Abstract
Epidermal growth factor receptor (EGFR) mutations are more common in non-small cell lung cancer (NSCLC) and in female patients of East Asian origin. Therefore, the present study investigated the presence of EGFR mutations in advanced NSCLC, and assessed its correlation with clinicopathologic factors, including the expression of estrogen receptor-β (ER-β) and patient prognosis. The present study performed a retrospective analysis of 83 patients with stage IIIB-IV NSCLC. The expression of ER-β and p53 were examined using immunohistochemical methods. EGFR mutations were evaluated using the amplification refractory mutation system. The expression of ER-β and p53 were detected in 37 (45.6%) and 48 (57.8%) of the patient tumors, respectively. EGFR mutations were identified in 36 (45.4%) cases. EGFR mutations were more frequently observed in ER-β-negative tumors (26/46; 56.5%), compared with ER-β-positive tumors (10/37; 27%). The expression of ER-β was significantly associated with EGFR mutations with an odds ratio (OR) of 0.241 (P=0.029). However, no significant correlation was observed between the expression of p53 and mutations in EGFR (OR=1.792; P=0.340). In addition, the expression of ER-β and lymph node metastasis were associated with poor prognosis, whereas EGFR mutations were significantly associated with favorable prognosis in terms of progression-free survival rates. However, there was no prognostic significance associated with the expression of p53. In conclusion, the expression of ER-β was significantly correlated with the presence of EGFR mutations. The expression of ER-β and mutations of EGFR were found to be prognostic factors for survival rates in patients with advanced NSCLC.
Keywords: epidermal growth factor receptor, estrogen receptor-β, non-small cell lung cancer, mutation, p53
Introduction
Lung cancer is a leading cause of cancer-associated mortality worldwide (1) and is difficult to cure, with a 5-year survival rate of only 1–26% (2). Although tobacco smoking is the predominant risk factor for lung cancer, ~25% of lung cancer cases are not attributable to tobacco use (3). The increase in lung cancer appears to be a consequence of a marked increase in the prevalence of non-small cell lung cancer (NSCLC). NSCLC is more common in females, is predominantly of the adenocarcinoma cell type, and has less of an association with smoking habits, compared with other histological subtypes of lung cancer (4–6). These clinicopathologic characteristics suggest that gender-dependent factors are involved in the cause and prognosis of NSCLC.
Previous laboratory (2,7–9) and clinical studies (10–13) have reported that estrogen affects the differentiation and maturation of the normal lung, and stimulates lung tumor growth in particular NSCLC tumors (8,14,15). Epidemiological studies have also suggested that exogenous and endogenous estrogen affects the development of lung cancer (16). Several studies have provided evidence to support a biological role for estrogens in lung carcinogenesis by the direct promotion of NSCLC cell proliferation through estrogen receptor (ER)-mediated signaling (2,17,18). ERs, including ER-α and ER-β, have been shown to be expressed in normal lung tissue and in lung carcinoma, particularly adenocarcinoma (8,19,20). The extent to which ER is expressed in lung tissue remains the subject of controversy, with minimal to almost ubiquitous expression previously reported (21).
In addition to transcriptional activation of estrogen-responsive genes, estrogen has been reported to transactivate growth factor signaling pathways, including the epidermal growth factor receptor (EGFR) pathway. This ER-EGFR signaling axis appears to be reciprocal, with EGFR signaling enhancing the activation of ER, and ER signaling enhancing that of EGFR (22). Following the observation of somatic EGFR mutations in NSCLC, several studies have reported higher mutation frequencies associated with adenocarcinoma, patients of East Asian ethnicity, women and non-smokers (23,24). Given the gender bias in the prevalence of EGFR mutations, interactions between the ER and EGFR pathways have been the subject of extensive investigation (12,25–27). However, the majority of studies have focused on stage I–III NSCLC, and few have examined their association with more advanced stages of this disease. In addition, the findings from these studies remain inconsistent.
The aim of the present study was to examine the frequency of EGFR gene mutations in advanced NSCLC, and to assess its correlation with clinicopathologic factors, including the expression of ER-β and patient prognosis.
Materials and methods
Patients
In the present study, a retrospective analysis of a total of 83 patients with advanced NSCLC was performed. The samples analyzed included 17 surgical specimens, 12 lung biopsy specimens, 15 bronchoscopic biopsy specimens, 24 pleural effusion specimens, 13 lymph node biopsy specimens and two bone biopsy specimens. These specimens were fresh frozen or tumors embedded in paraffin blocks. All patients were diagnosed as stage IIIB-IV according to the 1997 revised tumor-node-metastasis classification system of the International Union against Cancer (28). Tumor specimens were collected from the Anhui Provincial Hospital (Hefei, China) between August 2011 and August 2013. Written informed consent was provided by all patients. Approval was obtained from the institutional review board and ethics committee of Anhui Provincial Hospital. The clinical features of the patients are listed in Table I.
Table I.
Characteristics of the patients, according to EGFR status.
| Characteristic | Number (%) (n=83) | EGFR wild-type n (%) | EGFR mutant n (%) | P-value |
|---|---|---|---|---|
| Age (years) | 0.580 | |||
| ≤64 | 46 (55.4) | 26 (31.3) | 20 (24.1) | |
| >64 | 37 (45.6) | 21 (25.3) | 16 (19.3) | |
| Gender | <0.001 | |||
| Female | 30 (36.1) | 8 (9.6) | 22 (26.5) | |
| Male | 53 (63.9) | 39 (47) | 14 (16.9) | |
| Differentiation | 0.212 | |||
| Well | 25 (30.1) | 12 (14.5) | 13 (15.7) | |
| Poor | 58 (69.9) | 35 (42.2) | 23 (27.7) | |
| Smoking status | <0.001 | |||
| Never smoked | 38 (45.8) | 13 (15.7) | 25 (30.1) | |
| Current and former smokers | 45 (54.2) | 34 (41) | 11 (13.3) | |
| Histology | <0.001 | |||
| Adenocarcinoma | 62 (74.7) | 27 (32.5) | 35 (42.2) | |
| Squamous | 21 (25.3) | 20 (24.1) | 1 (1.2) | |
| Lymph node metastasis | 0.410 | |||
| Yes | 53 (63.9) | 31 (37.3) | 22 (26.5) | |
| No | 30 (36.1) | 16 (19.3) | 14 (16.9) | |
| Distant metastasis | 0.245 | |||
| Yes | 46 (55.4) | 24 (28.9) | 22 (26.5) | |
| No | 37 (45.6) | 23 (27.7) | 14 (16.9) | |
| Expression of estrogen receptor-β | 0.006 | |||
| Negative | 46 (55.4) | 20 (24.1) | 26 (31.3) | |
| Positive | 37 (45.6) | 27 (32.5) | 10 (12) | |
| Expression of p53 | 0.381 | |||
| Negative | 35 (42.2) | 21 (25.3) | 14 (16.9) | |
| Positive | 48 (57.8) | 26 (31.3) | 22 (26.5) |
EGFR, epidermal growth factor receptor.
EGFR mutation analysis
Mutations in exons 18–21 of the EGFR gene were detected using methods described previously (29). Briefly, genomic DNA was extracted and purified from either fresh-frozen tumors or tumors embedded in paraffin blocks using the QIAamp DNA FFPE Tissue kit (catalog no., 56404; Qiagen GmbH, Hilden, Germany). A 296-base pair GAPDH fragment was amplified as an internal control to ensure DNA integrity and for normalization. Primer pairs used were as follows: exon 18 sense, 5′-CAAATGAGCTGGCAAGTGCCGTGTC-3′ and antisense, 5′-GAGTTTCCCAAACACTCAGTGAAAC-3′; exon 19 sense, 5′-GCAATATCAGCCTTAGGTGCGGCTC-3′ and antisense, 5′-CATAGAAAGTGAACATTTAGGATGTG-3′; exon 20 sense, 5′-CCATGAGTACGTATTTTGAAACTC-3′ and antisense, 5′-CATATCCCCATGGCAAACTCTTGC-3′; exon 21 sense, 5′-CTAACGTTCGCCAGCCATAAGTCC-3′ and antisense, 5′-GCTGCGAGCTCACCCAGAATGTCTGG-3′. Genomic DNA was extracted and purified from either fresh-frozen tumors or tumors embedded in paraffin blocks using the QIAamp DNA FFPE Tissue kit (catalog no., 56404; Qiagen GmbH). The polymerase chain reaction (PCR) products were purified and labeled using the BigDye Terminator v3.1 Cycle Sequencing kit (Amoy Dx, Ltd., Xiamen, China), followed by sequencing in an ABI 3100 Genetic Analyzer (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All sequence variations were confirmed by multiple independent PCR amplifications and repeated sequencing reactions.
Immunohistochemical staining
Tissue sections (5-µm sections) obtained from the paraffin-embedded specimens were prepared on glass slides and then deparaffinized in xylene followed by a graded series of alcohol washes. The sections were placed in 0.1 mol/l citrate buffer (pH 6.0), autoclaved at 121°C for 10 min and treated with 3% H2O2 for 5 min to reduce endogenous peroxidase activity. The sections were then blocked with normal goat serum (Amoy Dx, Xiamen, China) for 15 min and then incubated with rabbit polyclonal antibodies targeting either ER-β (catalog no., SC-6820; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) or p53 (catalog no., 2527s; Cell Signaling Technology, Inc., Beverly, MA, USA) at room temperature for 30 min. The ER-β and p53 antibodies were diluted 1:500 and 1:10, respectively, in phosphate-buffered saline. Biotinylated secondary anti-mouse antibodies (dilution, 1:100; catalog no., 7076s; Cell Signaling Technology, Inc.) were applied to the sections for 20 min. Immunoperoxidase staining was subsequently performed using the EnVision kit (Dako Japan Co., Ltd., Kyoto, Japan) according to the manufacturer's protocol. Sections were observed using Olympus BX51 (Olympus, Tokyo, Japan).
Statistical analysis
All statistical analysis was performed using the SPSS 16.0 statistical software package (SPSS Inc., Chicago, IL, USA). All data are expressed as the mean ± standard deviation. The χ2-test was used to investigate associations between EGFR mutations and clinical characteristics, including the expression of ER-β, age, gender, smoking history, histological type, histological differentiation, lymph node metastasis and distant metastasis. Multivariate analysis was performed using logistic regression. Survival curves were estimated using the Kaplan-Meier method. Progression-free survival (PFS) was defined as the time of diagnosis to the time of recurrence or the date of the final follow-up. Significant differences among subgroups were compared using the log-rank test. Cox proportional hazard regression analysis was used to examine the effects of EGFR mutations, expression of ER-β and clinical variables on survival rates. Factors showing prognostic significance in the univariate analyses were entered as variables in the multivariate analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
EGFR mutation status in NSCLC tumors
Tumor specimens obtained from 83 patients with advanced NSCLC were analyzed for mutations in the EGFR gene. The tumors of 47 (56.6%) patients were identified as EGFR wild-type (EGFR-wt) and those of 36 (45.4%) patients were identified as EGFR mutant (EGFR-mut). It was found that >95% of the patients with EGFR-mut tumors had adenocarcinomas, and 42.6% of the patients with EGFR-wt tumors had squamous carcinomas. The percentage of former and current smokers was significantly higher in the EGFR-wt group, compared with the EGFR-mut group (72.3, vs. 30.6%, respectively), whereas the percentage of female patients was significantly higher in the EGFR-mut group, compared with the EGFR-wt group (61.1, vs. 17%, respectively).
Expression of ER-β and p53 in tumor specimens
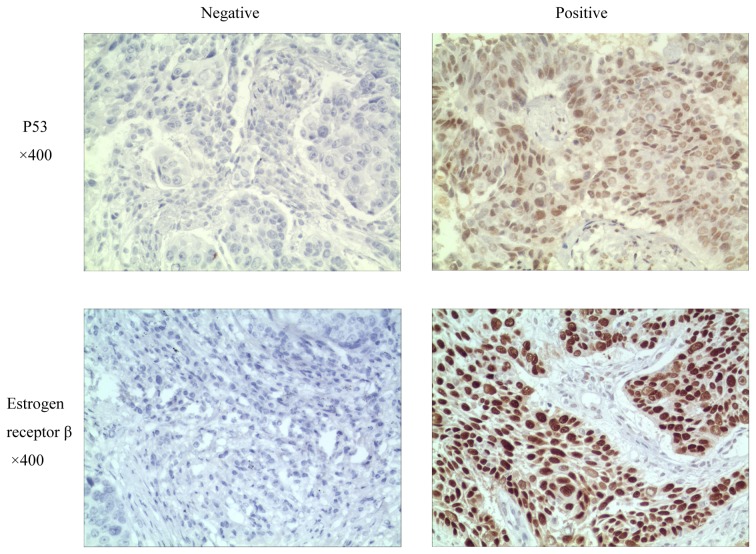
Immunohistochemistry was performed to evaluate the expression of ER-β and p53 in the tumors of the 83 patients with advanced NSCLC. Representative examples of positive and negative ER-β and p53 immunostaining are shown in Fig. 1. For each of these proteins, the patients were categorized into positive and negative expression groups depending upon whether or not protein staining was detectable in the tumor tissue samples. ER-β and p53 were expressed in 37 (45.6%) and 48 (57.8%) of the 83 patient tumors, respectively. The percentage of samples with detectable expression of ER-β was higher in the EGFR-wt patient group, compared with the EGFR-mut group (57.4, vs. 27.8%, respectively). However, the percentage of samples positive for the expression of p53 was similar for the EGFR-wt and EGFR-mut patient groups (55.3, vs. 61.1%, respectively). No significant correlation between the expression of p53 and EGFR mutations was found (Table I).
Figure 1.
Representative examples of estrogen receptor-β and p53 immunostaining in patient-derived tumor tissue sections. Examples of staining patterns scored as negative (left) and positive (right) for protein expression are shown for each protein.
Association between EGFR mutations and clinicopathologic factors
In the univariate analysis, mutations in the EGFR gene were significantly associated with gender, smoking status, histology and the expression of ER-β (Table I). No significant correlation was identified between EGFR mutations and well-differentiated tumors, lymph node metastasis and the expression of p53 (Table I). In the multivariate analysis, as shown in Table II, EGFR mutations were significantly associated with histology (OR 0.074; P=0.023) and the expression of ER-β (OR 0.241; P=0.029). However, no significant correlations were observed between EGFR mutations and either gender (OR 3.649; P=0.105) or smoking status (OR 0.493; P=0.360). The above findings indicated that there was a close correlation between the expression of ER-β and the presence of EGFR mutations.
Table II.
Multivariate analysis of predictive factors for epidermal growth factor receptor mutations.
| Characteristic | OR (95% CI) | P-value |
|---|---|---|
| Age: ≤64, vs. >64 years | 1.429 (0.424–4.823) | 0.565 |
| Gender: Female, vs. male | 3.649 (0.761–17.494) | 0.105 |
| Differentiation: Well, vs. poor | 3.061 (0.773–12.126) | 0.111 |
| Smoking status: Never smoked, vs. current/former smoker | 0.493 (0.108–2.245) | 0.360 |
| Histology: Squamous, vs. adenocarcinoma | 0.074 (0.008–0.698) | 0.023 |
| Lymph node metastasis: Yes, vs. no | 0.790 (0.209–2.986) | 0.729 |
| Distant metastasis: Yes, vs. no | 1.209 (0.329–4.447) | 0.775 |
| Expression of estrogen receptor-β: Negative, vs. positive | 0.241 (0.067–0.865) | 0.029 |
| Expression of p53: Negative, vs. positive | 1.792 (0.540–5.943) | 0.340 |
OR, odds ratio; CI, confidence interval.
Affect of clinicopathologic factors, expression of ER-β and EGFR mutations on PFS
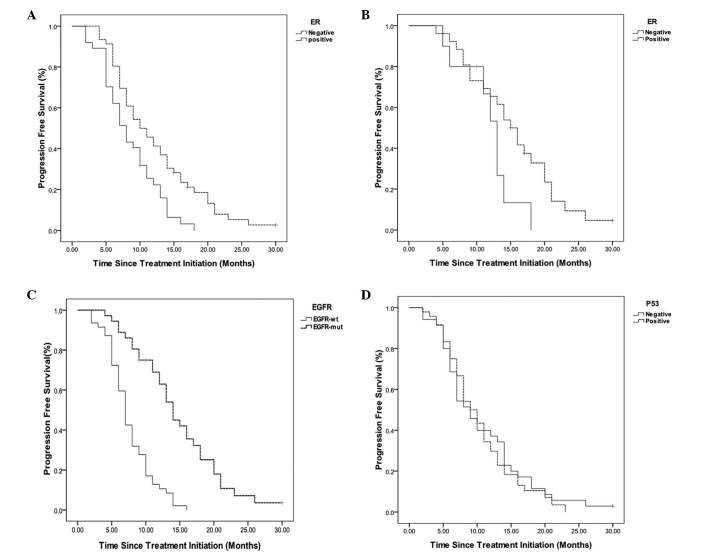
Subsequently, survival analysis was performed on the 83 advanced NSCLC patients. The expression of ER-β was associated with poor prognosis in terms of PFS. The median PFS was 8.5 months for the ER-β-postive expression group and 12.2 months for the ER-β-negative expression group (P=0.004; Fig. 2A). However, within the 36 patient EGFR-mut group, the ER-β expression status had no significant effect on PFS (P=0.085), as shown in Fig. 2B. EGFR mutations were associated with favorable prognosis in terms of PFS. The median PFS was 14.6 months in the EGFR-mut group, compared with 7.6 months in the EGFR-wt group (P<0.001; Fig. 2C). In addition, no significant correlation was observed between the expression of p53 and PFS (P=0.659; Fig. 2D). These data indicated that EGFR mutations and the expression of ER-β were significant determinants of prognosis in patients with advanced NSCLC.
Figure 2.
Kaplan-Meier PFS curves. (A) PFS curves for the 83 patients, stratified according to ER-β expression status. The median survival rate for ER-β-negative patients was 12.2 months, compared with 8.5 months for ER-β-positive patients (P=0.004). (B) PFS curves for the EGFR-mut subgroup of 36 patients, stratified according to ER-β expression status. The median survival rate for the ER-β-negative patients was 15.4 months, compared with 11.9 months for the ER-β-positive patients (P=0.085). (C) PFS curves for the 83 patients, stratified according to EGFR mutation status. The median survival rate of the EGFR-wt patients was 7.6 months, compared with 14.6 months for the EGFR-mut patients (P<0.001). (D) PFS curves for the 83 patients, stratified according to p53 expression status. The median survival rate for p53-negative patients was 10.8 months, compared with 10.4 months for p53-positive patients (P=0.659). PFS, progression-free survival; ER-β, estrogen receptor-β; EGFR, epidermal growth factor receptor; wt, wild-type; mut, mutant.
The results of the Cox regression analysis of clinical variables associated with PFS for the 83 patients are summarized in Table III. In the univariate analysis, survival rates were significantly associated with conventional prognostic factors, including smoking status (P=0.004), histology (P=0.004), lymph node metastasis (P=0.001), EGFR mutations (P<0.001) and expression of ER-β (P=0.007). The multivariate analysis revealed that EGFR mutations (P<0.001) and lymph node metastasis (P=0.003) were significant prognostic factors. However, no differences in PFS were observed with respect to histological type and the ER-β expression status.
Table III.
Results of the univariate and multivariate analyses of selected factors for PFS in all patients.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Gender (male, vs. female) | 0.624 (0.383–1.017) | 0.059 | ||
| Age (≤64, vs. >64 years) | 0.929 (0.593–1.456) | 0.749 | ||
| Smoking status (never, vs. current/former) | 2.012 (1.254–3.229) | 0.004 | 1.172 (0.682–2.012) | 0.566 |
| Histology (adenocarcinoma, vs. squamous) | 2.168 (1.273–3.695) | 0.004 | 1.111 (0.670–1.844) | 0.747 |
| Histological differentiation (well, vs. poor) | 0.873 (0.535–1.424) | 0.586 | ||
| Lymph node metastasis (yes, vs. no) | 2.576 (1.493–4.443) | 0.001 | 2.400 (1.353–4.257) | 0.003 |
| Distant metastasis (yes, vs. no) | 1.294 (0.824–2.032) | 0.264 | ||
| EGFR (mutated, vs. wild type) | 0.238 (0.139–0.409) | <0.001 | 0.284 (0.150–0.537) | <0.001 |
| Estrogen receptor-β (positive, vs. negative) | 1.924 (1.197–3.092) | 0.007 | 1.111 (0.670–1.844) | 0.684 |
| P53 (positive, vs. negative) | 1.102 (0.698–1.738) | 0.678 | ||
EGFR, epidermal growth factor receptor; HR, hazard ratio; CI, confidence interval.
Discussion
In the present study, a cohort of 83 patients with advanced NSCLC was analyzed. The results provided evidence for an association between the expression of ER-β and the presence of EGFR mutations, and demonstrated the prognostic significance of this association. The results showed that there was a significant correlation between EGFR mutations and clinicopathologic factors, including histology and the expression of ER-β. There was a significant trend toward improved PFS for patients with EGFR mutations, compared with those without mutations. It was also found that patients with ER-β tumors had a significantly poorer prognosis. These findings indicated that EGFR mutations and the expression of ER-β may represent clinically useful prognostic markers in advanced NSCLC.
In NSCLC cells, estrogen biosynthesis is driven by intrinsically expressed aromatase (30), resulting in the activation of ER signaling, and the promotion of tumor development and progression (2,7,31). Although the detection of ER-α and/or the expression of ER-β by immunohistochemical techniques has been associated with clinical outcome in certain studies (11,13,26), findings regarding the frequency of expression of these receptors in NSCLC are inconsistent (11,13,27,32). These differences may be a consequence of inter-study variations in factors, including patient cohort characteristics (13), the antibodies used and their associated dilutions. These discrepancies may obscure the significance of hormone receptor expression status on patient clinicopathologic characteristics and prognosis. In the present study, ER-β was found to be expressed in the tumors of 37 (45.6%) patients, and was more frequently associated with wild-type EGFR status. A significant negative correlation was identified between the expression of ER-β and EGFR mutations, which was consistent with previous reports (11,12,25,27). The present study also found that the expression of ER-β was significantly associated with shorter PFS in the 83 patients examined. However, no significant correlation between the expression of ER-β and PFS was found for the 36 EGFR-mut patients. These differences may be a consequence of the low number of patients with EGFR mutations in the present study. Although not significant, the PFS for the 36 EGFR-mut patients who were also negative for the expression of ER-β was longer, compared with that for the patients positively expressing ER-β (15.4, vs. 11.9 months, respectively). The above results indicated that female hormone-associated factors, including aromatase and the expression of ER-β, affect the outcomes for patients with NSCLC associated with EGFR mutations, suggesting that the ER and EGFR pathways contribute to the progression of NSCLC.
EGFR mutations are considered to be an early event in the pathogenesis of NSCLC, and are more commonly observed in women, non-smokers and in patients with adenocarcinoma (23,24,33). It is now universally accepted that the presence or absence of EGFR mutations can be used to define two types of NSCLC with differing biology, therapeutic options and outcomes (34). In the present study, 97.2% of EGFR-mut tumors were adenocarcinomas, and the EGFR mutation frequency was higher in non-smokers and female patients. These results are all consistent with those of previous reports (23,24). In addition, the present study found that patients with EGFR mutations had a significantly more favorable prognosis, compared with those without EGFR mutations. These data indicated that EGFR mutations wer the most important factor in determining the prognosis of patients with advanced NSCLC. The present study also evaluated the effect of gender, smoking history and the ER-β expression status on PFS in the 36 EGFR-mut patients; however, no significant differences were found (data not shown). This may have been due to the low number of EGFR-mut patients in the present study. Therefore, a clinical investigation involving a larger number of patients with EGFR mutations is required to further clarify the correlation between clinicopathologic factors and prognosis within this specific advanced NSCLC patient subgroup.
The p53 tumor suppressor protein regulates multiple important cellular processes, including cell-cycle arrest, senescence and apoptosis, and alterations in its activity are involved in tumorigenesis. Therefore, the loss of p53 function may lead to unchecked cellular proliferation, tumor growth and therapeutic resistance (35,36). The present study also investigated the effect of the expression of p53 in patients with advanced NSCLC. No correlation was found between the expression of p53 and the presence of EGFR mutations (Tables I and II). In addition, no significant correlation was observed between the expression of p53 and PFS. A previous study reported that non-disruptive mutations in the TP53 gene are an independent prognostic factor of shorter survival rates in advanced NSCLC (37), whereas another study showed that p53 mutations are significantly correlated with tumor relapse in patients with stage I disease (38). The tumor suppressor gene, TP53, is the most frequently mutated gene in NSCLC (39). Several of these mutations result in a stable protein, with significant loss of activity. However, the present study did not investigate the significance of p53 mutations. Clinical trials are warranted to determine the frequency, nature and prognostic significance of p53 mutations in patients with advanced NSCLC.
In conclusion, the present study demonstrated a significant correlation between the expression of ER-β and the presence of EGFR mutations in advanced NSCLC. EGFR mutations had a negative association with the expression of ER-β. The expression of ER-β was associated with poor prognosis, whereas EGFR mutations were associated with a more favorable patient outcome. These findings suggested that the expression of ER-β and presence of EGFR mutations may be used as surrogate markers for the prognosis of patients with advanced NSCLC.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 81172172). The authors would like to thank Mr. Guo Zhen-Li (Department of Respiratory Oncology, Anhui Provincial Cancer Hospital, Hefei, China) for tissue sample collection and Mr. Wang Xu (School of Public Health, Anhui Medical University, Hefei, China) for statistical analysis.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Pietras RJ, Márquez DC, Chen HW, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers-a different disease. Nat Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 4.Yano T, Haro A, Shikada Y, Maruyama R, Maehara Y. Non-small cell lung cancer in never smokers as a representative ‘non-smoking-associated lung cancer’: Epidemiology and clinical features. Int J Clin Oncol. 2011;16:287–293. doi: 10.1007/s10147-010-0160-8. [DOI] [PubMed] [Google Scholar]
- 5.Radzikowska E, Głaz P, Roszkowski K. Lung cancer in women: Age, smoking, histology, performance status, stage, initial treatment and survival. Population-based study of 20 561 cases. Ann Oncol. 2002;13:1087–1093. doi: 10.1093/annonc/mdf187. [DOI] [PubMed] [Google Scholar]
- 6.Yano T, Miura N, Takenaka T, Haro A, Okazaki H, Ohba T, Kouso H, Kometani T, Shoji F, Maehara Y. Never-smoking nonsmall cell lung cancer as a separate entity: Clinicopathologic features and survival. Cancer. 2008;113:1012–1018. doi: 10.1002/cncr.23679. [DOI] [PubMed] [Google Scholar]
- 7.Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;65:1459–1470. doi: 10.1158/0008-5472.CAN-04-1872. [DOI] [PubMed] [Google Scholar]
- 8.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried J. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–2150. [PubMed] [Google Scholar]
- 9.Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, Dubinett SM, Pietras RJ. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287–11291. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 10.Mah V, Seligson DB, Li A, Márquez DC, Wistuba II, Elshimali Y, Fishbein MC, Chia D, Pietras RJ, Goodglick L. Aromatase expression predicts survival in women with early-stage non-small cell lung cancer. Cancer Res. 2007;67:10484–10490. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raso MG, Behrens C, Herynk MH, Liu S, Prudkin L, Ozburn NC, Woods DM, Tang X, Mehran RJ, Moran C, et al. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res. 2009;15:5359–5368. doi: 10.1158/1078-0432.CCR-09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nose N, Sugio K, Oyama T, Nozoe T, Uramoto H, Iwata T, Onitsuka T, Yasumoto K. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol. 2009;27:411–417. doi: 10.1200/JCO.2008.18.3251. [DOI] [PubMed] [Google Scholar]
- 13.Stabile LP, Dacic S, Land SR, Lenzner DE, Dhir R, Acquafondata M, Landreneau RJ, Grandis JR, Siegfried JM. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res. 2011;17:154–164. doi: 10.1158/1078-0432.CCR-10-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegfried JM, Hershberger PA, Stabile LP. Estrogen receptor signaling in lung cancer. Semin Oncol. 2009;36:524–531. doi: 10.1053/j.seminoncol.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Márquez-Garbán DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids. 2007;72:135–143. doi: 10.1016/j.steroids.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Inoue M, Sobue T, Tsugane S. Reproductive factors, hormone use and the risk of lung cancer among middle-aged never-smoking Japanese women: A large-scale population-based cohort study. Int J Cancer. 2005;117:662–666. doi: 10.1002/ijc.21229. [DOI] [PubMed] [Google Scholar]
- 17.Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried JM, Nichols M. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res. 2005;65:1598–1605. doi: 10.1158/0008-5472.CAN-04-2694. [DOI] [PubMed] [Google Scholar]
- 18.Mollerup S, Jørgensen K, Berge G, Haugen A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung cancer. 2002;37:153–159. doi: 10.1016/S0169-5002(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 19.Kawai H, Ishii A, Washiya K, Konno T, Kon H, Yamaya C, Ono I, Minamiya Y, Ogawa J. Estrogen receptor alpha and beta are prognostic factors in non-small cell lung cancer. Clin Cancer Res. 2005;11:5084–5089. doi: 10.1158/1078-0432.CCR-05-0200. [DOI] [PubMed] [Google Scholar]
- 20.Alì G, Donati V, Loggini B, Servadio A, Dell'Omodarme M, Prati MC, Camacci T, Lucchi M, Melfi F, Mussi A, Fontanini G. Different estrogen receptor beta expression in distinct histologic subtypes of lung adenocarcinoma. Hum Pathol. 2008;39:1465–1473. doi: 10.1016/j.humpath.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Toh CK, Ahmad B, Soong R, Chuah KL, Tan SH, Hee SW, Leong SS, Tan EH, Lim WT. Correlation between epidermal growth factor receptor mutations and expression of female hormone receptors in East-Asian lung adenocarcinomas. J Thorac Oncol. 2010;5:17–22. doi: 10.1097/JTO.0b013e3181c0a602. [DOI] [PubMed] [Google Scholar]
- 22.Pietras RJ, Márquez-Garbán DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res. 2007;13:4672–4676. doi: 10.1158/1078-0432.CCR-07-1373. [DOI] [PubMed] [Google Scholar]
- 23.Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 24.Kosaka T, Yatabe Y, Endoh H, Kuwano H, Takahashi T, Mitsudomi T. Mutations of the epidermal growth factor receptor gene in lung cancer: Biological and clinical implications. Cancer Res. 2004;64:8919–8923. doi: 10.1158/0008-5472.CAN-04-2818. [DOI] [PubMed] [Google Scholar]
- 25.Garon EB, Pietras RJ, Finn RS, Kamranpour N, Pitts S, Márquez-Garbán DC, Desai AJ, Dering J, Hosmer W, von Euw EM, et al. Antiestrogen fulvestrant enhances the antiproliferative effects of epidermal growth factor receptor inhibitors in human non-small cell lung cancer. J Thorac Oncol. 2013;8:270–278. doi: 10.1097/JTO.0b013e31827d525c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nose N, Uramoto H, Iwata T, Hanagiri T, Yasumoto K. Expression of estrogen receptor beta predicts a clinical response and longer progression-free survival after treatment with EGFR-TKI for adenocarcinoma of the lung. Lung Cancer. 2011;71:350–355. doi: 10.1016/j.lungcan.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Sun HB, Zheng Y, Ou W, Fang Q, Li P, Ye X, Zhang BB, Yang H, Wang SY. Association between hormone receptor expression and epidermal growth factor receptor mutation in patients operated on for non-small cell lung cancer. Ann Thorac Surg. 2011;91:1562–1567. doi: 10.1016/j.athoracsur.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q, Zhang XC, Chen ZH, Yin XL, Yang JJ, Xu CR, Yan HH, Chen HJ, Su J, Zhong WZ, et al. Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:3316–3321. doi: 10.1200/JCO.2010.33.3757. [DOI] [PubMed] [Google Scholar]
- 30.Niikawa H, Suzuki T, Miki Y, Suzuki S, Nagasaki S, Akahira J, Honma S, Evans DB, Hayashi S, Kondo T, Sasano H. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res. 2008;14:4417–4426. doi: 10.1158/1078-0432.CCR-07-1950. [DOI] [PubMed] [Google Scholar]
- 31.Hershberger PA, Stabile LP, Kanterewicz B, Rothstein ME, Gubish CT, Land S, Shuai Y, Siegfried JM, Nichols M. Estrogen receptor beta (ERbeta) subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J Steroid Biochem Mol Biol. 2009;116:102–109. doi: 10.1016/j.jsbmb.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miki Y, Abe K, Suzuki S, Suzuki T, Sasano H. Suppression of estrogen actions in human lung cancer. Mol Cell Endocrinol. 2011;340:168–174. doi: 10.1016/j.mce.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, Mahmood U, Mitchell A, Sun Y, Al-Hashem R, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 34.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, Majem M, Lopez-Vivanco G, Isla D, Provencio M, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 35.Levine AJ, Oren M. The first 30 years of p53: Growing ever more complex. Nat Rev Cancer. 2009;9:749–758. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: Drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 37.Molina-Vila MA, Bertran-Alamillo J, Gascó A, Mayo-de-las-Casas C, Sánchez-Ronco M, Pujantell-Pastor L, Bonanno L, Favaretto AG, Cardona AF, Vergnenègre A, et al. Nondisruptive p53 mutations are associated with shorter survival in patients with advanced non-small cell lung cancer. Clin Cancer Res. 2014;20:4647–4659. doi: 10.1158/1078-0432.CCR-13-2391. [DOI] [PubMed] [Google Scholar]
- 38.Lin MW, Wu CT, Shih JY, Chang YL, Yang PC. Clinicopathologic characteristics and prognostic significance of EGFR and p53 mutations in surgically resected lung adenocarcinomas ≤2 cm in maximal dimension. J Surg Oncol. 2014;110:99–106. doi: 10.1002/jso.23628. [DOI] [PubMed] [Google Scholar]
- 39.Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, Shepherd R, Leung K, Menzies A, et al. COSMIC: Mining complete cancer genomes in the catalogue of somatic mutations in cancer. Nucleic Acids Res. 2011;39(Database issue):D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]