Abstract
Oridonin is an active constituent isolated from the traditional Chinese herb Rabdosia rubescens, which exerts antitumor effects in experimental and clinical settings. However, its antitumor effects and underlying mechanisms on prostate cancer cells have not yet been clearly identified. In the present study, the androgen-independent prostate cancer PC3 and DU145 cell lines were used as models to investigate the effects and possible mechanisms of oridonin on cellular proliferation and apoptosis. Results demonstrated that oridonin inhibited cellular proliferation, and was able to significantly induce G2/M cell cycle arrest and apoptosis. Detailed signaling pathway analysis by western blotting demonstrated that the expression levels of p53 and p21 were upregulated, whereas the expression of cyclin-dependent kinase 1 was downregulated following oridonin treatment, which led to cell cycle arrest in the G2/M phase. Oridonin also upregulated the proteolytic cleaved forms of caspase-3, caspase-9 and poly (ADP-ribose) polymerase. Furthermore, the protein expression levels of B-cell lymphoma 2 were decreased and those of Bcl-2-associated X protein were increased following oridonin treatment. In addition, oridonin treatment significantly inhibited the expression of phosphoiniositide-3 kinase (PI3K) p85 subunit and the phosphorylation of Akt. The downstream gene murine double minute 2 was also downregulated, which may contribute to the elevated expression of p53 following oridonin treatment. In conclusion, the results of the present study suggested that oridonin is able to inactivate the PI3K/Akt pathway and activate p53 pathways in prostate cancer cells, resulting in the suppression of proliferation and the induction of caspase-mediated apoptosis.
Keywords: oridonin, prostate cancer, proliferation, apoptosis, phosphoinositide 3-kinase/Akt
Introduction
Prostate cancer is one of the most common types of cancer and the second leading cause of cancer-associated mortality in men (1,2). Orchiectomy, and hormone and radiation therapy are the commonly employed modes of treatment for prostate cancer (3). However, prostate cancer begins as an androgen-dependent tumor that may eventually progress to an androgen-independent stage, which is resistant to the current treatment regimens (4,5). The loss of androgen sensitivity generally leads to the adaptation of cells to an environment without androgens, and an alternative pathway of signal transduction (6). Therefore, identifying drugs that may override androgen-independent prostate cancer cells is an important topic of study.
Oridonin is a diterpenoid isolated from the traditional Chinese herb Rabdosia rubescens, which has been widely used for thousands of years in traditional Chinese medicine (7,8) and has been reported to be useful in the treatment of several types of tumor (9,10). The preventative effects of R. rubescens are considered to be as a result of the antibacterial and anti-inflammatory functions of oridonin (11,12). Numerous studies have demonstrated that oridonin possesses antiproliferative and apoptotic activities in various cancer cells, including breast, colorectal and hepatocellular carcinoma cells (13–15). Oridonin administration has also been reported to induce growth inhibition in prostate cancer cells, mainly by inducing cellular apoptosis (16–18). However, the exact effects and the underlying mechanisms of oridonin against prostate cancer cells remain poorly characterized.
The present study examined the potential effects of oridonin on cultured PC3 and DU145 androgen-independent prostate cancer cells, and focused mainly on the underlying cellular mechanisms. The results demonstrated that oridonin was able to inactivate the phosphoiniositide-3 kinase (PI3K)/Akt pathway and activate p53 pathways to promote growth inhibition and apoptosis in prostate cancer cells, thus suggesting that oridonin may be considered a candidate antineoplastic drug for future prostate cancer therapeutics.
Materials and methods
Cell lines and cell culture
The PC3 and DU145 human prostate cancer cell lines were purchased from American Type Culture Collection (Manassas, VA, USA), and were cultured in F12 (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and minimum essential medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), respectively. The media were supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.) and 100 U/ml penicillin-streptomycin. Each cell line was maintained at 37°C in an atmosphere containing 5% CO2.
MTT assay
The viability of the cells was assessed using an MTT assay (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) according to the manufacturer's protocol. Briefly, 104 cells were collected and seeded in a 96-well plate overnight at 37°C, and then treated with oridonin (Chengdu Must Bio-Technology Co., Ltd., Sichuan, China) at the concentrations of 20, 40 and 60 µM. Following a 24, 36 or 48 h incubation at 37°C, 100 µl 0.5 mg/ml MTT was added to each well and plates were incubated at 37°C for 4 h. Following the removal of the medium, 150 µl dimethyl sulfoxide (DMSO) was added to dissolve formazan crystals and the absorbance of each well was measured using a microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a test wavelength of 490 nm, with a reference wavelength of 630 nm. Each treatment was performed in triplicate wells, and a control group consisting of cells grown in culture medium containing DMSO was included. Each experiment was repeated at least 3 times.
Flow cytometric analysis of cell cycle distribution and apoptosis
For flow cytometric analysis, 5×105 cells were cultured in 60 mm dishes overnight at 37°C. Subsequently, 10, 20 and 40 µM oridonin were used to treat PC3 cells, and 15, 30 and 60 µM oridonin were selected to treat DU145 cells. Subsequently, cells were incubated for a further 24 h at 37°C. Cells were then harvested by trypsinization, washed twice with PBS, fixed in 70% ethanol overnight at 4°C, and then incubated with 0.5 ml propidium iodide (PI)/Triton X-100 staining solution with Ribonuclease A for 30 min at room temperature. The percentage of cells in the G1, S and G2/M phases was measured with a FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA), and analyzed using CellQuest Pro software version 5.1 (BD Biosciences).
For apoptotic analysis, 5×105 cells were seeded in a 6-well plate overnight at 37°C. Then PC3 cells were treated with 10, 20 or 40 µM oridonin or DMSO, and DU145 cells were treated with 15, 30 or 60 µM oridonin or DMSO. Following a 24 h incubation at 37°C, cells were trypsinized and resuspended in 500 µl binding buffer, followed by the addition of Annexin V-fluorescein isothiocyanate (FITC)/PI into the binding buffer; the cells were then incubated in the dark for 5 min at room temperature. The labeled cells were analyzed by flow cytometry using a FACScan flow cytometer (BD Biosciences) and the CellQuest Pro software version 5.1 (BD Biosciences).
Western blot analysis
Following the indicated treatments, the cells were washed with PBS and harvested in radioimmunoprecipitation assay buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS and 50 mM Tris) containing 10 mg/ml aprotinin, 5 mg/ml leupeptin and 1 mM phenylmethylsulfonyl fluoride. Protein concentrations were determined using the Bradford-Coomassie dye binding assay. In total, 50 µg of protein was loaded and separated by 12% SDS-PAGE, and proteins were then transferred onto polyvinylidene difluoride membranes (Merck Millipore). The membranes were blocked in blocking buffer (5% milk in TBS and 0.1% Tween-20) for 1 h at room temperature and then incubated overnight at 4°C with the respective primary antibody (in 5% milk) followed by a secondary antibody. Antibodies against p53 (dilution, 1:500; #SC-47698), p21 (dilution, 1:500; #SC-817), cyclin-dependent kinase 1 (CDK1; dilution, 1:1,000; #SC-53219), β-actin (dilution, 1:2,000; #SC-47778), B-cell lymphoma 2 (Bcl-2; dilution, 1:1,000; #SC-509), Bcl-2-associated X protein (Bax; dilution, 1:1,000; #SC-20067), murine double minute 2 (MDM2; dilution, 1:500; #SC-965), goat anti-mouse immunoglobulin G(IgG)-horseradish peroxidase (HRP; dilution, 1:3,000; #SC-2005) and mouse anti-rabbit IgG-HRP (dilution, 1:5,000; #SC-2357) were purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA). Antibodies against PI3K (dilution, 1:1,000; #4292), phosphorylated (p)-Akt (dilution, 1:1,000; #9271), Akt (dilution, 1:1,000; #9272), cleaved caspase-3 (dilution, 1:1,000; #9661), cleaved caspase-9 (dilution 1:1,000; #9501) and cleaved poly (ADP-ribose) polymerase (PARP; dilution, 1:1,000; #9541) were purchased from Cell Signaling Technology Inc., (Danvers, MA, USA). Immunoblots were developed using the enhanced chemiluminescence western blot substrate kit (Pierce; Thermo Fisher Scientific, Inc.) and were exposed to Kodak BioMax MR film (Kodak, Rochester, NY, USA). Equal protein loading was confirmed by probing blots with a β-actin antibody. Image J software (version 1.42q; National Institutes of Health, Bethesda, MD, USA) was used to quantify band intensities.
Statistical analysis
Data are presented as the mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance followed by Dunnett's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Oridonin suppresses prostate cancer cell proliferation
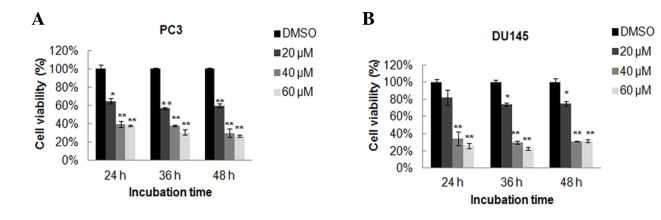
To investigate the possible effects of oridonin on prostate cancer cells, the PC3 and DU145 cells were treated with oridonin, and its effect on cell proliferation was determined by MTT assay. Results demonstrated that oridonin effectively inhibited cell proliferation in a dose-dependent manner in the two cell lines (Fig. 1). However, the two prostate cancer cell lines exhibited different sensitivity to oridonin treatment. It appeared that PC3 cells were more sensitive to oridonin treatment than DU145 cells. A significant inhibitory effect was observed at 24 h post-treatment with 20 µM oridonin in PC3 cells (P<0.05), whereas DU145 cells treated with 20 µM oridonin exhibited a slight, non-significant decrease in cell viability, compared with the DMSO control group. Nevertheless, high doses of oridonin (40 or 60 µM) produced significant toxicity to the cells even following a short exposure in PC3 and DU145 cells (P<0.01 vs. the DMSO control group). The half-maximal inhibitory concentration values of oridonin at 24 h were ~30 and 35 µM, respectively in PC3 and DU145 cells. To avoid the toxicity of high concentrations of oridonin in prostate cancer cells, we treated the cells with varying concentrations of oridonin according to their respective sensitivity to oridonin treatment. In the subsequent assays, 10, 20 and 40 µM oridonin were used to treat PC3 cells, whereas 15, 30 and 60 µM oridonin were selected to treat DU145 cells.
Figure 1.
Oridonin inhibits the proliferation of prostate cancer cells. (A) PC3 cells and (B) DU145 cells were treated with 20, 40 or 60 µM oridonin or DMSO for 24, 36 or 48 h. Cell viability was determined by MTT assay. Data are presented as the mean ± standard deviation from 3 independent experiments. *P<0.05, **P<0.01 vs. the DMSO control group. DMSO, dimethyl sulfoxide.
Oridonin induces cell cycle arrest at G2/M phase in prostate cancer cells
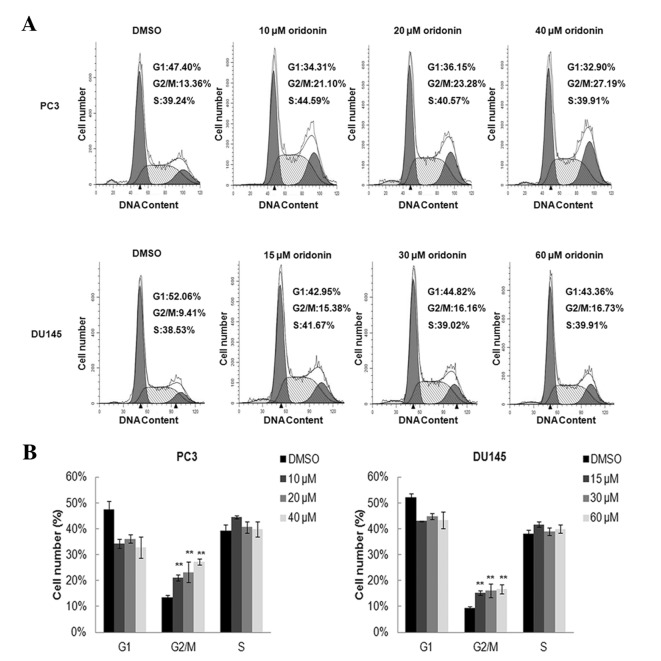
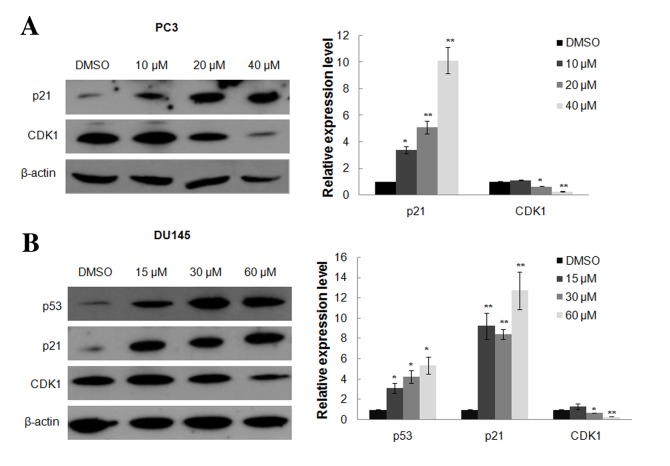
Based on the growth inhibition detected in PC3 and DU145 cells in response to oridonin treatment, the effects of oridonin on cell cycle regulation were investigated. The two cell lines treated with either 0.1% DMSO or various concentration of oridonin for 24 h were analyzed by flow cytometry. The representative histograms of cell cycle distribution in the control, 10, 20 and 40 µM oridonin-treated PC3 cells and 15, 30 and 60 µM oridonin-treated DU145 cells are presented in Fig. 2A. Oridonin treatment induced marked G2/M arrest even in cells treated with a low dose of oridonin (10 µM for PC3 cells or 15 µM for DU145 cells). The percentage of cells in the G2/M phase increased from 13.36±0.91 to 27.19±1.15% in PC3 cells treated with 40 µM oridonin, and from 9.41±0.57 to 16.73±1.79% in DU145 cells treated with 60 µM oridonin (P<0.01; Fig. 2B). The expression of cell cycle-related proteins, including p53, p21 and CDK1, were detected by western blot analysis. Results demonstrated that p21 expression was markedly upregulated following oridonin treatment, whereas CDK1 expression was decreased, particularly in PC3 cells treated with 40 µM oridonin and DU145 cells treated with 60 µM oridonin compared with the DMSO control group (P<0.01; Fig. 3A and B). Oridonin also elevated p53 protein level in p53 mutant DU145 cells (P<0.05 vs. the DMSO control group; Fig. 3B).
Figure 2.
Oridonin induces G2/M cell cycle arrest in prostate cancer cells. PC3 and DU145 cells were treated with various concentrations of oridonin or DMSO for 24 h and were then stained with propidium iodide. DNA contents were analyzed by flow cytometry. (A) Results are representative of 3 independent experiments. (B) Proportions of G1, S and G2/M phase cells are indicated and presented as the mean ± standard deviation from 3 independent experiments. **P<0.01 vs. the DMSO control group. DMSO, dimethyl sulfoxide.
Figure 3.
Oridonin treatment induces alterations in the expression of cell cycle regulators. (A) PC3 and (B) DU145 cells were treated with various concentrations of oridonin or DMSO for 24 h, and were then collected and subjected to western blot analysis. β-actin was used as a protein-loading control. The representative western blotting figures (left) and the densitometric analysis (right) of the expression levels of p53, p21 and CDK1 are presented. *P<0.05, **P<0.01 vs. the DMSO control group. DMSO, dimethyl sulfoxide; CDK1, cyclin-dependent kinase 1.
Oridonin induces apoptosis of prostate cancer cells
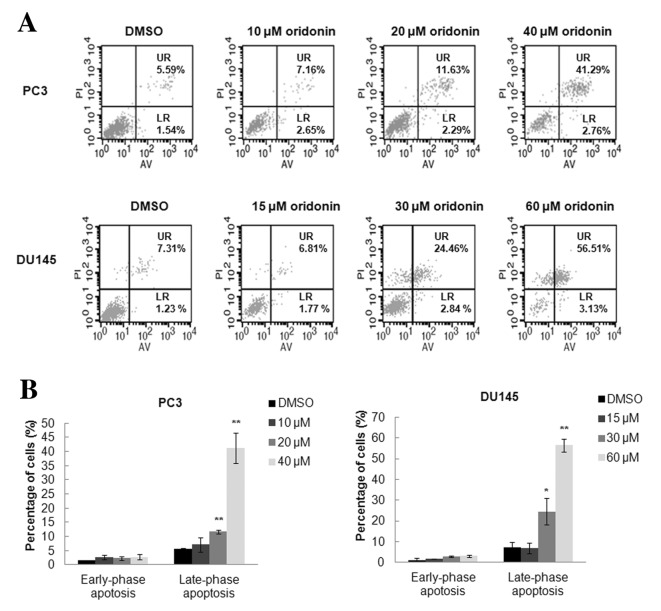
To investigate the occurrence of apoptosis following oridonin treatment, flow cytometric analysis was performed using Annexin V-FITC/PI double staining. Results demonstrated that treatment with oridonin for 24 h significantly induced late-phase apoptosis but only had a slight effect on early-phase apoptosis. Fig. 4A demonstrates representative results of 3 independent experiments. The percentage of PC3 cells in late-phase apoptosis was significantly increased following treatment with 20 µM (11.63±0.74%) or 40 µM (41.29±5.31%) oridonin compared with the control group (5.59±0.21%; both P<0.01). The percentage of DU145 cells in late-phase apoptosis was also increased following treatment with 30 µM (24.46±6.39%; P<0.05) or 60 µM (56.51±3.05%; P<0.01) oridonin compared with in the control group (7.31±2.38%) (Fig. 4B).
Figure 4.
Oridonin induces apoptosis of prostate cancer cells. PC3 and DU145 cells were treated with various concentrations of oridonin or DMSO for 24 h and then underwent Annexin V-fluorescein isothiocyanate/propidium iodide double staining. Cell apoptosis was analyzed by flow cytometry. (A) Results are representative of 3 independent experiments. (B) Percentage of apoptotic cells is presented as the mean ± standard deviation from 3 independent experiments. *P<0.05, **P<0.01 vs. the DMSO control group. DMSO, dimethyl sulfoxide; UR, late-phase apoptotic cells; LR, early-phase apoptotic cells; AV, Annexin V; PI, propidium iodide.
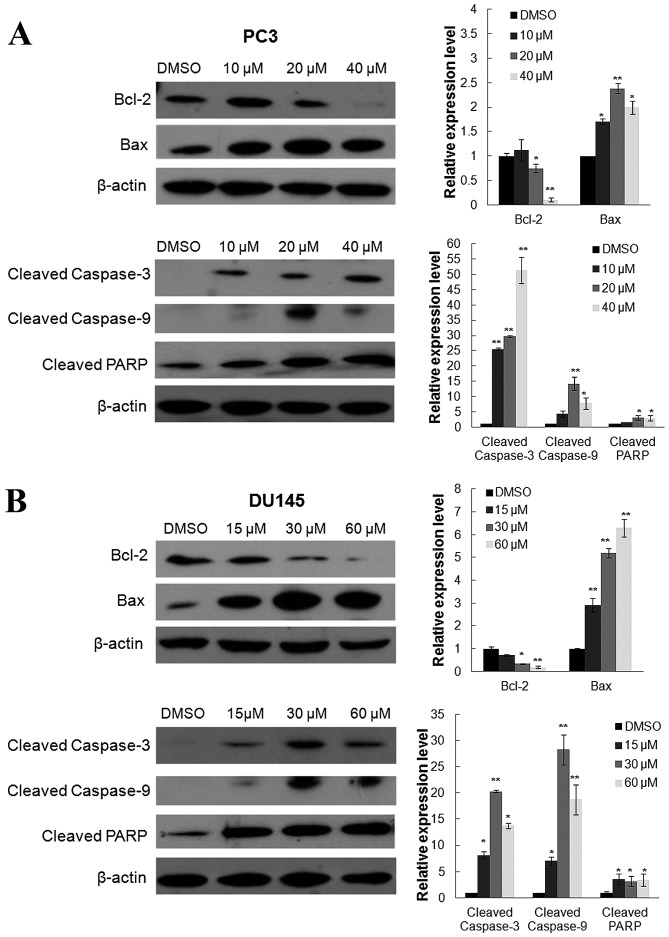
Oridonin activates caspase family proteins
Since the aforementioned results indicated that oridonin was able to induce apoptosis in prostate cancer cells, the present study investigated its effects on the expression of apoptotic proteins, Bax and Bcl-2, by western blot analysis. As presented in Fig. 5A and B, the expression levels of proapoptotic protein Bax were significantly increased in PC3 and DU145 cells following oridonin treatment for 24 h compared with the control. However, the expression levels of the anti-apoptotic protein Bcl-2 were markedly decreased in the two cell lines. In addition, to elucidate the molecular mechanisms underlying oridonin-induced apoptosis in prostate cancer cells, the pivotal initiators and executors of apoptosis (caspase-3, caspase-9 and PARP) were investigated. Compared with the DMSO control, oridonin treatment significantly altered the expression of cleaved caspase-3, −9 and PARP (Fig. 5). In PC3 cells, the cleaved caspase-3 expression was significantly increased following treatment with 10, 20 or 40 µM oridonin, with 25.55-, 29.66- or 51.33-fold increases, respectively, when compared with the control group (all P<0.01). The cleaved caspase-9 (14.28-fold increase; P<0.01) and PARP (3.12-fold increase; P<0.05) levels peaked following treatment with 20 µM oridonin, which was followed by a decrease after treatment with 40 µM oridonin (cleaved caspase-9, 7.80-fold decrease; PARP, 2.95-fold decrease; both P<0.05) compared with the DMSO control group. In DU145 cells, the cleaved PARP level was markedly upregulated following oridonin treatment. The cleaved caspase-3 (20.30-fold increase) and −9 (28.23-fold increase) levels peaked following treatment with 30 µM oridonin (both P<0.01), which was followed by a decrease after treatment with 60 µM oridonin (cleaved caspase-3, 13.80-fold decrease; P<0.05; cleaved caspase-3, 18.75-fold decrease; P<0.01) compared with the DMSO control group.
Figure 5.
Effects of oridonin treatment on apoptotic protein and caspase family protein expression. (A) PC3 and (B) DU145 cells were treated with various concentrations of oridonin or DMSO for 24 h, and were then collected and subjected to western blot analysis. β-actin was used as a protein-loading control. The representative figures (left) and the densitometric analysis (right) of the expression levels of Bcl-2, Bax, cleaved caspase-3, −9 and PARP are presented. *P<0.05, **P<0.01 vs. the DMSO control group. DMSO, dimethyl sulfoxide; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; PARP, poly (ADP-ribose) polymerase.
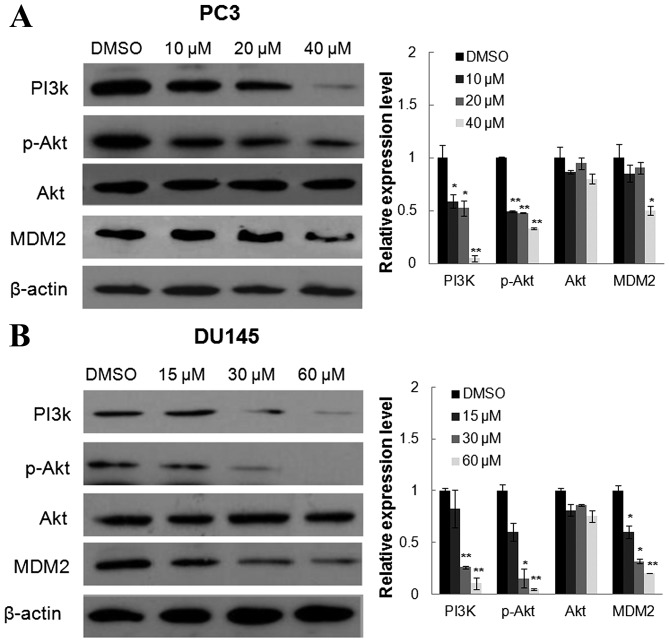
Oridonin inhibits the PI3K/Akt pathway
To determine whether the PI3K/Akt survival pathway is involved in the anticancer effects of oridonin, the present study examined the protein expression of PI3K p85 subunit and the phosphorylation of Akt following oridonin treatment. As shown in Fig. 6, oridonin significantly decreased the levels of PI3K p85 subunit and the phosphorylation of Akt at Ser473 sites, however there was no significant difference in the expression of total Akt. The present study also examined the effects of oridonin on MDM2 expression. MDM2 is an Akt downstream target and a mediator of the degradation of p53 protein. The protein levels of MDM2 were decreased following treatment with oridonin, which may contribute to the accumulation of the p53 protein.
Figure 6.
Concentration-dependent effects of oridonin on protein expression levels of PI3K, Akt, p-Akt and MDM2. (A) PC3 and (B) DU145 cells were treated with various concentrations of oridonin or DMSO for 24 h, and were then collected and subjected to western blot analysis. β-actin was used as a protein-loading control. The representative figures and the densitometric analysis of the expression levels of PI3K, p-Akt, Akt and MDM2 are presented. *P<0.05, **P<0.01 vs. the DMSO control group. DMSO, dimethyl sulfoxide; PI3K, phosphoinositide 3-kinase; p, phosphorylated; MDM2, murine double minute 2.
Discussion
Previous studies have demonstrated that oridonin exerts anticancer properties in various types of human cancer, including breast, colorectal and hepatocellular cancer (13–15). The present study demonstrated that oridonin inhibited the proliferation of PC3 and DU145 prostate cancer cells and induced cell cycle arrest in the G2/M phase. In addition, the present study investigated the expression of proteins involved in cell cycle progression, including CDK1 and p21 by western blot analysis. The results indicated that oridonin-induced G2/M phase arrest was at least partially caused by deactivation of CDK1 and upregulation of p21. CDK1 transcription is normally stable throughout the cell cycle, but is modulated in some pathological conditions (19).
It has previously been reported that p53 negatively regulates CDK1 transcription (20). In response to stimuli, p53 also transactivates p21, which directly binds to and inhibits the activity of the cyclin B1/CDK1 complex, thus inducing G2/M phase arrest (21). PC3 and DU145 cells exhibit varying p53 statuses: PC3 is p53 null, whereas DU145 is a p53 mutant (22). Following oridonin treatment for 24 h, the present study detected a marked increase in p53 protein levels in DU145 cells. A previous study reported that alterations in the p53 gene in tumor cells result in defective checkpoint function (22). The present results suggested that oridonin may induce G2-checkpoint arrest in prostate cancer cells irrespective of p53 status. In addition, oridonin could induce apoptosis of arrested prostate cancer cells.
The majority of the currently used anticancer drugs mediate their effects via the induction of apoptosis in cancer cells; therefore, apoptotic induction is considered one of the major mechanisms for the targeted therapy of various types of cancer, including prostate cancer (23). Apoptosis is largely initiated either from mitochondria (the intrinsic pathway) or through cell death receptors (the extrinsic pathway), followed by recruitment of the caspase cascade, ultimately resulting in apoptosis. The present study focused on whether the mitochondria-dependent pathway was involved in oridonin-induced apoptosis. In PC3 and DU145 cells, oridonin upregulated the expression of the proapoptotic protein Bax, and decreased Bcl-2 expression. An increase in the Bax/Bcl-2 ratio activates caspase-9 and its downstream effectors caspase-3 and PARP; these findings clearly indicated that the mitochondrial signaling pathway is involved in oridonin-induced apoptosis of prostate cancer cells. However, the active caspase-9 was downregulated, whereas the active caspase-3 was still increased following treatment with 40 µM oridonin in PC3 cells. It is probable that other upstream caspases, such as caspase-8 and caspase-10, activate caspase-3 and induce cell apoptosis through an extrinsic apoptotic pathway in prostate cancer cells. Previous studies have reported that oridonin may induce cell apoptosis through the mitochondria-mediated intrinsic and the death receptor-mediated extrinsic pathway in laryngeal and breast cancer cells (24,25).
Among the pathways associated with apoptotic resistance, the PI3K/Akt pathway is considered the convergent point for various stimuli generated at the cell surface (26,27). The PI3K/Akt pathway can be activated by growth factors and specific extracellular signals, and is associated with the regulation of cell proliferation, differentiation and apoptosis (28–30). There is substantial evidence to suggest that activation of the PI3K/Akt pathway is a common occurrence in advanced prostate cancer (31–33). It has previously been reported that PI3K/Akt is involved in oridonin-induced apoptosis in numerous cancer cells (34,35). The present results demonstrated that Akt is constitutively phosphorylated at Ser473 sites in the 2 prostate cancer cells, and that oridonin may decrease the expression of PI3K p85 subunit and the phosphorylation of Akt at Ser473 sites. As the downstream target of Akt, MDM2 was also decreased following treatment with oridonin. MDM2 serves as an E3 ubiquitin ligase that conjugates a chain of ubiquitin molecules onto p53, targeting p53 for proteasome-mediated degradation (36–38). In addition, MDM2 binding with a region of p53 that overlaps with its transactivation domain, may suppress p53 activity (39–41). Therefore, in response to oridonin treatment, inactivation of PI3K/Akt was accompanied by downregulation of MDM2 and the accumulation of transcriptionally active p53. These results suggested that the PI3K/Akt and p53 pathways may be involved in oridonin-induced G2/M arrest and apoptosis in prostate cancer cells.
In conclusion, oridonin induces inactivation of the PI3K/Akt signaling pathway in prostate cells, which mediates inhibition of proliferation and induction of apoptosis (Fig. 7). Within the context of oridonin-treated DU145 cells, PI3K/Akt inactivation facilitates the accumulation of p53 protein through the downregulation of MDM2. Subsequently, p53 accumulation triggers G2/M phase arrest by upregulating p21 and inhibiting the activity of the cyclin B1/CDK1 complex or negatively regulating CDK1 transcription. Conversely, p53 could also initiate apoptosis by upregulating Bax whilst downregulating Bcl-2 expression, which contributes to the activation of caspase-9, caspase-3 and PARP, and additionally results in apoptotic occurrence. Oridonin may also induce G2/M phase arrest and trigger apoptosis in a p53-independent manner, predominantly via the PI3K/Akt-mediated p21 pathway and the PI3K/Akt-mediated Bax pathway. The present study hypothesized that oridonin may effectively inhibit cell proliferation and induce apoptosis via multiple pathways in prostate cancer cells, thus suggesting it may be used as a valuable component in therapeutic strategies to treat prostate cancer.
Figure 7.
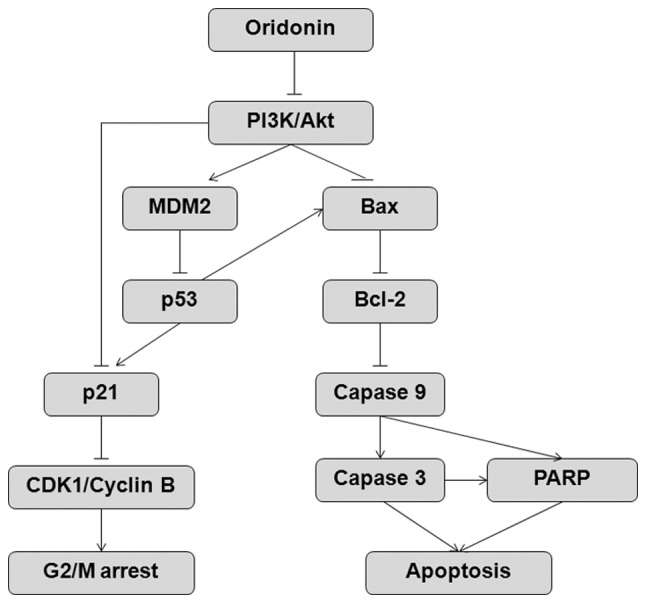
The proposed mechanism for oridonin-induced G2/M arrest and apoptosis in prostate cancer cells. PI3K, phosphoinositide 3-kinase; MDM2, murine double minute 2; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X protein; CDK1, cyclin-dependent kinase 1; PARP, poly (ADP-ribose) polymerase.
Acknowledgements
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81502303, 81071525, 81372320 and 81570804), the Natural Science Foundation of Jiangsu Educational Committee (grant no. 15KJB310006), the Key Laboratory for Laboratory Medicine of Jiangsu Province of China (grant no. XK201114), the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, and the Key Project of Science and Technology Development Foundation of Nanjing Medical University (grant no. 2014NJMUZD009).
References
- 1.Hammond LA, Brown G, Keedwell RG, Durham J, Chandraratna RA. The prospects of retinoids in the treatment of prostate cancer. Anticancer Drugs. 2002;13:781–790. doi: 10.1097/00001813-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 3.Abdollah F, Sun M, Thuret R, Jeldres C, Tian Z, Briganti A, Shariat SF, Perrotte P, Rigatti P, Montorsi F, Karakiewicz PI. A competing-risks analysis of survival after alternative treatment modalities for prostate cancer patients: 1988–2006. Eur Urol. 2011;59:88–95. doi: 10.1016/j.eururo.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Smith MR, Nelson JB. Future therapies in hormone-refractory prostate cancer. Urology. 2005;65(5 Suppl):S9–S17. doi: 10.1016/j.urology.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 5.Kibel AS. An interdisciplinary approach to treating prostate cancer. Urology. 2005;65(6 Suppl):S13–S18. doi: 10.1016/j.urology.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 6.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 7.Marks LS, DiPaola RS, Nelson P, Chen S, Heber D, Belldegrun AS, Lowe FC, Fan J, Leaders FE, Jr, Pantuck AJ, Tyler VE. PC-SPES: Herbal formulation for prostate cancer. Urology. 2002;60:369–377. doi: 10.1016/S0090-4295(02)01913-1. [DOI] [PubMed] [Google Scholar]
- 8.de la Taille A, Hayek OR, Burchardt M, Burchardt T, Katz AE. Role of herbal compounds (PC-SPES) in hormone-refractory prostate cancer: Two case reports. J Altern Complement Med. 2000;6:449–451. doi: 10.1089/acm.2000.6.449. [DOI] [PubMed] [Google Scholar]
- 9.Hsiao WL, Liu L. The role of traditional Chinese herbal medicines in cancer therapy-from TCM theory to mechanistic insights. Planta Med. 2010;76:1118–1131. doi: 10.1055/s-0030-1250186. [DOI] [PubMed] [Google Scholar]
- 10.Salminen A, Lehtonen M, Suuronen T, Kaarniranta K, Huuskonen J. Terpenoids: Natural inhibitors of NF-kappaB signaling with anti-inflammatory and anticancer potential. Cell Mol Life Sci. 2008;65:2979–2999. doi: 10.1007/s00018-008-8103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Y, Xue Y, Wang Y, Feng D, Lin S, Xu L. Multiple-modulation effects of Oridonin on the production of proinflammatory cytokines and neurotrophic factors in LPS-activated microglia. Int Immunopharmacol. 2009;9:360–365. doi: 10.1016/j.intimp.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z, Ouyang L, Peng H, Zhang WZ. Oridonin: Targeting programmed cell death pathways as an anti-tumour agent. Cell Prolif. 2012;45:499–507. doi: 10.1111/j.1365-2184.2012.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Y, Zhang T, Li J, Deng H, Song Y, Zhai D, Peng Y, Lu X, Liu M, Zhao Y, Yi Z. Oridonin inhibits tumor growth and metastasis through anti-angiogenesis by blocking the Notch signaling. PLoS One. 2014;9:e113830. doi: 10.1371/journal.pone.0113830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin H, Tan X, Liu X, Ding Y. Downregulation of AP-1 gene expression is an initial event in the oridonin-mediated inhibition of colorectal cancer: Studies in vitroin vivo. J Gastroenterol Hepatol. 2011;26:706–715. doi: 10.1111/j.1440-1746.2010.06500.x. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Ye Y, Pan SY, Zhu GY, Li YW, Fong DW, Yu ZL. Proteomic identification of proteins involved in the anticancer activities of oridonin in HepG2 cells. Phytomedicine. 2011;18:163–169. doi: 10.1016/j.phymed.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Zhang X, Xue W, Yangyang Y, Xu D, Zhao Y, Lou H. Effects of oridonin nanosuspension on cell proliferation and apoptosis of human prostatic carcinoma PC-3 cell line. Int J Nanomedicine. 2010;5:735–742. doi: 10.2147/IJN.S13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Dou QP. Targeting apoptosis pathway with natural terpenoids: Implications for treatment of breast and prostate cancer. Curr Drug Targets. 2010;11:733–744. doi: 10.2174/138945010791170842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Li X, Wang J, Ye Z, Li JC. Oridonin up-regulates expression of P21 and induces autophagy and apoptosis in human prostate cancer cells. Int J Biol Sci. 2012;8:901–912. doi: 10.7150/ijbs.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castedo M, Perfettini JL, Roumier T, Kroemer G. Cyclin-dependent kinase-1: Linking apoptosis to cell cycle and mitotic catastrophe. Cell Death Differ. 2002;9:1287–1293. doi: 10.1038/sj.cdd.4401130. [DOI] [PubMed] [Google Scholar]
- 20.Yun J, Chae HD, Choy HE, Chung J, Yoo HS, Han MH, Shin DY. p53 negatively regulates cdc2 transcription via the CCAAT-binding NF-Y transcription factor. J Biol Chem. 1999;274:29677–29682. doi: 10.1074/jbc.274.42.29677. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera S, Ikejima T. Oridonin induces G2/M arrest and apoptosis via activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK survival pathway in murine fibrosarcoma L929 cells. Arch Biochem Biophys. 2009;490:70–75. doi: 10.1016/j.abb.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 22.Mad'arová J, Lukesová M, Hlobilková A, Strnad M, Vojtesek B, Lenobel R, Hajdúch M, Murray PG, Perera S, Kolár Z. Synthetic inhibitors of CDKs induce different responses in androgen sensitive and androgen insensitive prostatic cancer cell lines. Mol Pathol. 2002;55:227–234. doi: 10.1136/mp.55.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Zhu D, Lou Y. A novel anticancer agent, icaritin, induced cell growth inhibition, G1 arrest and mitochondrial transmembrane potential drop in human prostate carcinoma PC-3 cells. Eur J Pharmacol. 2007;564:26–36. doi: 10.1016/j.ejphar.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 24.Kang N, Zhang JH, Qiu F, Tashiro S, Onodera S, Ikejima T. Inhibition of EGFR signaling augments oridonin-induced apoptosis in human laryngeal cancer cells via enhancing oxidative stress coincident with activation of both the intrinsic and extrinsic apoptotic pathways. Cancer Lett. 2010;294:147–158. doi: 10.1016/j.canlet.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 25.Wang S, Zhong Z, Wan J, Tan W, Wu G, Chen M, Wang Y. Oridonin induces apoptosis, inhibits migration and invasion on highly-metastatic human breast cancer cells. Am J Chin Med. 2013;41:177–196. doi: 10.1142/S0192415X13500134. [DOI] [PubMed] [Google Scholar]
- 26.Diaz-Montero CM, Wygant JN, McIntyre BW. PI3-K/Akt-mediated anoikis resistance of human osteosarcoma cells requires Src activation. Eur J Cancer. 2006;42:1491–1500. doi: 10.1016/j.ejca.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 27.Kim BJ, Lee YS, Lee SY, Park SY, Dieplinger H, Ryu SH, Yea K, Choi S, Lee SH, Koh JM, Kim GS. Afamin secreted from nonresorbing osteoclasts acts as a chemokine for preosteoblasts via the Akt-signaling pathway. Bone. 2012;51:431–440. doi: 10.1016/j.bone.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 28.Cantrell DA. Phosphoinositide 3-kinase signalling pathways. J Cell Sci. 2001;114:1439–1445. doi: 10.1242/jcs.114.8.1439. [DOI] [PubMed] [Google Scholar]
- 29.Liang D, Xiang L, Yang M, Zhang X, Guo B, Chen Y, Yang L, Cao J. ZnT7 can protect MC3T3-E1 cells from oxidative stress-induced apoptosis via PI3K/Akt and MAPK/ERK signaling pathways. Cell Signal. 2013;25:1126–1135. doi: 10.1016/j.cellsig.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Kitase Y, Barragan L, Qing H, Kondoh S, Jiang JX, Johnson ML, Bonewald LF. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J Bone Miner Res. 2010;25:2657–2668. doi: 10.1002/jbmr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shukla S, Maclennan GT, Hartman DJ, Fu P, Resnick MI, Gupta S. Activation of PI3K-Akt signaling pathway promotes prostate cancer cell invasion. Int J Cancer. 2007;121:1424–1432. doi: 10.1002/ijc.22862. [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Zhu G, Getzenberg RH, Veltri RW. The upregulation of PI3K/Akt and MAP kinase pathways is associated with resistance of microtubule-targeting drugs in prostate cancer. J Cell Biochem. 2015;116:1341–1349. doi: 10.1002/jcb.25091. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Dong X. Complex impacts of PI3K/AKT inhibitors to androgen receptor gene expression in prostate cancer cells. PLoS One. 2014;9:e108780. doi: 10.1371/journal.pone.0108780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin S, Shen JN, Wang J, Huang G, Zhou JG. Oridonin induced apoptosis through Akt and MAPKs signaling pathways in human osteosarcoma cells. Cancer Biol Ther. 2007;6:261–268. doi: 10.4161/cbt.6.2.3621. [DOI] [PubMed] [Google Scholar]
- 35.Hu HZ, Yang YB, Xu XD, Shen HW, Shu YM, Ren Z, Li XM, Shen HM, Zeng HT. Oridonin induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa cell line. Acta Pharmacol Sin. 2007;28:1819–1826. doi: 10.1111/j.1745-7254.2007.00667.x. [DOI] [PubMed] [Google Scholar]
- 36.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 37.Honda R, Tanaka H, Yasuda H. Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53. FEBS Lett. 1997;420:25–27. doi: 10.1016/S0014-5793(97)01480-4. [DOI] [PubMed] [Google Scholar]
- 38.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 39.Momand J, Zambetti GP, Olson DC, George D, Levine AJ. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell. 1992;69:1237–1245. doi: 10.1016/0092-8674(92)90644-R. [DOI] [PubMed] [Google Scholar]
- 40.Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993;362:857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- 41.Thut CJ, Goodrich JA, Tjian R. Repression of p53-mediated transcription by MDM2: A dual mechanism. Genes Dev. 1997;11:1974–1986. doi: 10.1101/gad.11.15.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]