.C. and P.B.) associated with the study.
Diagnosis and tissue samples
Tumor tissue, samples with germ cell neoplasias in situ (GCNIS), and normal testicular tissue were evaluated in all cases, when available. The present study included tumor specimens from 228 patients prior to the administration of systemic therapy. Primary testicular tumor specimens were obtained in 205 (89.9%) patients. Biopsies of metastatic sites were available in 23 (10.1%) cases.
The TGCTs were classified according to the World Health Organization criteria (19). Since the normal testicular biopsies from non-cancer patients were not available for our analysis, normal tissue adjacent to testicular tumor was used for evaluation of CA IX expression, as described in previous studies (20–22).
Tissue microarray construction
According to tumor histology, one or two representative tumor areas from each histological subtype of germ cell tumor (from 1–6 cores from each tumor) were identified on haematoxylin and eosin stained sections. Samples from normal testicular tissue and germ cell neoplasia in situ were also marked, if present. Sections were matched to their corresponding wax blocks (the donor blocks), and 3-mm diameter cores of the tumor were removed from these donor blocks with the multipurpose sampling tool Harris Uni-Core (Sigma-Aldrich, Steinheim, Germany) and inserted into the recipient master block. The recipient block was cut into 5-µm sections that were transferred to coated slides.
Immunohistochemical staining
Deparaffinized slides were rehydrated in phosphate buffered saline solution (10 mM, pH 7.2). The tissue epitopes were demasked using the automated water bath heating process in Dako PT Link (Dako, Glostrup, Denmark); the slides were incubated in TRIS-EDTA retrieval solution (10 mM TRIS, 1 mM EDTA pH 9.0) at 98°C for 20 min. This step was introduced because of low intensity of staining when the standard CA IX immunohistochemical staining protocol was used (23). Increasing the concentration, nor the extension of incubation time with the M75 primary antibody, did improve the staining result, however, pretreatment with TRIS-EDTA substantially strengthened the reaction signal. The slides were subsequently incubated for 1 h at room temperature with the monoclonal antibody M75 against the N-terminal domain of the human CA IX protein (23,24) diluted 1:100 in Dako REAL antibody diluent (Dako, Glostrup, Denmark) and immunostained using anti-mouse/anti-rabbit immune-peroxidase polymer (EnVision FLEX/HRP, Dako, Glostrup, Denmark) for 30 min at room temperature, according to the manufacturer's instructions. The reaction was visualized with a diaminobenzidine substrate-chromogen solution (DAB, Dako Cytomation, Denmark) for 5 min, and slides were counter-stained with haematoxylin. The trophoblast staining in human placenta served as positive control. Additional testicular tumors specimens stained with omission of the primary antibody served as negative control.
Immunohistochemistry scoring
Two observes (Z.C. and P.B.) who were blinded to clinicopathological data conducted an independent analysis of the tumor cores. In cases of disagreement, the result was reached by consensus. CA IX expression was stratified as negative or positive (any staining).
Statistical analysis
The patients' characteristics were summarized as mean or median (range) values for continuous variables and frequency (percentage) for categorical variables, respectively. Statistical analysis was performed using non-parametric tests as the distribution of the CA IX expression was significantly different from the normal distribution (Shapiro-Wilk test). Analyses of differences in distributions of CA IX expression between the two groups of patients were performed using the Mann-Whitney U test, whereas Fisher's exact test or the χ2 test were used when CA IX expression was categorized as ‘absent’ or ‘present’ according to the aforementioned criteria. Median follow-up period was calculated as a median observation time of all patients and of those still alive at the time of their last follow-up. Progression-free survival (PFS) was calculated from the date of the starting treatment with systemic therapy to the date of progression or death or the date of the last adequate follow-up. Overall survival (OS) was calculated from the date of starting treatment with systemic therapy to the date of death or last follow-up. PFS and OS were estimated using the Kaplan-Meier product limit method and were compared with the log-rank test. A multivariate Cox proportional hazards model for PFS and OS was used to assess differences in outcome on the basis of the CA IX expression in primary tumor and/or biopsy of metastatic site and prognosis according to the IGCCCG (International Germ Cell Collaborative Group) criteria (1997) (19). All presented P-values were two sided. Values of P<0.05 were considered to indicate a statistically significant difference. Statistical analyses were performed using NCSS 2007 software developed by Hintze J (2007) (NCSS, LLC, Kaysville, UT, USA) (25).
Results
Patient characteristics
Patients characteristics are summarized in Table I. The mean age of patients enrolled into this study was 30 years (range, 16–67 years). The majority of patients had non-seminomatous primary testicular tumor, and had good prognosis according to the IGCCCG criteria. All patients were treated with cisplatin-based chemotherapy. No extragonadal germ cell tumors were included.
Table I.
Patient characteristics (n=228).
| Characteristics | No. | % |
|---|---|---|
| Age (years) | ||
| Median (range) | 30 (16–67) | |
| Histology | ||
| Seminoma | 44 | 19.3 |
| Non-seminoma | 184 | 80.7 |
| IGCCCG risk group prognosis | ||
| Good | 173 | 75.8 |
| Intermediate | 25 | 11.0 |
| Poor | 30 | 13.2 |
| Sites of metastases | ||
| Retroperitoneum | 159 | 69.7 |
| Mediastinum | 23 | 10.1 |
| Lungs | 52 | 22.8 |
| Liver | 12 | 5.3 |
| Brain | 3 | 1.3 |
| Other | 11 | 4.8 |
| Non-pulmonary visceral metastases | 16 | 7.0 |
| No. of metastatic sites | ||
| 0 | 64 | 28.1 |
| 1 | 98 | 43.0 |
| 2 | 34 | 14.9 |
| >3 | 32 | 14.0 |
| Mean (range) of pretreatments markers | ||
| AFP mIU/ml | 1168.0 (0.0–60570.0) | |
| -HCG IU/ml | 15980.0 (0.0–929000.0) | |
| LDH (mkat/l) | 11.0 (0.0–88.6) | |
-HCG, -human chorionic gonadotropin; AFP, α-fetoprotein; LDH, lactate dehydrogenase; IGCCCG, international germ cell consensus classification group.
In total, 228 patient specimens were analyzed for CA IX expression using immunohistochemical analysis. CA IX staining was evaluated in 321 tumor specimens from primary testicular tumors (205 patients, Table II), in 23 specimens from metastatic sites sampled post chemotherapy, and in 107 adjacent normal testicular tissues. GCNIS adjacent to testicular tumor was present in 76 patients.
Table II.
CA IX expression in different histologic subtypes of the primary germ cell tumors (n=205).
| CA IX expression | ||||||
|---|---|---|---|---|---|---|
| Absent | Present | |||||
| Histological subtype | No. | No. | % | No. | % | P-value |
| Healthy testis | 107 | 107 | 100.0 | 0 | 0.0 | N/A |
| Testicular germ cell tumors | 205 | 143 | 69.8 | 62 | 30.2 | <0.001 |
| Seminoma | 75 | 58 | 77.3 | 17 | 22.7 | <0.001 |
| Embryonal carcinoma | 118 | 104 | 88.1 | 14 | 11.9 | <0.001 |
| Yolc sac tumor | 36 | 28 | 77.8 | 8 | 22.2 | <0.001 |
| Choriocarcinoma | 13 | 12 | 92.3 | 1 | 7.7 | 0.11 |
| Teratoma | 59 | 36 | 61.0 | 23 | 39.0 | <0.001 |
| GCNIS | 76 | 76 | 100.0 | 0 | 0.0 | N/A |
GCNIS, germ cell neoplasia in situ; CA IX, carbonic anhydrase IX.
The analyzed cohort of primary testicular tumors included 49 pure seminomas, 79 non-seminomas (57 embryonal carcinomas, 12 yolk sac tumors, 9 teratomas, 1 choriocarcinoma) and 76 mixed germ cell tumors (Table III). Six cases of seminomas were clinically considered as non-seminomas based on positivity of alpha-fetoprotein.
Table III.
Composition of mixed testicular germ cell tumors (n=76).
| No. of patients | Histological subtype |
|---|---|
| 22 | EC/TER |
| 15 | EC/SEM |
| 6 | EC/YST/TER |
| 6 | YST/TER |
| 5 | EC/YST |
| 4 | EC/ChC/TER |
| 4 | SEM/TER |
| 3 | EC/SEM/TER |
| 3 | EC/ChC |
| 3 | YST/ChC/TER |
| 1 | EC/SEM/YST |
| 1 | EC/SEM/YST/TER |
| 1 | SEM/YST |
| 1 | EC/SEM/ChC |
| 1 | YST/ChC |
EC, embryonal carcinoma; SEM, seminoma; YST, yolk sac tumour; ChC, choriocarcinoma; TER, teratoma.
Association between CA IX expression and patients/tumor characteristics
CA IX staining of various TGCTs histological subtypes and normal tissue adjacent to testicular tumor is presented in Fig. 1. Whereas normal testicular tissue adjacent to testicular tumor did not show any CA IX staining, CA IX expression was detected in all histological subtypes of TGCTs (Table II). The highest frequency of the CA IX expression was in teratoma samples, with decreasing trend in seminoma, embryonal carcinoma, yolk sac tumor and choriocarcinoma. In choriocarcinomas, the CA IX staining was observed only in one (7.7%) specimen, while no CA IX expression was observed in GCNIS. CA IX expression was detected in 17 seminomas (22.7), 14 embryonal carcinomas (11.9), 8 yolk sac tumors (22.2) and 23 teratomas (39.0) compared to 0 (0.0) of normal testicular tissue adjacent to testicular tumor (P<0.001; Table II).
Figure 1.
Immunohistochemical detection of CA IX expression in testicular germ cell tumors. (A) Seminoma showed focal moderate membrane CA IX positivity (brown). (B) Yolk sac tumor with focal strong cytoplasmic CA IX positivity. (C) Embryonal carcinoma with focal strong cytoplasmic CA IX positivity. (D) Mature teratoma with strong cytoplasmic CA IX positivity in epithelial component and negativity in mesenchymal component. Magnification, upper ×40, lower ×400. CA IX, carbonic anhydrase IX.
The CA IX staining pattern was largely focal in the tumor tissue, with prevailing membrane positivity in seminomas and embryonal carcinomas and cytoplasmic staining in the other histological subtypes. Mesenchymal cells in teratomas and in the tumor stroma exhibited focal positivity, the intercellular matrix was negative (Fig. 1). Areas of cells neighboring with necrosis did not show increased CA IX expression.
In addition, we analyzed the relationship between the CA IX expression in primary tumors and clinicopathological features (Table IV). We did not find any significant association of the CA IX expression in primary tumors with patients/tumor characteristic, such as tumor histology, IGCCCG risk group, number and localization of metastatic sites and S-stage (Table V). The Spearman's test did not show any significant correlation between the intratumoral CA IX expression and LDH level (Spearman's correlation index, P=0.216).
Table IV.
Patient characteristics according to the CA IX expression in the primary tumors (n=205).
| CA IX expression | ||||||
|---|---|---|---|---|---|---|
| Absent | Present | |||||
| Variable | No. | No. | % | No. | % | P-value |
| All patients | 205 | 143 | 69.8 | 62 | 30.2 | N/A |
| Histology | ||||||
| Seminoma | 40 | 28 | 70.0 | 12 | 30.0 | 0.567 |
| Non-seminoma | 165 | 115 | 69.7 | 50 | 30.3 | |
| IGCCCG risk group | ||||||
| Good/Intermediate prognosis | 180 | 127 | 70.6 | 53 | 29.4 | 0.325 |
| Poor prognosis | 25 | 16 | 64.0 | 9 | 36.0 | |
| Number of metastatic sites | ||||||
| 0 | 58 | 38 | 65.5 | 20 | 34.5 | 0.841 |
| ≥1 | 147 | 105 | 71.4 | 42 | 28.6 | |
| Retroperitoneal LN metastases | ||||||
| Absent | 62 | 40 | 64.5 | 22 | 35.5 | 0.892 |
| Present | 143 | 103 | 72.0 | 40 | 28.0 | |
| Mediastinal LN metastases | ||||||
| Absent | 187 | 133 | 71.1 | 54 | 28.9 | 0.136 |
| Present | 18 | 10 | 55.6 | 8 | 44.4 | |
| Lung metastases | ||||||
| Absent | 160 | 114 | 71.3 | 46 | 28.8 | 0.242 |
| Present | 45 | 29 | 64.4 | 16 | 35.6 | |
| Liver | ||||||
| Absent | 194 | 136 | 70.1 | 58 | 29.9 | 0.438 |
| Present | 11 | 7 | 63.6 | 4 | 36.4 | |
| Brain | ||||||
| Absent | 204 | 142 | 69.6 | 62 | 30.4 | 1.000 |
| Present | 1 | 1 | 100.0 | 0 | 0.0 | |
| Non-pulmonary visceral metastases | ||||||
| Absent | 191 | 134 | 70.2 | 57 | 29.8 | 0.423 |
| Present | 14 | 9 | 64.3 | 5 | 35.7 | |
| S-stage | ||||||
| 0-II | 186 | 132 | 71.0 | 54 | 29.0 | 0.178 |
| III | 19 | 11 | 57.9 | 8 | 42.1 | |
CA IX, carbonic anhydrase IX; IGCCCG, international germ cell consensus classification group; LN, lymph node.
Table V.
Multivariate analysis of the potential prognostic value of CA IX.
| Progression free survival | Overall survival | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| CA IX expression in primary tumor high vs. low | 1.650 (0.963–2.826) | 0.068 | 1.613 (0.844–3.080) | 0.148 |
| IGCCCG risk group poor vs. good/intermediate prognosis | 5.260 (3.005–9.209) | <0.001 | 8.282 (4.286–16.005) | <0.001 |
CA IX, carbonic anhydrase IX; IGCCCG, international germ cell consensus classification group; HR, hazard ratio; CI, confidence interval.
Prognostic value of CA IX
The median follow-up time was 82.3 months (0.3–289.1 months) for all 228 patients and 92.6 months (14.2–289.1 months) for patients still alive. To the date of last follow-up, 56 patients (24.6%) experienced disease progression and 38 patients (16.7%) had succumbed. The estimated 2-year and 5-year PFS survival was 80.3% (95% CI, 75.1–85.4%) and 78.3% (95% CI, 72.9–83.7%), while the estimated 2-year and 5-year OS survival was 90.3% (95% CI, 86.5–94.2%) and 85.1% (95% CI, 80.4–89.8%), respectively.
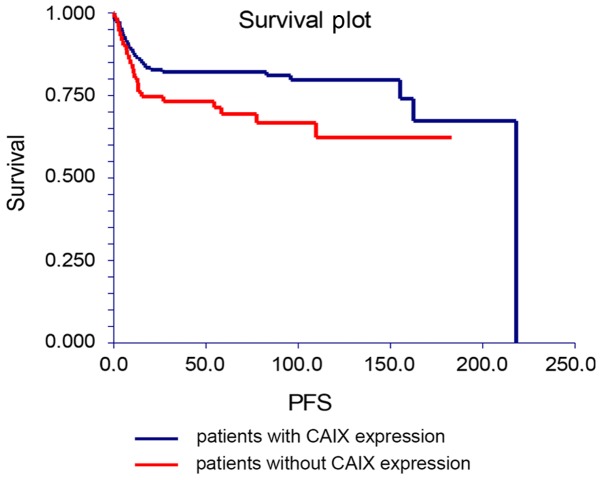
In univariate analysis, patients without CA IX expression in analyzed tumor specimens had significantly better PFS, in contrast to patients with the CA IX expression [hazard ratio (HR), 0.57; 95% CI, 0.32–1.02; P=0.0365; Fig. 2]. Moreover, there was a trend for association between the CA IX expression and OS in this patients' cohort (HR, 0.58; 95% CI, 0.29–1.16; P=0.0876; Fig. 3). Multivariate analysis revealed that CA IX expression in tumor tissues was associated with PFS independently of the IGCCCG risk group, however this correlation did not reach statistical significance (P=0.0682). Moreover, no significant association was shown between the IGCCCG risk group and the CA IX expression as an independent prognostic factor for overall survival (Table V).
Figure 2.
Kaplan-Meier estimates of probabilities of progression-free survival according to CA IX expression in TGCT patients (n=228; HR, 0.57; 95% CI, 0.32–1.02; P=0.037). CA IX, carbonic anhydrase IX; TGCT, testicular germ cell tumors; PFS, progression free survival; HR, hazard ratio; CI, confidence interval.
Figure 3.
Kaplan-Meier estimates of probabilities of overall survival according to CA IX expression in TGCT patients (n=228; HR, 0.58; 95% CI, 0.29–1.16; P=0.088). CA IX, carbonic anhydrase IX; TGCT, testicular germ cell tumors; OS, overall survival; HR, hazard ratio; CI, confidence interval.
Therefore, exploratory subgroup analysis was performed to reveal a potential subgroup-related prognostic value of CA IX (Table VI). This analysis demonstrated that the tumor CA IX expression correlated with the worse PFS in non-seminoma patients, patients with one or more metastatic lesions and patients with retroperitoneal lymph node metastases. Furthermore, the absence of the CA IX expression in primary tumors was significantly associated with better PFS in patients without lung, brain and non-pulmonary visceral metastases.
Table VI.
Prognostic value of CA IX as an independent indicator in different patient subgroups.
| Variable | No. | PFS HR (95% CI) | P-value | OS HR (95% CI) | P-value |
|---|---|---|---|---|---|
| Histology | |||||
| Seminoma | 44 | 2.46 (0.45–13.48) | 0.396 | 1.00 (0.09–11.00) | 0.997 |
| Non-seminoma | 184 | 0.47 (0.25–0.87) | 0.006 | 0.54 (0.26–1.12) | 0.067 |
| IGCCCG group | |||||
| Good prognosis | 173 | 0.68 (0.29–1.62) | 0.342 | 0.56 (0.16–1.94) | 0.305 |
| Intermediate prognosis | 25 | 0.49 (0.14–1.72) | 0.258 | 0.88 (0.22–3.53) | 0.857 |
| Poor prognosis | 30 | 0.73 (0.29–1.88) | 0.499 | 0.73 (0.27–1.99) | 0.518 |
| Number of metastatic sites | |||||
| 0 | 64 | 0.79 (0.14–4.46) | 0.756 | 0.43 (0.05–3.53) | 0.317 |
| ≥1 | 164 | 0.50 (0.27–0.94) | 0.013 | 0.56 (0.27–1.19) | 0.099 |
| Reroperitoneal LN metastases | |||||
| Present | 159 | 0.49 (0.26–0.94) | 0.014 | 0.58 (0.27–1.24) | 0.120 |
| Absent | 69 | 0.65 (0.14–3.05) | 0.536 | 0.33 (0.05–2.11) | 0.141 |
| Mediastinal LN metastases | |||||
| Present | 23 | 0.43 (0.13–1.41) | 0.161 | 0.48 (0.12–1.94) | 0.303 |
| Absent | 205 | 0.68 (0.35–1.31) | 0.210 | 0.60 (0.26–1.37) | 0.1785 |
| Lung metastases | |||||
| Present | 52 | 0.82 (0.35–1.92) | 0.636 | 0.69 (0.28–1.68) | 0.379 |
| Absent | 176 | 0.48 (0.22–1.05) | 0.035 | 0.50 (0.17–1.49) | 0.162 |
| Liver metastases | |||||
| Present | 12 | 0.30 (0.05–2.01) | 0.115 | 0.32 (0.05–2.05) | 0.130 |
| Absent | 216 | 0.60 (0.33–1.12) | 0.076 | 0.62 (0.29–1.32) | 0.176 |
| Brain metastases | |||||
| Present | 3 | NA | NA | NA | NA |
| Absent | 225 | 0.54 (0.30–0.98) | 0.024 | 0.53 (0.26–1.08) | 0.052 |
| Non-pulmonary visceral metastases | |||||
| Present | 16 | 0.52 (0.10–2.64) | 0.355 | 0.53 (0.11–2.68) | 0.377 |
| Absent | 217 | 0.56 (0.30–1.04) | 0.041 | 0.55 (0.25–1.21) | 0.099 |
| S-stage | |||||
| 0-II | 206 | 0.57 (0.29–1.12) | 0.070 | 0.61 (0.26–1.45) | 0.224 |
| III | 22 | 0.62 (0.20–1.91) | 0.368 | 0.51 (0.16–1.67) | 0.219 |
P-values in bold indicate statistically significant differences. CA IX, carbonic anhydrase IX; IGCCCG, international germ cell consensus classification group; HR, hazard ratio; CI, confidence interval; PFS, progression free survival; OS, overall survival; LN, lymph node.
Discussion
Carbonic anhydrase IX (CA IX) is a hypoxia-inducible enzyme that is important in cancer development, progression, acidification and metastasis (8). There is increasing evidence that overexpression of CA IX in a variety of cancers correlates with an unfavorable outcome, and is related to a decrease in the progression free survival following successful therapy. Therefore, it is considered as a surrogate tumor biomarker (26).
The present translational study demonstrated significantly increased CA IX expression in TGCTs, in contrast to its absence in normal testicular tissue adjacent to germ cell tumors. CA IX expression was demonstrated in all histological subtypes, with the highest expression in teratomas. These findings can be explained by the mesodermal origin of all CA IX expressing cells. Moreover, our results are supported by the detection of CA IX expression in the flat surface epithelium (modified mesothelium) of all male and female genital organs (27). Only one of thirteen choriocarcinoma specimens was positive in CA IX staining. This result is consistent with the study of Donato et al (28), where choriocarcinoma tumors were predominately negative in CA IX. In contrast to the results published by Donato et al (28), the present study detected CA IX expression not only in teratomas and embryonal carcinomas, but also in seminomas and in yolk sac tumors. On the other hand we identified no CA IX expression in germ cell neoplasia in situ. GCNIS represents a precursor lesion for invasive TGCT of the adult testis (29). Thus, we may suppose that CA IX expression does not belong to early events in the pathogenesis of TGCTs. We also failed to detect any significant association between the CA IX expression and the patients/tumor characteristics. However, our data indicated the value of CA IX in the prognosis of progression-free survival, since CA IX expression in analyzed patients tumor specimens correlated with the significantly worse PFS. These findings are in agreement with previous reports on the prognostic value of CA IX in the wide variety of human carcinomas, including upper gastrointestinal cancer, breast cancer, ovarian and cervical cancer, nasopharyngeal cancer, lung and rectal cancer (30–36). The relationship between the CA IX expression and progression-free survival in TGCT patients was confirmed by the subgroup analysis of non-seminoma patients, patients with one or more metastatic site as well as patients with retroperitoneal lymph node metastases. Moreover, the analysis of patients without lung, brain and non-pulmonary visceral metastases showed a similar association. On the other hand, we did not observe any association between the CA IX expression and unfavorable outcome in patients with brain metastases, non-pulmonary visceral metastases and in patients with S-stage 3. It is therefore possible that the poor prognosis of these groups of patients is related to other pathways than that driven by hypoxia. Based on these findings, we propose that CA IX expression, mainly in patients with metastatic disease but without high-risk features (specifically, patients without brain and non-pulmonary visceral metastases and/or S-stage 3) may serve as prognostic marker of inferior outcome. This suggestion is also supported by the observation, that the hypoxic microenvironment plays a role in inferior outcome and chemoresistance due to increased burden of circulating tumor cells (16). Experimental data suggest that the tumor cells overexpress CA IX in order to maintain the intracellular pH and thus preserve their survival in hypoxia (37). The acidification of extracellular space mediated by this mechanism contributes to tumor cell invasion, development of metastases and therefore to worse progression-free survival (38,39). The study has some limitations, including the relative under-representation of choriocarcinoma and yolk sac tumor and the selection of tissue samples into the tissue microarray. For this reason we performed whole tissue section immunohistochemical staining of several TGCT cases, and identified corresponding staining pattern with that in the tissue array. Notably, the TRIS-EDTA pretreatment of the slides considerably increased the sensitivity of CA IX expression detection in the studied tumor specimens.
In conclusion, the present translational study demonstrated significant overexpression of CA IX in TGCTs when compared to normal testicular tissue. We detected for the first time an association between CA IX expression in primary tumor tissue and worse progression-free survival in patients with TGCTs. Higher CA IX expression correlating with inferior outcome was found predominantly in patients with metastatic disease. These results suggest that CA IX expression may serve as an important predictive factor associated with disease recurrence and poor progression-free survival time in patients with advanced testicular cancer.
Acknowledgements
The authors of the present study would like to acknowledge our collaborators: Dr Antol M from the Department of Pathology, Regional Hospital and Health Centre, Levoča, Slovakia, Dr Benko J from the Department of Pathology, University Hospital in Nové Zámky, Slovakia, Dr Danis D and Dr Galbavy S from the Department of Pathology, Slovak Medical University, Bratislava, Slovakia, Dr Durcansky D from the Department of Pathology, Regional Hospital and Health Centre, Prievidza, Slovakia, Dr Fiala P from the Department of Pathology, Faculty of Medicine, Pavol Jozef Šafárik University in Košice, Slovakia, Dr Gogora M from the Department of Pathology, Regional Hospital and Health Centre, Ilava, Slovakia, Dr Hudcovsky P from the Department of Pathology, Regional Hospital and Health Centre, Šaľa, Slovakia, Dr Macuch J from the Department of Clinical Pathology, National Cancer Institute, Bratislava, Slovakia, Dr Martanovic P from the Department of Forensic Medicine, Faculty of Medicine, Comenius University, Bratislava, Slovakia, Dr Ondrias F from the Department of Pathology, University Hospital Bratislava, Ruzinov, Slovakia, Dr Plank L from the Department of Pathology, Comenius University, Jessenius Medical Faculty and Martin´s Faculty Hospital, Martin, Slovakia and Dr Svajdler M from the Department of Pathology, Louis Pasteur University Hospital in Kosice, Slovakia. We would like to acknowledge Mrs. Daniela Jantekova, from the Population Registry of Slovak Republic, Bratislava, Slovakia for help with updating the patient database, and Dr Maria Reckova, 2nd Department of Oncology, Faculty of Medicine, Comenius University, Bratislava, Slovakia for discussions and critical input. We would like to acknowledge Mrs. Zlatica Pekova from the Department of Oncology, National Cancer Institute, Bratislava, Slovakia for administration support, and Mrs. Emilia Klincova and Mr. Ludovit Gaspar from the Department of Pathology, Faculty of Medicine, Comenius University, Bratislava, Slovakia for excellent technical assistance. The present study was supported by the Slovak Research and Development Agency (Bratislava, Slovakia; grant nos. APVV-0016-11 and APVV-15-0086), the State Budget of the Slovak Republic (Bratislava, Slovakia; grant no. ITMS 26240220071) and the Slovak Scientific Grant Agency (Bratislava, Slovakia; grant no. VEGA-2/0108/16).
References
- 1.Rijlaarsdam MA, Looijenga LH. An oncofetal and developmental perspective on testicular germ cell cancer. Semin Cancer Biol. 2014;29:59–74. doi: 10.1016/j.semcancer.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Trabert B, Chen J, Devesa SS, Bray F, McGlynn KA. International patterns and trends in testicular cancer incidence, overall and by histologic subtype, 1973–2007. Andrology. 2015;3:4–12. doi: 10.1111/andr.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–684. doi: 10.1001/jama.299.6.672. [DOI] [PubMed] [Google Scholar]
- 4.Voutsadakis IA. The chemosensitivity of testicular germ cell tumors. Cell Oncol (Dordr) 2014;37:79–94. doi: 10.1007/s13402-014-0168-6. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Agarwal N, Beard C, Bhayani S, Bolger GB, Buyyounouski MK, Carducci MA, Chang SS, Choueiri TK, Gupta S, et al. Testicular cancer. J Natl Compr Canc Netw. 2012;10:502–535. doi: 10.6004/jnccn.2012.0050. [DOI] [PubMed] [Google Scholar]
- 6.Mardiak J, Sálek T, Sycova-Milá Z, Obertová J, Hlavatá Z, Mego M, Recková M, Koza I. Paclitaxel plus ifosfamide and cisplatin in second-line treatment of germ cell tumors: A phase II study. Neoplasma. 2005;52:497–501. [PubMed] [Google Scholar]
- 7.Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, Rich JN. Hypoxia inducible factors in cancer stem cells. Br J Cancer. 2010;102:789–795. doi: 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: From biology to clinical use. Semin Cancer Biol. 2015;31:52–64. doi: 10.1016/j.semcancer.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 9.McIntyre A, Patiar S, Wigfield S, Li JL, Ledaki I, Turley H, Leek R, Snell C, Gatter K, Sly WS, et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin Cancer Res. 2012;18:3100–3111. doi: 10.1158/1078-0432.CCR-11-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jubb AM, Buffa FM, Harris AL. Assessment of tumour hypoxia for prediction of response to therapy and cancer prognosis. J Cell Mol Med. 2010;14:18–29. doi: 10.1111/j.1582-4934.2009.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan EY, Yan M, Campo L, Han C, Takano E, Turley H, Candiloro I, Pezzella F, Gatter KC, Millar EK, et al. The key hypoxia regulated gene CA IX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br J Cancer. 2009;100:405–411. doi: 10.1038/sj.bjc.6604844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Generali D, Fox SB, Berruti A, Brizzi MP, Campo L, Bonardi S, Wigfield SM, Bruzzi P, Bersiga A, Allevi G, et al. Role of carbonic anhydrase IX expression in prediction of the efficacy and outcome of primary epirubicin/tamoxifen therapy for breast cancer. Endocr Relat Cancer. 2006;13:921–930. doi: 10.1677/erc.1.01216. [DOI] [PubMed] [Google Scholar]
- 13.McCord AM, Jamal M, Williams ES, Camphausen K, Tofilon PJ. CD133+ glioblastoma stem-like cells are radiosensitive with a defective DNA damage response compared with established cell lines. Clin Cancer Res. 2009;15:5145–5153. doi: 10.1158/1078-0432.CCR-09-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lock FE, McDonald PS, Lou Y, Serrano I, Chafe SC, Ostlund C, Aparicio S, Winum JY, Supuran CT, Dedhar S. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32:5210–5219. doi: 10.1038/onc.2012.550. [DOI] [PubMed] [Google Scholar]
- 15.Ledaki I, McIntyre A, Wigfield S, Buffa F, McGowan S, Baban D, Li JL, Harris AL. Carbonic anhydrase IX induction defines a heterogenous cancer cell response to hypoxia and mediates stem cell-like properties and sensitivity to HDAC inhibition. Oncotarget. 2015;6:19413–19427. doi: 10.18632/oncotarget.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conley SJ, Gheordunescu E, Kakarala P, Newman B, Korkaya H, Heath AN, Clouthier SG, Wicha MS. Antiangiogenic agents increase breast cancer stem cells via the generation of tumor hypoxia. Proc Natl Acad Sci USA. 2012;109:2784–2789. doi: 10.1073/pnas.1018866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeung TM, Gandhi SC, Bodmer WF. Hypoxia and lineage specification of cell line-derived colorectal cancer stem cells. Proc Natl Acad Sci USA. 2011;108:4382–4387. doi: 10.1073/pnas.1014519107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheikine Y, Genega E, Melamed J, Lee P, Reuter VE, YE H. Molecular genetics of testicular germ cell tumors. Am J Cancer Res. 2012;2:153–167. [PMC free article] [PubMed] [Google Scholar]
- 19.Ulbright TM, Amin MB, Balzer B, Berney DM, Epstein JI, Guo C, Idrees MT, Looijenga LHJ, Paner G, et al. Germ cell tumors. In: Moch H, Humphrey PA, Reuter VE, Ulbright TM, editors. WHO Classification of tumours of the urinary system and male genital organs. IARC Press; Lyon: 2016. pp. 185–258. [Google Scholar]
- 20.Barbagallo F, Paronetto MP, Franco R, Chieffi P, Dolci S, Fry AM, Geremia R, Sette C. Increased expression and nuclear localization of the centrosomal kinase Nek2 in human testicular seminomas. J Pathol. 2009;217:431–441. doi: 10.1002/path.2471. [DOI] [PubMed] [Google Scholar]
- 21.Ulisse S, Baldini E, Mottolese M, Sentinelli S, Gargiulo P, Valentina B, Sorrenti S, Di Benedetto A, De Antoni E, D'Armiento M. Increased expression of urokinase plasminogen activator and its cognate receptor in human seminomas. BMC Cancer. 2010;10:151. doi: 10.1186/1471-2407-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mego M, Cierna Z, Svetlovska D, Macak D, Machalekova K, Miskovska V, Chovanec M, Usakova V, Obertova J, Babal P, Mardiak J. PARP expression in germ cell tumours. J Clin Pathol. 2013;66:607–612. doi: 10.1136/jclinpath-2012-201088. [DOI] [PubMed] [Google Scholar]
- 23.Takacova M, Bullova P, Simko V, Skvarkova L, Poturnajova M, Feketeova L, Babal P, Kivela AJ, Kuopio T, Kopacek J, et al. Expression pattern of carbonic anhydrase IX in medullary thyroid carcinoma supports a role for ret-mediated activation of the HIF pathway. Am J Pathol. 2014;184:953–965. doi: 10.1016/j.ajpath.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Pastoreková S, Závadová Z, Kostál M, Babusíková O, Závada J. A novel quasi-viral agent, MaTu, is a two-component system. Virology. 1992;187:620–626. doi: 10.1016/0042-6822(92)90464-Z. [DOI] [PubMed] [Google Scholar]
- 25.Hintze J. NCSS 2007. NCSS, LLC, Kaysville, UT. 2007 [Google Scholar]
- 26.Tafreshi NK, Lloyd MC, Bui MM, Gillies RJ, Morse DL. Carbonic anhydrase IX as an imaging and therapeutic target for tumors and metastases. Subcell Biochem. 2014;75:221–254. doi: 10.1007/978-94-007-7359-2_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liao SY, Lerman MI, Stanbridge EJ. Expression of transmembrane carbonic anhydrases, CA IX and CAXII, in human development. BMC Dev Biol. 2009;922 doi: 10.1186/1471-213X-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donato DP, Johnson MT, Yang XJ, Zynger DL. Expression of carbonic anhydrase IX in genitourinary and adrenal tumours. Histopathology. 2011;59:1229–1239. doi: 10.1111/j.1365-2559.2011.04074.x. [DOI] [PubMed] [Google Scholar]
- 29.van Echten J, Oosterhuis JW, Looijenga LH, van de Pol M, Wiersema J, te Meerman GJ, Koops H Schaffordt, Sleijfer DT, de Jong B. No recurrent structural abnormalities apart from i(12p) in primary germ cell tumors of the adult testis. Gene Chromosomes Cancer. 1995;14:133–144. doi: 10.1002/gcc.2870140208. [DOI] [PubMed] [Google Scholar]
- 30.Loncaster JA, Harris AL, Davidson SE, Logue JP, Hunter RD, Wycoff CC, Pastorek J, Ratcliffe PJ, Stratford IJ, West CM. Carbonic anhydrase (CA IX) expression, a potential new intrinsic marker of hypoxia: Correlations with tumor oxygen measurements and prognosis in locally advanced carcinoma of the cervix. Cancer Res. 2001;61:6394–6399. [PubMed] [Google Scholar]
- 31.Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, Zee B, Mo F, Teo PM, Huang DP, et al. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–2604. [PubMed] [Google Scholar]
- 32.Driessen A, Landuyt W, Pastorekova S, Moons J, Goethals L, Haustermans K, Nafteux P, Penninckx F, Geboes K, Lerut T, Ectors N. Expression of carbonic anhydrase IX (CA IX), a hypoxia-related protein, rather than vascular-endothelial growth factor (VEGF), a pro-angiogenic factor, correlates with an extremely poor prognosis in esophageal and gastric adenocarcinomas. Ann Surg. 2006;243:334–340. doi: 10.1097/01.sla.0000201452.09591.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hynninen P, Vaskivuo L, Saarnio J, Haapasalo H, Kivelä J, Pastoreková S, Pastorek J, Waheed A, Sly WS, Puistola U, Parkkila S. Expression of transmembrane carbonic anhydrases IX and XII in ovarian tumours. Histopathology. 2006;49:594–602. doi: 10.1111/j.1365-2559.2006.02523.x. [DOI] [PubMed] [Google Scholar]
- 34.Hussain SA, Rea DW, Palmer DH. Reply: Randomised studies with translational end points are required to further elucidate the prognostic and predictive value of CA IX. Br J Cancer. 2007;96:1310. doi: 10.1038/sj.bjc.6603716. [DOI] [Google Scholar]
- 35.Korkeila E, Talvinen K, Jaakkola PM, Minn H, Syrjänen K, Sundström J, Pyrhönen S. Reply: Expression of carbonic anhydrase IX suggests poor response to therapy in rectal cancer. Br J Cancer. 2009;101:373. doi: 10.1038/sj.bjc.6605151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giatromanolaki A, Koukourakis MI, Sivridis E, Turley H, Talks K, Pezzella F, Gatter KC, Harris AL. Relation of hypoxia inducible factor 1alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer. 2001;85:881–890. doi: 10.1054/bjoc.2001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene. 2010;29:6509–6521. doi: 10.1038/onc.2010.455. [DOI] [PubMed] [Google Scholar]
- 38.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 39.Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8:2032–2038. doi: 10.1021/mp200292c. [DOI] [PMC free article] [PubMed] [Google Scholar]