Abstract
Langerhansmporal histiocytosis (LCH) refers to a group of diseases that are characterized by the primary pathogenesis of an abnormal polyclonal proliferation of Langerhans cells that affect different structures of the human body, including the temporal bone. Thus far, the etiology of LCH remains unclear. Diagnosis of LCH is based on a synthetic analysis of the clinical presentations, as well as on features of imaging and histopathology. The clinical features, diagnosis, treatment and prognosis of LCH remain obscure, and temporal LCH is often confused with ear inflammatory lesions and malignant tumors. There are several therapeutic modalities for temporal bone LCH that include surgery, chemotherapy, radiotherapy and steroidal injections. The present study reports the case of an infant presenting a 1-month history of worsening left facial paralysis and a slowly enlarging post auricular mass followed by a 1-month history of postauricular swelling in the left ear. Computed tomography demonstrated a large mass of organized tissue. Moreover, the diagnosis of LCH was confirmed by histopathological and immunohistochemical examinations. The patient also suffered from multiple organ failure, including the liver, kidney, lymphatic system, skin, hematopoietic system and lungs. Following surgical intervention with mastoidotympanectomy, the parents of the patient refused further chemotherapy, and the patient succumbed to the disease ~6 months later.
Keywords: Langerhans' cell histiocytosis, temporal bone, etiology, diagnosis, therapeutic modalities, mastoidotympanectomy, prognosis
Introduction
Langerhans' cell histiocytosis (LCH) is a rare disease with variable clinical appearance. The etiopathogenesis of LCH is still unclear and remains to be investigated. Despite the fact that it had been widely discussed that viral or genetic predisposing factors make serious contributions to this disease, no conclusive proof has ever been provided (1). In 1998, Weintrarb et al (2) demonstrated that p53 was detected in all LCH biopsy specimens, particularly in Langerhans cells; according to their report, the inactivation of p53 led to an uncontrolled proliferation of LCH cells. The annual incidence of LCH is ~5.4 cases per one million people, and a male predominance could be observed. LCH is primarily a pediatric disease, which is rarely reported in adults. Moreover, the peak age of the patients range between 1 and 4 years old, and patients with focal lesions are generally older (0.1–15.1 years) than those with multisystemic disease (0.09–14.8 years) (3). Histiocytosis of the temporal bone occurs in 15–60% of the sample cases, and it is higher when radiological findings are included in asymptomatic patients; its bilateral occurrence is described in up to 30% of the cases (4) and is more frequent in the multisystemic diseases (5,6). The characteristics of LCH are local invasive growth that easily relapses following treatment, and possible disseminated malignant tumors (7). Moreover, the disease has complicated clinical manifestations of which bone damage is the most common (8). The clinical features, diagnosis, treatment and prognosis of the LCH remain obscure, and it is easy to confuse temporal LCH with ear inflammatory lesions and malignant tumors (8). Thus, the aim of the present study is to illustrate the clinical presentations, diagnosis, management and prognosis of this disease.
Case report
A 20-month-old Chinese boy presented with a 1-month history of worsening left facial paralysis and imbalance and progressive hearing loss, which co-presented with purulence of the left external auditory meatus and a slowly enlarging post auricular mass, swelling of the ear and the temporal bone. Free-field pure-tone audiometry showed moderate conductive deafness and there was no pathological nystagmus. The blood routine examination revealed leukocytosis (27.2/ml). The computed tomography scan revealed a destructive bone lesion involving the left posterior external canal, middle and posterior cranial fossa, and the lateral semicircular canal (Fig. 1).
Figure 1.
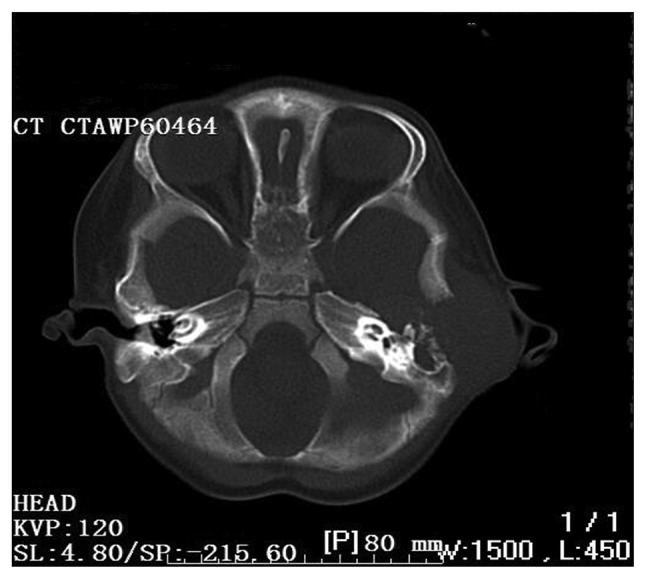
An axial computed tomographic scan revealing a destructive bone lesion involving the left posterior external canal, middle and posterior crani fossa, lateral semicircular canal and the facial nerve.
The cutaneous infiltrates were located in the papules of the mid-line of the trunk and the flexor areas of the limbs. Moreover, radiological images demonstrated a micronodular infiltrate of the lungs and evidence of nodes that were revealed in the liver. The levels of bilirubin and aminotransferases in the serum were also increasing. The patient was initially diagnosed with malignant granuloma of the ear or inflammatory lesions of the ear. Moreover, surgical biopsies were obtained via a mastoidectomy approach. During surgery, vast destruction of the temporal bone involving the left posterior external canal, middle and posterior cranial fossa, and the lateral semicircular canal were confirmed, and fibrous inflammatory tissue interrupted by necrotic and purulent material was found. Intraoperative histology revealed bony and fibrotic tissue with interposed infiltrate containing lymphocytes, eosinophilic granulocytes, and typical Langerhans histiocytes. A final diagnosis of LCH was made by a surgical pathological examination that revealed numerous ovoid Langerhans' histocytic cells with grooved and lobulated nuclei and fine chromatin, which were positive for CD1/S-100 by immunohistochemical staining (Figs. 2 and 3). Following surgery, further chemotherapy was planned, however the parents of the boy refused any additional therapy and removed him from the hospital, and the boy succumbed to the disease 6 months later.
Figure 2.
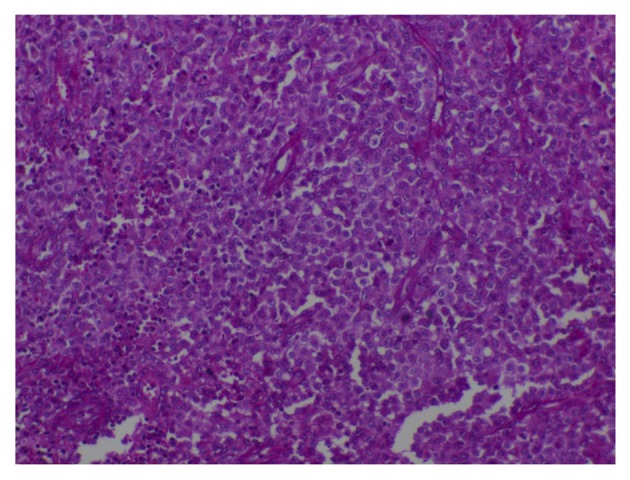
High-power hematoxylin and eosin stained image demonstrating numerous ovoid and histiocytic cells with grooved and lobulated nuclei. Eosinophils and giant cells are presented (original magnification, ×400).
Figure 3.
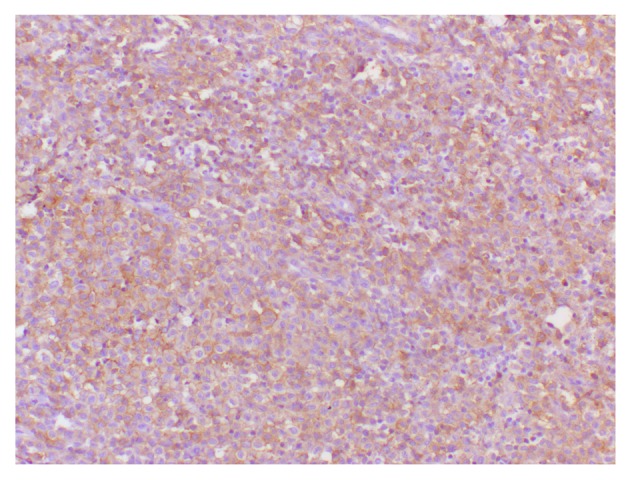
Immunohistochemical observations demonstrated histiocytic cells that were positive for CD1a expression, which was consistent for cells of Langerhans' origin. The image depicts an immunoperoxidase staining technique (original magnification, ×400).
Discussion
LCH is a rare disorder that is associated with a wide spectrum of presenting symptoms and variable clinical behavior and prognosis. In addition to the temporal bone, the head and neck area involving the orbital bone, the scalp, the mandible, the maxillary bone and the facial skin may be involved when LCH is evident. Clinical manifestations varied depending on the site of involvement; thus, there is no characteristic pathognomonic presentation that specifically identifies LCH (9). The classical disease is divided into three overlapping states called eosin ophitic granuloma, Hand-Schüller-Christian disease, and Letterer-Siwe disease (10).
LCH is capable of affecting reticuloendothelial cells anywhere in the body, and as such, multiple organs may be involved.
The regions that are most frequently involved include the bones, skin, liver, lung, spleen, hypophysis, lymph nodes and bone marrow (11). Moreover, cutaneous lesions are observed in almost 50% of patients, and can be the only sign of the disease or evidence of a multisystem involvement. Rash is one of the most common effects, and the preferred location is the mid-line of the trunk and the flexor areas of the limbs. Furthermore, the scalp has erythematous lesions that may evolve into petechiae that ulcerate and form crusts (12).
Pulmonary involvement is observed in 20–40% of patients and manifests clinically as a cough, dyspnea and tachypnea amongst other presentations (13). In addition, radiological images commonly reveal a micronodular presence of cystic infiltrates. The main complication of gastrointestinal lesions is bleeding (13). In addition, the levels of aminotransferases and bilirubin increase when hepatic cells are involved (13). Moreover, the hematological alterations may indicate a lesion in the bone marrow or in the spleen. Enlarged cervical lymph nodes are affected either in a single isolated organ presentation of the disease or as part of a multisystem involved disease (14).
LCH can also be easily misdiagnosed because of the common involvement of the external and middle ear (15). A previous study has reported a misdiagnosis rate of ~72.7% (15/22) for LCH, primarily due to the clinical presentation resembling that caused by other more common conditions (15). Moreover, diagnosis of LCH should be based on structured and synthetic analyses of the clinical manifestations, the clinical features of imaging and their histopathology. Pathologically, LCH is characterized by multinucleated Langerhans' cells, histiocytes and eosinophils, despite the hallmark cell of LCH being Langerhans' cell histiocyte (16). The gold standard for the diagnosis of the disease requires the presence of Birbeck granules on an electron microscopic examination; however, Birbeck granules are not exclusively observed in this disease, since they have already been identified in other inflammatory conditions of the lymph nodes (16). However, in certain cases, immunohistochemistry can be fundamental in establishing a diagnosis. For example, a diagnosis can be established with the use of the cell surface or cytoplasmic expression of CD1a, S100 and/or CD45 immunostaining on histopathological specimens (17).
According to the diagnostic criteria of the international organization cell association for LCH in 1987, diagnosis of LCH was divided into three degrees of staging. Firstly, the preliminary diagnosis relies on the results of the clinical laboratory, including bone and lung X-rays and common pathological changes (e.g., abnormal Langerhans cells are shown to be present in pathological sections). Secondly, the diagnosis can be made if analysis by immunohistochemistry demonstrates a positive result for S-100 protein based on the preliminary diagnosis. Third-degree diagnosis is made from a detailed examination of the preliminary diagnosis, along with ultrastructural examination that shows Birbeck particles or a positive result for CD1a expression by immunohistochemistry (18).
An ideal therapy for LCH has not yet been established. However, the objectives of the treatment include improvement of clinical symptoms and prevention of complications (19). There are several treatment modalities for temporal bone LCH, including surgery, radiotherapy, steroidal injections and chemotherapy. In addition, they can be used alone or in combination depending on the severity and extent of the disease (20). It is noteworthy that ~10–20% of patients have revealed a spontaneous regression of the disease. Moreover, it is accepted that, if possible, a surgical resection of the lesions is preferred as a treatment. Nevertheless, a number of authors prefer the application of chemotherapy. Different protocols, in addition to the use of systemic corticosteroids, cytostatic therapies like 2-chlorodesoxiademosin (2CdA-cladribine), cyclosporine, thalidomide and myeloablative therapies were prescribed (19,21–23). Single system disease can be treated with local injectable intratumoral steroids (11,18). Following surgery, numerous investigators recommend low-dose radiotherapy (10–20 Gy) in cases in which the tissue involved cannot be completely excised (21,24).
Although LCH is very critical, the prognosis is generally good if vital organs are not involved and correct diagnosis and a timely rational therapy can be made. The indices of response, recurrence and complications may vary due to different schemes. Moreover, the prognosis of temporal bone LCH is excellent in patients with limited organ involvement (3). Besides the extent of the disease, the age of the patient at the time of diagnosis is an important factor. In addition, children <2 years of age have a worse outcome. In addition, the presence of cervical lymph nodes and scalp involvement presents as a poorer prognosis and an indicator for recurrence. Finally, the most relevant prognostic factors are multisystem involvement and vital organ dysfunction. In general, the patients with target organ lesions have a worse outcome and a higher incidence of complications and risk of death (25).
In conclusion, the case of this young male patient described in the present case report <2 years of age and suffered from temporal bone LCH. In addition, the patient also suffered from multiple organ failure including that of the liver, kidney, lymphatic system, skin and hematopoietic system. Therefore, patients with lesions in target organs have worse outcomes and a higher incidence of complications and mortality.
References
- 1.Campos MK, Viana MB, de Oliveira BM, Ribeiro DD, Silva CM. Langerhans cell histiocytosis: A 16-year experience. J Pediatr (Rio J) 2007;83:79–86. doi: 10.2223/JPED.1581. [DOI] [PubMed] [Google Scholar]
- 2.Weintraub M, Bhatia KG, Chandra RS, Magrath IT, Ladisch S. p53 expression in Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 1998;20:12–17. doi: 10.1097/00043426-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Saliba I, Sidani K, El Fata F, Arcand P, Quintal MC, Abela A. Langerhans' cell histiocytosis of the temporal bone in children. Int J Pediatr Otorhinolaryngol. 2008;72:775–786. doi: 10.1016/j.ijporl.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Cochrane LA, Prince M, Clarke K. Langerhans' cell histiocytosis in the paediatric population: Presentation and treatment of head and neck manifestations. J Otolaryngol. 2003;32:33–37. doi: 10.2310/7070.2003.35266. [DOI] [PubMed] [Google Scholar]
- 5.DiNardo LJ, Wetmore RF. Head and neck manifestations of histiocytosis-X in children. Laryngoscope. 1989;99:721–724. doi: 10.1288/00005537-198907000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Irving RM, Broadbent V, Jones NS. Langerhans' cell histiocytosis in childhood: Management of head and neck manifestations. Laryngoscope. 1994;104:64–70. doi: 10.1288/00005537-199401000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Kleinjung T, Woenckhaus M, Bachthaler M, Wolff JE, Wolf SR. Langerhans' cell histiocytosis with bilateral temporal bone involvement. Am J Otolaryngol. 2003;24:265–270. doi: 10.1016/S0196-0709(03)00049-8. [DOI] [PubMed] [Google Scholar]
- 8.Vezina JP, Audet N, Fradet G. Cerebrospinal fluid otorrhoea: A rare presentation of Langerhans' cell histiocytosis of the temporal bone. J Laryngol Otol. 2010;124:545–548. doi: 10.1017/S0022215109992295. [DOI] [PubMed] [Google Scholar]
- 9.Grant GA, Kim DK, Shaw CM, Berger MS. Solitary eosinophilic granuloma of the temporal lobe: Case report and review of the literature. Brain Tumor Pathol. 1999;16:55–59. doi: 10.1007/BF02478903. [DOI] [PubMed] [Google Scholar]
- 10.Isaacs H., Jr Fetal and neonatal histiocytoses. Pediatr Blood Cancer. 2006;47:123–129. doi: 10.1002/pbc.20725. [DOI] [PubMed] [Google Scholar]
- 11.Ha SY, Helms P, Fletcher M, Broadbent V, Pritchard J. Lung involvement in Langerhans' cell histiocytosis: Prevalence, clinical features, and outcome. Pediatrics. 1992;89:466–469. [PubMed] [Google Scholar]
- 12.Nanduri VR, Pritchard J, Chong WK, Phelps PD, Sirimanna K, Bailey CM. Labyrinthine involvement in Langerhans' cell histiocytosis. Int J Pediatr Otorhinolaryngol. 1998;46:109–115. doi: 10.1016/S0165-5876(98)00116-5. [DOI] [PubMed] [Google Scholar]
- 13.Ha SY, Helms P, Fletcher M, Broadbent B, Pritchard J. Lung involvement in Lanerhans' cell histiocytosis: Prevalence, clinical features, and outcome. Pediatrics. 1992;89:466–469. [PubMed] [Google Scholar]
- 14.Chen L, Wang WQ, Xu H, Chi FL. Langerhans cell histiocytosis of the temporal bone: 22 cases analysis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;45:212–216. (In Chinese) [PubMed] [Google Scholar]
- 15.Brown CW, Jarvis JG, Letts M, Carpenter B. Treatment and outcome of vertebral Langerhans cell histiocytosis at the Children's Hospital of Eastern Ontario. Can J Surg. 2005;48:230–236. [PMC free article] [PubMed] [Google Scholar]
- 16.Herwig MC, Wojno T, Zhang Q, Grossniklaus HE. Langerhans cell histiocytosis of the orbit: Five clinicopathologic cases and review of the literature. Surv Ophthalmol. 2013;58:330–340. doi: 10.1016/j.survophthal.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasper EM, Aguirre-Padilla DH, Alter RY, Anderson M. Histiocytosis X: Characteristics, behavior, and treatments as illustrated in a case series. Surg Neurol Int. 2011;2:57. doi: 10.4103/2152-7806.80122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Barros Fernandes Humberlo, Granjeiro RC, de Negreiros Júnior J. Langerhans Cell Histiocytosis in Otorhinolaryngology. 2009;13:444–449. [Google Scholar]
- 19.Bayazit Y, Sirikci A, Bayaram M, Kanlikama M, Demir A, Bakir K. Eosinophilic granuloma of the temporal bone. Auris Nasus Larynx. 2001;28:99–102. doi: 10.1016/S0385-8146(00)00078-X. [DOI] [PubMed] [Google Scholar]
- 20.Mosnier I, Rondini-Gilli E, Crosara PT, Belmatoug N, Cyna-Gorse F, Cazals-Hatem D, Abbey-Toby A, Bozorg-Grayeli A, Sterkers O. Langerhans' cell histiocytosis of the labyrinth in adults. Otol Neurotol. 2004;25:27–32. doi: 10.1097/00129492-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Martini A, Aimoni C, Trevisani M, Marangoni P. Langerhans' cell histiocytosis: Report of a case with temporal localization. Int J Pediatr Otorhinolaryngol. 2000;55:51–56. doi: 10.1016/S0165-5876(00)00374-8. [DOI] [PubMed] [Google Scholar]
- 22.Wu JJ, Huang DB, Pang KR, Hsu S, Tyring SK. Thalidomide: Dermatological indications, mechanisms of action and side-effects. Br J Dermatol. 2005;153:254–273. doi: 10.1111/j.1365-2133.2005.06747.x. [DOI] [PubMed] [Google Scholar]
- 23.Dornfeld S, Winkler C, Dörr W, Herrmann T. The radiotherapy of histiocytosis X. A case report of an eosinophilic granuloma in adulthood and a review of the literature. Strahlenther Onkol. 1998;174:534–535. doi: 10.1007/BF03038987. (In German) [DOI] [PubMed] [Google Scholar]
- 24.Heyd R, Strassmann G, Donnerstag F, Martin T, Zamboglou N. Radiotherapy in Langerhans-cell histiocytosis. 2 case reports and review of the literature. Rontgenpraxis. 1999;53:51–61. (In German) [PubMed] [Google Scholar]
- 25.Howarth DM, Gilchrist GS, Mullan BP, Wiseman GA, Edmonson JH, Schomberg PJ. Langerhans cell histiocytosis: Diagnosis, natural history, management, and outcome. Cancer. 1999;85:2278–2290. doi: 10.1002/(SICI)1097-0142(19990515)85:10<2278::AID-CNCR25>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]


