Abstract
Turner syndrome is a genetic disorder that results from an abnormal or missing X chromosome in females and is typically associated with impairments in visuospatial, but not verbal, information processing. These visuospatial processing impairments may be exacerbated with increased task demands, such as those engaged during working memory (WM). While previous studies have examined spatial WM function in Turner syndrome, none have directly compared the neural correlates of spatial and verbal WM processes across the encoding, maintenance and retrieval phases. We employed both neurocognitive assessments and functional MRI (fMRI) to examine the neural circuitry underlying both verbal and visuospatial WM functions in individuals with Turner syndrome and normal controls. We furthermore examined the vulnerability of task-related fMRI activation to distracters presented during WM maintenance. Fifteen healthy female volunteers and eight individuals with Turner syndrome performed a delayed-response WM task during fMRI scanning. Neurocognitive tests revealed impaired performance across both verbal and spatial domains in Turner syndrome, with greater impairment on tasks with WM demands. Frontoparietal regions in controls showed significantly sustained levels of activation during visuospatial WM. This sustained activation was significantly reduced in the group with Turner syndrome. Domain-specific activation of temporal regions, in contrast, did not differ between the two groups. Sensory distraction during the WM maintenance phase did not differentially alter frontoparietal activation between the two groups. The results reveal impaired frontoparietal circuitry recruitment during visuospatial executive processing in Turner syndrome, suggesting a significant role for the X chromosome in the development of these pathways.
Keywords: executive functions, fMRI, spatial working memory, Turner syndrome, verbal working memory
Keywords: fMRI = functional MRI, IFG = inferior frontal gyri, IPS = intraparietal sulci, ITG = inferior temporal gyri, MFG = middle frontal gyri, ROI = regions of interest, RT = reaction time, WM = working memory
Introduction
Turner syndrome is a genetic disorder that affects ∼1 in 2500 live-born girls (Lippe, 1996; Saenger, 1996; Ranke and Saenger, 2001) and results from an abnormal or missing second sex (i.e. X) chromosome. Individuals with Turner syndrome typically demonstrate an uneven profile of cognitive strengths and weaknesses, with greater difficulties in visuospatial processing accompanied by relative preservation of verbal skills (McCauley et al., 1987), suggestive of right hemisphere syndrome (Rovet, 1995; Buchanan et al., 1998). Cognitive deficits in Turner syndrome appear to become most pronounced under high task demand conditions, such as working memory (WM), particularly in visuospatial domains (Buchanan et al., 1998). These deficits may reflect a selective impairment in the engagement of higher-order executive control regions, such as the prefrontal cortex, with parietal regions during processing of visuospatial information.
Structural imaging studies have provided evidence of prefrontal and parietal pathology associated with higher-order visuospatial processing in Turner syndrome. Reduced parietal, parietal-occipital (Murphy et al., 1993; Reiss et al., 1995) and prefrontal volumes have been reported in Turner syndrome (Ross and Zinn, 1999). Furthermore, impaired performance on higher-order cognitive tasks in individuals with Turner syndrome, such as arithmetic calculation, has recently been linked to selective structural pathology in the intraparietal sulcus (Molko et al., 2003). Functional imaging studies using PET and functional MRI (fMRI) methods have also reported reduced activation in parietal regions both at rest (Clark et al., 1990) and during abnormal engagement of parietal and prefrontal areas in more challenging tasks (Tamm et al., 2003; Kesler et al., 2004). Haberecht et al. (2001) found that subjects with Turner syndrome showed decreased activation in the dorsolateral prefrontal cortex, caudate and inferior parietal lobes during the high-load, but not low-load, condition of a visuospatial WM task. The consistent abnormalities across functional and structural neuroimaging studies suggest deficient engagement of frontoparietal circuits in association with visuospatial executive dysfunction in Turner syndrome.
In the present study, we employed multiple assessment strategies to determine the integrity of WM functions in individuals with Turner syndrome when compared with a control group. Neurocognitive measurement and fMRI were used to determine the integrity of visual discrimination, visuospatial functions and working memory. For the fMRI, we employed a visuospatial delayed-recognition task to examine haemodynamic responses during the encoding, maintenance and retrieval phases of WM, both with and without distraction. As previous studies have suggested more significant deficits in visuospatial than verbal processing domains, we also designed a verbal WM task with a distracter condition to enable comparison of processing domains. We hypothesized that subjects with Turner syndrome would perform less capably than controls on the visuospatial tasks across assessment methods. We further hypothesized that differential activations between the group with Turner syndrome and controls would be demonstrated during the visuospatial, but not verbal, WM task, and that these differences would involve the frontoparietal circuitry. Finally, given the postulated executive function impairments in Turner syndrome, we predicted that the differences between the groups would become more pronounced in the presence of distracters. The distracter condition requires the ability to suppress task-irrelevant perceptual information that interferes with WM maintenance.
Material and methods
Subjects
Ten females with monosomic (45,X) Turner syndrome (age range 14–29 years, mean = 21.4 years, all right-handed) and 15 control female subjects (age range 19–26 years, mean = 22.3 years, 14 right-handed) were recruited for this study. All individuals with Turner syndrome were recruited through the UNC Pediatric Endocrinology Turner Syndrome Clinic. All participants with Turner syndrome had begun oestrogen replacement therapy. Volunteers with Turner syndrome were excluded if they had a karyotype other than 45,X. Individuals with Turner syndrome were also excluded from participation if they had a history of a significant neurological disorder or injury, a history of drug or alcohol abuse disorders, an estimated verbal IQ less than 85 as measured by the Wechsler Abbreviated Scales of Intelligence (WASI) Vocabulary Subtest (The Psychological Corporation, 1998) or a major medical condition not typically associated with Turner syndrome. For the fMRI, one participant with Turner syndrome was excluded because of excessive head motion (>5 mm) during the scan, and one additional participant with Turner syndrome was excluded owing to artefact from a metal dental appliance. Therefore, eight volunteers with Turner syndrome participated in the fMRI portion of the study. All participants included in the fMRI analyses had average head movement displacement values within one voxel (4 mm in either direction). All 10 volunteers with Turner syndrome participated in the neurocognitive assessments, in addition to 10 healthy controls. Female control subjects were excluded for a history of a significant neurological disorder or injury, estimated verbal IQ less than 85, history of treatment for a major psychiatric illness, history of chronic drug or alcohol abuse or a significant, chronic medical condition. This study was approved by the Duke University Medical Center Institutional Review Board and the University of North Carolina at Chapel Hill Committee on the Protection of the Rights of Human Subjects. All participants provided informed consent according to the Declaration of Helsinki.
Stimuli and tasks
Neurocognitive assessment
Visual processing was assessed in all subjects with a battery of tasks with demonstrated reliability and validity. Tasks were selected to determine the integrity of visual discrimination functions, visuospatial functions and WM. In addition to providing evidence for the presence of the characteristic phenotypical presentation in Turner syndrome, these tasks were selected to provide a clinical correlate to measures employed in the fMRI paradigm.
Visual discrimination ability was tested using the Benton Face Recognition Test, which tests the subject's ability to identify faces, while visuospatial functions were tested using the Benton Judgment of Line Orientation Test (Benton et al., 1983). Visuospatial WM was assessed using the Wechsler Memory Scale—III Spatial Span Subtest, which consists of a board with nine blocks attached to it in no clear identifiable pattern (The Psychological Corporation, 1998). The examiner pointed to a sequence of blocks starting from a span of two, and then instructed the individual to point to the same blocks in the same order. If successful, the spans increased by one block each time. If not successful, a second trial of the same span was administered. Testing was discontinued if both trials at any particular span length failed. For standardization purposes, both the forward and backward recall conditions of the Spatial Span Subtest were assessed in this study, but only the backward recall Spatial Span component was employed as the measure of WM, given the increased demands on visual WM and executive functions.
Verbal WM was assessed using the Woodcock–Johnson-III Numbers Reversed Subtest, in which participants were asked to repeat a series of increasingly longer digit sequences in reverse order. The score was the total number of correct responses (Woodcock et al., 2001). The backwards digit span test measures the ability to encode a series of verbally presented digits, maintain them in WM, transform the order of the items and recall them. Recalling the digits in backwards order demands a greater WM load than in the forward recall condition, requiring an additional executive component. This verbal WM test provides a complementary measure to the spatial WM component of the spatial span task.
In addition, the Vocabulary Subtest from the WASI was administered to estimate verbal IQ. This measure was used to screen participants with low verbal IQ, in accordance with our inclusion/exclusion criteria, and was examined as a possible covariate in subsequent analyses.
Functional MRI working memory task
Following Jha and McCarthy (2000), a delayed-response task was developed in which a visual stimulus array (S1) was presented for 4 s, followed by a delay interval of 17 s, followed then by a probe stimulus (S2) presented for 4 s, to which the subject responded with a button press. A total of 10 S1–S2 trials were presented in each run, lasting ∼8 min. The inter-trial interval (ITI), as measured from the end of the S2 period to the beginning of the S1 period in the following trial, was 18.5 s. An experimental session consisted of eight runs and lasted no longer than 2 h.
Stimuli were presented using an LCD projector and were back-projected upon a 10-inch wide screen located within the magnet bore directly behind the subject's head. All stimuli were presented using the CIGAL display environment (Vovyodic, 1999) and were viewed using mirrored glasses. Responses were acquired using a fibre-optic button box. Accuracy and reaction times (RTs) were recorded by the experimental control software. Within each experimental session, both the neural correlates of spatial and verbal WM function (manipulation of domain specificity) and the effects of distracters on the maintenance period activity (manipulation of distractibility) were assessed.
Domain specificity of WM was manipulated using a delayed-response design by presenting two different types of stimuli to be remembered: verbal and spatial. In condition 1 (verbal WM), four 5-letter words were presented at S1 for 1 s each (Fig. 1). S2 consisted of a single 5-letter word presented for 4 s that was, or was not, present in the S1 memory set. Using a forced response paradigm, subjects indicated whether the S2 stimulus was present in the S1 memory set by pressing a button on the response box. Words were chosen that could not be easily associated with a visual or object representation. In condition 2 (spatial WM), S1 was composed of 4 squares presented for 1 s each, appearing randomly in 1 of 12 possible locations (Fig. 1). At S2, the square was presented for 4 s and appeared in 1 of the 12 possible locations. Subjects indicated by button press whether the S2 stimulus appeared in one of the same locations as the S1 stimuli.
Fig. 1.
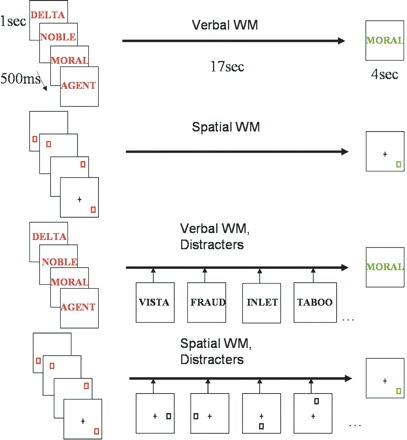
Verbal and spatial delayed-response tasks. The memory array (S1) was presented for 4 s, and a delay of 17 s ensued, followed by a probe stimulus (S2) for 4 s. On half of the trials, distracters were presented during the delay period.
We also examined the effects of distractibility upon verbal and spatial WM. Distracters occurred on half of the trials and were randomly distributed. The tasks were identical to those described above as conditions 1 and 2, with the addition of distracters presented during the maintenance period. Accordingly, condition 3 (verbal distracters) was identical to condition 1, with the addition of a series of briefly presented words appearing on the screen during the delay interval. A total of nine distracters were presented for a duration of 1 s each, with 500 ms interstimulus intervals. Subjects were asked to simply monitor the words and were not required to produce a response (perceptual interference). In condition 4 (spatial distracters), subjects were presented with squares that appeared in a series of random locations on the screen during the delay period. Again, the goal of the secondary monitoring task presented during the maintenance interval was to introduce a perceptual distractibility or interference condition.
Neurocognitive data analysis
The neurocognitive testing procedures were obtained on 20 of the subjects, 10 with Turner syndrome and 10 controls. Two individuals with Turner syndrome were included in these neurocognitive testing results, but not in the fMRI analyses, because of excessive head motion and artefacts. Group differences in age, race and vocabulary were examined, and subsequent univariate analyses were performed to examine group differences on the four neurocognitive measures.
Imaging behavioural data analysis
RT and accuracy (% correct) measures were obtained from all 8 subjects with Turner syndrome and from 14 control subjects. Repeated-measures analysis of variance (ANOVA) tests were run on RT and % correct measurements to assess the effects of domain, distraction and group (control versus Turner syndrome).
Functional MRI acquisition and analyses
The scans were performed on a General Electric 1.5 T NVi system. Imaging began with the acquisition of a T1-weighted sagittal localizer series to identify the anterior (AC) and posterior commissures (PC) and to prescribe contiguous oblique slices parallel to the AC–PC plane. These series were followed by the acquisition of 68 slices of high-resolution T1-weighted structural images [repetition time (TR) = 12.3 ms, echo time (TE) = 5.4 ms, field of view (FOV) = 24 cm, 256 × 256 matrix, slice thickness 1.9 mm], oriented parallel to the AC–PC. Thirty-four contiguous functional images sensitive to BOLD contrast were acquired at the same slice locations as the structural images (TE = 30 ms, 24 cm FOV, 64 × 64 image matrix, 90° flip angle, TR = 1.5 s, slice thickness = 3.8 mm, yielding 3.8 mm3 isotropic voxels).
The time courses of the voxels in each slice were realigned with the onset of the TR, in order to compensate for the interleaved slice acquisition order. Images were preprocessed using SPM99 (http://www.fil.ion.ucl.ac.uk/spm). Volumes were spatially aligned to a reference volume using translation and rigid rotation, in order to correct for head movements. Individual subjects' functional images were co-registered with their high-resolution anatomical images. The data were normalized to standard Talairach coordinates, using the standard SPM/MNI T1 template image. Images were resampled using sinc interpolation and smoothed with an 8 mm Gaussian kernel to improve the signal-to-noise ratio.
For the voxel-based analysis, epochs beginning 4 time points prior to S1-onset and continuing for 29 time points following S1 were excised from the continuous time series of non-normalized co-registered raw images for each condition. Signal averaging was performed on these excised epochs with separate average epochs created for each experimental condition. The average MR signal values were then converted to per cent signal change relative to a pre-S1 baseline, defined as the four time points preceding S1. These time course analyses were performed on the raw, TR-aligned functional data that had not undergone smoothing or normalization.
For the anatomical regions of interest (ROI) analysis, selected structures in frontoparietal and frontotemporal regions associated with functional and structural abnormalities in Turner syndrome were drawn by a single observer on each individual subject's high-resolution structural MRIs (see Supplementary Fig. 1S available at Brain online). Selected ROIs included the left and right middle frontal gyri (MFG), inferior frontal gyri (IFG), intraparietal sulci (IPS) and inferior temporal gyri (ITG). ROIs were drawn using a three-dimensional interactive image segmentation program (IRIS/SNAP) (Ho et al., 2002) using landmarks from the Duvernoy's Human Brain Atlas (1999). All ROI analyses were performed on individual subjects' non-normalized brains. No ROIs were found to differ significantly in size (i.e. number of voxels) between controls and the group with Turner syndrome.
Using custom MATLAB software, ROIs were interrogated with a function that returned the number of voxels within the selected ROI that correlated significantly with an ideal haemodynamic response waveform (t > 1.96, P < 0.05) (Jha and McCarthy, 2000). This measurement of per cent activated voxels reflected the spatial extent of activation relative to ROI size. Relative per cent signal change was calculated for each ROI, by interrogating them with a function that returned the average time-varying signal change of the voxels within the selected regions. Differences between the waveforms evoked by the four different experimental conditions were tested by measuring the signal over particular time periods, allowing for the separate assessment of condition and population effects in the S1 period, delay period and S2 period. The per cent signal change at four different latencies in each epoch (time points 9, 12, 18 and 24 relative to epoch onset) were selected as measures of peak haemodynamic responses associated with the encoding (time points 9 and 12), maintenance (time point 18) and retrieval (time point 24) phases of the WM trials.
Repeated-measures ANOVAs were performed on the peak amplitudes at the selected time points to examine group, anatomical structure, hemisphere and experimental condition differences. Quantitative statistical analyses were performed on the dependent measure (% signal change) with group as a between-subjects variable, and task phase (encode, maintain and retrieve) and hemisphere as within-subject repeated measures. Post hoc analyses were then conducted to assess the direction and pairwise effects at each level. Repeated-measures ANOVAs were used to identify group differences in the peak measures.
Results
Neurocognitive testing results
Initial group comparisons indicated that individuals with Turner syndrome and controls had significantly different verbal IQs [t(18) = 6.97, P < 0.0001], with the controls having a higher level of vocabulary (Turner syndrome mean T-score = 54.20, SD = 5.88; controls mean T-score = 68.40, SD = 5.60). No significant differences were found for age (Turner syndrome mean = 19.8, controls mean = 22.9) or race, although there was a strong trend for the Turner syndrome group to be younger [t(18) = 2.05, P < 0.06]. Given these findings, subsequent analyses were co-varied for estimated IQ and chronological age.
Univariate ANCOVAs (analysis of covariance), controlling for estimated IQ and age, revealed that the group with Turner syndrome performed more poorly than controls on every task except for Facial Recognition (Table 1). On the Judgment of Line Orientation Test, WJ-III Numbers Reversed and WMS-III Spatial Span Reversed, the Turner syndrome group performed significantly lower than the control group. Effect sizes were moderate to large, ranging from 0.63 for WMS-III Spatial Span Reversed to 0.72 for WJ-III Numbers Reversed. These findings suggest that while visuospatial processing may be disrupted in Turner syndrome, consistent with the classic Turner syndrome phenotype, WM functions—both verbal and visuospatial—may also be significantly impaired.
Table 1.
Performance on neurocognitive tests
| Turner syndrome |
Normal controls |
|||||||
|---|---|---|---|---|---|---|---|---|
| Tasks |
Mean |
SD |
Mean |
SD |
F(3, 15) |
Effect size |
||
| Facial recognition | 22.22 | 2.64 | 24.70 | 1.49 | 2.90 | 0.37 | ||
| Judgement of line orientation | 20.67 | 7.43 | 26.70 | 2.26 | 11.75*** | 0.70 | ||
| WMS-III spatial span reverse | 5.56 | 2.13 | 9.50 | 1.84 | 8.50** | 0.63 | ||
| WJ-III numbers reversed | 14.11 | 5.58 | 24.30 | 4.17 | 12.77*** | 0.72 | ||
Comparisons of the Turner syndrome group versus the control group on the neurocognitive measures controlling for age and estimated verbal IQ (**P < 0.01, ***P < 0.0001). All scores are reported as raw scores.
Imaging behavioural results
ANOVA tests were performed on the dependent variables of accuracy (% correct) and RT, to assess the between-subject factor of group (controls versus Turner syndrome) and the within-subject factors of domain (verbal versus spatial) and distraction. Behavioural results for accuracy and RT are displayed in Fig. 2A and 2B. Participants with Turner syndrome were less accurate (83% correct, SD = 15.69) than controls [91% correct, SD = 10.88, F(1,21) = 13.39; P < 0.002] and had slower response times (1865 ms, SD = 465 ms) than controls [1349 ms, SD = 442 ms, F(1,21) = 14.36; P = 0.001]. For both groups, accuracy was better for the verbal WM condition (96% correct, SD = 5.22) than the spatial condition (79% correct, SD = 13.47), and RTs were faster for the verbal (1267 ms, SD = 434 ms) than the spatial WM conditions (1807 ms, SD = 440 ms) [domain effect on accuracy, F(1,21) = 48.1; P < 0.0001, and RT, F(1,21) = 55.23; P < 0.0001]. Although the individuals with Turner syndrome were less accurate in the spatial WM task than in the verbal WM task, this effect did not reach significance [group by domain interaction, F(1,21) = 2.49; P = 0.13]. There was also no significant group by domain interaction in the RT data [F(1,21) = 0.3; P = 0.59].
Fig. 2.
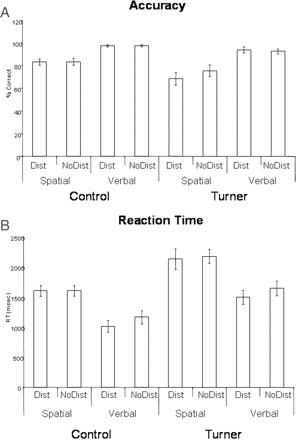
Behavioural results for spatial and verbal WM, with and without distraction (Dist versus NoDist). (A) Mean per cent correct with standard error bars. (B) Mean RT with standard error bars.
Distraction had a significant overall effect on RT [F(1,21) = 8.0; P = 0.01], but had no significant effect on accuracy. No significant interactions were found between group and distraction for either accuracy or RT. Accuracy on the spatial task was more vulnerable to distraction than the verbal task [domain by distracter interaction, F(1,21) = 4.8; P < 0.04]. In addition, RTs on the spatial task were more vulnerable to distraction than the verbal task [domain by distracter interaction, F(1,21) = 4.98; P = 0.037].
Functional MRI results
We performed time-course analyses on the significantly activated voxels in each ROI to compare the haemodynamic changes between groups and conditions throughout the WM trials. Group and condition effects were assessed for the encoding (time points 9 and 12), maintenance (time point 18) and retrieval (time point 24) phases of the trials for each ROI. All significant statistics for each time point, including the effects of group and domain are displayed in Table 2, with hemispheric laterality effects displayed in Table 3 (see Supplementary Figs 2S and 3S for group average activation maps). Statistics for the distracter condition are displayed in Supplementary Table 1S.
Table 2.
Effects of group and domain on fMRI per cent signal change
| 9 |
12 |
18 |
24 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test |
ROI |
F
|
P
|
F
|
P
|
F
|
P
|
F
|
P
|
||||
| Group | ITG | 5.71 | 0.026 | 6.56 | 0.018 | ||||||||
| IPS | 5.9 | 0.024 | |||||||||||
| Domain | ITG | 9.41 | 0.006 | 4.87 | 0.039 | ||||||||
| IPS | 44.45 | <0.0001 | 44.04 | <0.0001 | 21.77 | <0.0001 | 84.06 | <0.0001 | |||||
| MFG | 9.1 | 0.007 | 8.16 | 0.009 | |||||||||
| Group by domain | IPS | 7.27 | 0.014 | ||||||||||
| MFG | 6.71 | 0.017 | 6.4 | 0.019 | 4.25 | 0.052 | |||||||
| IFG | 6.36 | 0.02 | 15.78 | 0.0007 | 4.71 | 0.042 | |||||||
F and P values for the encode (time point 9 and 12), maintain (time point 18) and retrieve (time point 24) periods of the task, for significantly active voxels in each ROI. ROIs that were not active for a given test at any time point are not displayed.
Table 3.
Hemispheric effects on fMRI per cent signal change
| 9 |
12 |
18 |
24 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Test |
ROI |
F
|
P
|
F
|
P
|
F
|
P
|
F
|
P
|
||||
| Hemisphere | ITG | 4.39 | 0.048 | 4.21 | 0.053 | ||||||||
| IPS | 14.76 | 0.001 | |||||||||||
| MFG | 13.8 | 0.001 | |||||||||||
| IFG | 14.03 | 0.001 | |||||||||||
| Hemisphere by group | ITG | 5.54 | 0.028 | 7.21 | 0.014 | 6.53 | 0.018 | 4.28 | 0.051 | ||||
| IFG | 5.13 | 0.034 | |||||||||||
| Hemisphere by domain | ITG | 24.04 | <0.0001 | 22.85 | <0.0001 | 8.97 | 0.007 | ||||||
| IPS | 14.74 | 0.001 | |||||||||||
| MFG | 16.14 | 0.001 | 18.03 | 0.0004 | 10.96 | 0.003 | |||||||
| IFG | 39.71 | <0.0001 | 22.65 | <0.0001 | 5.25 | 0.032 | 5.94 | 0.024 | |||||
Laterality effects: F and P values for the encode (time point 9 and 12), maintain (time point 18) and retrieve (time point 24) periods of the task, for significantly active voxels in each ROI. ROIs that were not active for a given test at any time point are not displayed. Only those interactions that were significant at any time point are displayed.
Intraparietal sulcus
Consistent with posterior parietal cortex involvement in spatial processing, the IPS was activated significantly more strongly by the spatial domain than by the verbal domain at all time points (domain effect, Table 2), with significantly greater right-hemisphere dominance during the spatial task (hemisphere effect, Table 3). The average time course of activation in the IPS during each WM task is displayed in Fig. 3, for both the control and Turner syndrome groups. As reflected in the time-course graph of the controls' activation of the IPS (Fig. 3A), during the visuospatial WM task, they displayed highly sustained levels of activation throughout the maintenance period. The maintenance-phase activation in the group with Turner syndrome, in contrast, was significantly decreased relative to controls (Fig. 3B). During the verbal WM condition, however, the two groups showed similar patterns of haemodynamic change throughout the task. The two groups activated the IPS differentially according to domain during the maintenance phase, as measured by time point 18 activation (group by domain interaction, Table 2). Consistent with our hypothesis, the group with Turner syndrome therefore showed different parietal activation patterns on the spatial, but not verbal, task.
Fig. 3.
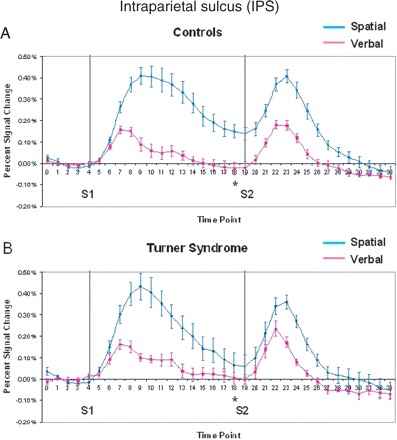
Mean per cent signal change within active voxels for the intraparietal sulcus in (A) controls and (B) Turner syndrome. Grey lines indicate onset of S1 and S2 periods. Asterisks indicate those tested time points (i.e. 9, 12, 18, 24) that had significant group by domain interactions.
Controlling for age had no effect on the group by domain interaction in the IPS [F(1,20) = 6.5; P = 0.02], but when accuracy was controlled for, the interaction did not reach significance [F(1,17) = 2.22; P = 0.15], suggesting that group differences in IPS maintenance levels were partially explained by their differences in performance. Contrary to our hypothesis that group differences would be enhanced in the presence of distracters, no group by distracter interactions were found.
Middle frontal gyrus
The MFG was activated significantly more strongly by the spatial task than the verbal task (domain effect, Table 2) at time points 12 and 24, with significantly greater right-hemisphere dominance during the spatial task (Table 3). The average time course of activation in the MFG during each WM task is displayed in Fig. 4 for both groups. Controls displayed sustained activation during the maintenance phase of the spatial WM task, while activation during the verbal WM task returned to baseline between the encoding and retrieval phases (Fig. 4A). In the group with Turner syndrome, the MFG showed less differentiation in activation related to domain, and showed slightly greater activation during verbal WM maintenance compared with the spatial task (Fig. 4B). The two groups therefore activated the MFG differentially according to domain during the maintenance and retrieval phases (group by domain interaction at time points 12, 18 and 24, Table 2).
Fig. 4.
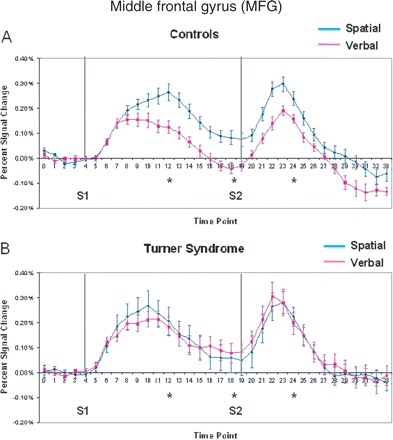
Mean per cent signal change within active voxels for the middle frontal gyrus in (A) controls and (B) Turner syndrome. Grey lines indicate onset of S1 and S2 periods. Asterisks indicate those tested time points that had significant group by domain interactions.
No significant effects of age or performance were found on MFG activation. Also, no significant group by distracter interactions were found.
Inferior frontal gyrus
The IFG did not show overall significantly different activation patterns depending on the task domain (Table 2). The average time course of activation in the IFG during each WM task is displayed in Fig. 5 for both groups. While controls showed a more similar pattern of activation for both verbal and spatial tasks, with slightly greater activation to the spatial task (Fig. 5A), the individuals with Turner syndrome showed larger activation to the verbal task relative to the spatial task (Fig. 5B). Although controls and subjects with Turner syndrome did not differ in their overall IFG activation levels, the two groups activated the IFG differentially according to domain for time points 12, 18 and 24 (group by domain interaction, Table 2).
Fig. 5.
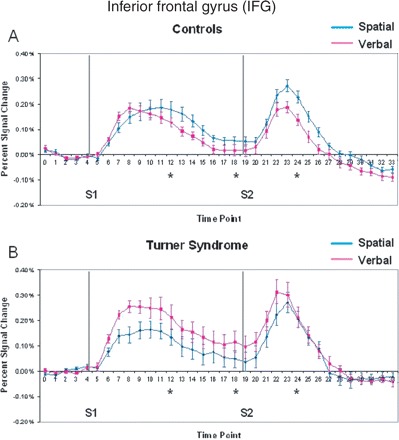
Mean per cent signal change within active voxels for the inferior frontal gyrus in (A) controls and (B) Turner syndrome. Grey lines indicate onset of S1 and S2 periods. Asterisks indicate those tested time points that had significant group by domain interactions.
No significant effects of age or performance were found on IFG activation. A significant group by distraction effect was found (see supplementary Table 1S). Finally, the IFG activation patterns indicated that controls showed more right-lateralized activation at encoding, whereas subjects with Turner syndrome showed more left-lateralized activation (hemisphere by group interaction, Table 3).
Inferior temporal gyrus
Consistent with temporal cortex involvement in verbal processing, the ITG activated more strongly to the verbal than spatial task for both groups at encoding (Table 2), and showed significantly greater left-hemisphere dominance during the verbal task (hemisphere by domain, Table 3). The average time course of activation in the ITG during each WM task is displayed in Fig. 6 for both groups. The group with Turner syndrome showed overall greater activation in the ITG compared with controls (group effect, Table 2). Both the controls and individuals with Turner syndrome showed greater activation at encoding to the verbal task, but the group with Turner syndrome showed a larger increase in this encoding-related verbal activation (Fig. 6A and B). Despite the greater activation during the verbal task in subjects with Turner syndrome relative to controls, the group by domain interaction did not reach significance.
Fig. 6.
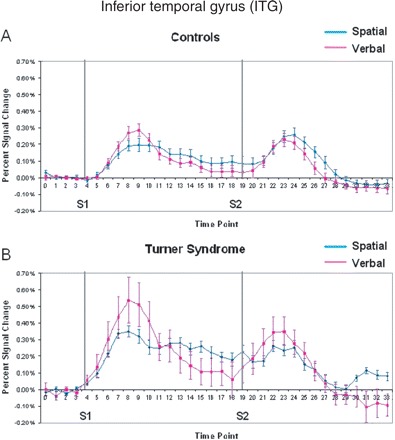
Mean per cent signal change within active voxels for the inferior temporal gyrus in (A) controls and (B) Turner syndrome. Grey lines indicate onset of S1 and S2 periods.
No significant effects of age or performance were found on ITG activation. A significant group by distraction effect was found, which differed by task domain (see Supplementary Table 1S). Hemispheric effects showed a bias in the ITG at encoding and maintenance during the verbal WM task for both groups, but the subjects with Turner syndrome showed significantly greater left-side bias on the verbal task relative to controls (hemisphere by group interaction, Table 3).
Discussion
In the present study, we examined neurobehavioural correlates of verbal and spatial WM processes in healthy controls and in individuals with Turner syndrome, with further examination of vulnerability to distraction in both groups. Our results produced the classic phenotypical profile of visuospatial weaknesses that has been proliferated in the literature on children and adults with Turner syndrome. However, our findings also revealed significant WM differences suggestive of executive dysfunction across methods of measurement that may co-exist with or underlie the visuospatial weaknesses in this population. More specifically, our findings demonstrated changes in prefrontal and posterior parietal function in Turner syndrome, which were particularly pronounced during the period of visuospatial WM maintenance.
The patterns of activation in frontoparietal regions indicated that the subjects with Turner syndrome engaged these regions to a lesser degree, particularly during spatial WM performance, whereas they were able to engage these regions normally during verbal WM. Several additional studies have similarly found deficient engagement of these regions in Turner syndrome during spatial WM (e.g. Haberecht et al., 2001; Kesler et al., 2004). The data in the current study suggests that the inability of individuals with Turner syndrome to engage prefrontal and posterior parietal regions may be primarily explained by dysfunction within the maintenance phase, whereas these regions may function normally in Turner syndrome during WM encoding and retrieval processes. Although the present study is limited by the relatively small sample size of participants with Turner syndrome, this lack of sustained engagement of posterior parietal regions was observed consistently across individuals in the Turner syndrome group. Within the group with Turner syndrome, only 2 out of 8 individuals activated posterior parietal regions above baseline levels during the WM delay, whereas 13 out of 15 control participants showed sustained activity. While additional studies will certainly be needed to support these findings of abnormal spatial WM delay activity, this pattern showing lack of parietal engagement appears to be relatively robust across individuals.
Because the participants with Turner syndrome performed more poorly than controls on the WM tasks, it is possible that group differences in brain activation may be partially explained by task performance. Indeed, a covariance analysis of our data indicated that WM performance significantly predicted activation in the intraparietal sulcus, although it did not predict activation in the other ROI. Analyses comparing correct versus incorrect trials indicated that while the group with Turner syndrome was able to adequately engage parietal regions during their correct trials in a similar manner to controls, their incorrect trials were associated with abnormally low levels of delay period activation during the spatial WM delay phase. Posterior parietal dysfunction during spatial WM maintenance in Turner syndrome may therefore be an important factor underlying their visuospatial cognitive difficulties. As there is considerable cognitive variability among individuals with Turner syndrome, future studies may further clarify the relationship between individual visuospatial abilities and the degree to which these parietal mechanisms are engaged.
Analyses of the behavioural results indicated that although the individuals with Turner syndrome showed the expected differences on the spatial neurocognitive tasks and on the spatial WM task completed in the fMRI scanner, the group with Turner syndrome also had lower estimated verbal IQ and concomitant weaker verbal WM. On the surface, these findings would seem to contrast with the Turner syndrome literature that typically characterizes their deficits as primarily in the visuospatial domain. A potential reason for this finding could be that our sample of participants with Turner syndrome was biased towards having greater verbal deficits than controls, related to the sample size. However, these deficits would support the presence of higher-order executive dysfunctions (e.g. working memory) that may in turn contribute to the significantly poorer verbal performance in the group with Turner syndrome when compared with controls. Other studies of Turner syndrome have indeed found retrieval impairments during verbal fluency tasks with relatively large executive demands (Temple, 2002), and poorer performance on verbal executive tasks requiring cognitive flexibility and WM (Kirk et al., 2005). It is possible therefore that a global executive function deficit in Turner syndrome may be responsible for poorer performance on a multitude of cognitively demanding tasks that require planning, attention shifting and WM. This executive dysfunction in Turner syndrome may explain multiple aspects of the neurocognitive profile, such as arithmetic deficits (e.g. Rovet et al., 1993), attentional abilities (e.g. Romans et al., 1998) and inhibitory control (Tamm et al., 2003). The executive function deficit in Turner syndrome may therefore only be apparent on tasks with sufficient complexity to engage prefrontal mechanisms.
We directly manipulated executive requirements of our task by including a distracter condition where irrelevant stimuli were presented during the WM delay. However, contrary to our hypotheses, our results indicated that the presence of distracters on both tasks did not significantly affect performance in either group, nor did the distracters largely affect the Turner syndrome group's activation patterns more than controls. It is possible that because the distracters involved passive viewing of perceptual stimuli, they may not have provided significant interference to require the engagement of the executive attention component of WM. While lower level sensory processing mechanisms may be intact in Turner syndrome, it is possible that increasing the executive load of the task to a level sufficient to interfere with WM rehearsal could lead to greater sensitivity in Turner syndrome.
In addition to frontoparietal dysfunction in the group with Turner syndrome, our results also revealed a pattern of over-engagement of inferior frontal and temporal regions in subjects with Turner syndrome compared with controls, suggesting that the neurophysiological differences in Turner syndrome may not be restricted to frontoparietal regions. Several structural imaging studies have found specific temporal lobe abnormalities in Turner syndrome, such as alterations in temporal lobe fibre tracts (Molko et al., 2004) and larger superior temporal gyri, suggesting the disruption of neural pruning mechanisms during development (Kesler et al., 2003). These temporal lobe abnormalities have been suggested to possibly underlie deficits in language tasks such as semantic fluency in Turner syndrome (Rae et al., 2004), which may require greater executive demand than reading tasks where individuals with Turner syndrome have been found to perform normally (Temple and Carney, 1996). Structural and functional changes may therefore be apparent in multiple brain regions in Turner syndrome, which may possibly underlie some of their executive deficits or potentially reflect compensatory mechanisms.
Because of the observed changes in brain structure and function in Turner syndrome, the X chromosome has been proposed to play a particularly significant role in the development of circuitry underlying visuospatial and executive function (Skuse, 2005). It has been hypothesized that the brains of individuals with Turner syndrome develop abnormally during gestation, as a result of missing genetic material responsible for neural pruning mechanisms (Rae et al., 2004). The cognitive deficits in Turner syndrome may arise from a reduced dosage of a gene or genes on the X chromosome (Buchanan et al., 1998), as evidenced by studies showing that individuals with a higher percentage of normal 46,XX cells perform better on visuospatial processing tasks than those with the 45,X karyotype (Murphy et al., 1993). Furthermore, mapping of deletions using molecular markers has shown that a 2 Mb critical region of the X chromosome is associated with visuospatial deficits in Turner syndrome (Ross et al., 2000). Cognitive assessments of women with fragile X syndrome have also suggested specific deficits in visuospatial ability and executive function (Bennetto et al., 2001), affecting the development of complex cognitive functions (Cornish et al., 2004). Future studies combining neuroimaging with molecular genetic techniques will help in elucidating the relationships between neurobiological and genetic markers of the cognitive deficits associated with X chromosome abnormalities.
Our findings support the characteristic phenotypic description of visuospatial dysfunction in Turner syndrome found in the literature, but they also extend this description by providing evidence of frontal-temporal and frontal-parietal involvement as well, thus contributing to the complex pathophysiology inherent in Turner syndrome. The results suggest that executive function impairments in Turner syndrome may affect a multitude of cognitive tasks that have a sufficient level of complexity or difficulty. However, the frontoparietal circuitry may be particularly vulnerable to dysfunction during visuospatial WM in Turner syndrome and is characterized by a relative lack of sustained engagement during the maintenance of spatial information.
Supplementary material
See supplementary material available at Brain online.
Supplementary Material
Acknowledgments
We thank V. Allen Santos for assistance in patient recruitment and Dr Joseph Piven for valuable contributions to the research. This research was supported by the National Institutes of Health grant 1R03HD40249. Funding to pay the Open Access publication charges for this article was provided by the University of North Carolina at Chapel Hill, Department of Psychiatry.
References
- Bennetto L, Pennington BF, Porter D, Taylor AK, Hagerman RJ. Profile of cognitive functioning in women with the fragile X mutation. Neuropsychology 2001; 15: 290–9. [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Varney NR, Spreen O. Contributions to neuropsychological assessment: a clinical manual. New York: Oxford University Press; 1983.
- Buchanan L, Pavlovic J, Rovet J. A rexamination of the visuospatial deficit in turner syndrome: contributions of working memory. Dev Neuropsychol 1998; 14: 341–67. [Google Scholar]
- Clark C, Klonoff H, Hayden M. Regional cerebral glucose metabolism in Turner syndrome. Can J Neurol Sci 1990; 17: 140–4. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Turk J, Wilding J, Sudhalter V, Munir F, Kooy F, et al. Annotation: deconstructing the attention deficit in fragile X syndrome: a developmental neuropsychological approach. J Child Psychol Psychiatry 2004; 45: 1042–53. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human brain: surface, blood supply, and three-dimensional sectional anatomy. New York: Springer-Verlag/Wien; 1999.
- Haberecht MF, Menon V, Warsofsky IS, White CD, Dyer-Friedman J, Glover GH, et al. Functional neuroanatomy of visuo-spatial working memory in Turner syndrome. Hum Brain Mapp 2001; 14: 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S, Bullit, E, Gerig, G. Level set evolution with region competition: automatic 3-D segmentation of brain tumors. Proceedings of the 16th International Conference On Pattern Recognition. ICPR: IEEE Computer Society; 2002.
- Jha AP, McCarthy G. The influence of memory load upon delay-interval activity in a working-memory task: an event-related functional MRI study. J Cogn Neurosci 2000; 12 (Suppl 2): 90–105. [DOI] [PubMed] [Google Scholar]
- Kesler SR, Blasey CM, Brown WE, Yankowitz J, Zeng SM, Bender BG, et al. Effects of X-monosomy and X-linked imprinting on superior temporal gyrus morphology in Turner syndrome. Biol Psychiatry 2003; 54: 636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesler SR, Haberecht MF, Menon V, Warsofsky IS, Dyer-Friedman J, Neely EK, et al. Functional neuroanatomy of spatial orientation processing in Turner syndrome. Cereb Cortex 2004; 14: 174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk JW, Mazzocco MM, Kover ST. Assessing executive dysfunction in girls with fragile X or Turner syndrome using the Contingency Naming Test (CNT). Dev Neuropsychol 2005; 28: 755–77. [DOI] [PubMed] [Google Scholar]
- Lippe BM. Turner syndrome. In: Sperling MA, editor. Pediatric endocrinology. Philadelphia, PA: W.B. Saunders Co; 1996. p. 387–421.
- McCauley E, Kay T, Ito J, Treder R. The Turner syndrome: cognitive deficits, affective discrimination, and behavior problems. Child Dev 1987; 58: 464–73. [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, Le Bihan D, et al. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron 2003; 40: 847–58. [DOI] [PubMed] [Google Scholar]
- Molko N, Cachia A, Riviere D, Mangin JF, Bruandet M, LeBihan D, et al. Brain anatomy in Turner syndrome: evidence for impaired social and spatial-numerical networks. Cereb Cortex 2004; 14: 840–50. [DOI] [PubMed] [Google Scholar]
- Murphy DG, DeCarli C, Daly E, Haxby JV, Allen G, White BJ, et al. X-chromosome effects on female brain: a magnetic resonance imaging study of Turner's syndrome. Lancet 1993; 342: 1197–200. [DOI] [PubMed] [Google Scholar]
- Rae C, Joy P, Harasty J, Kemp A, Kuan S, Christodoulou J, et al. Enlarged temporal lobes in Turner syndrome: an X-chromosome effect? Cereb Cortex 2004; 14: 156–64. [DOI] [PubMed] [Google Scholar]
- Ranke MB, Saenger P. Turner's syndrome. Lancet 2001; 358: 309–14. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Mazzocco MM, Greenlaw R, Freund LS, Ross JL. Neurodevelopmental effects of X monosomy: a volumetric imaging study. Ann Neurol 1995; 38: 731–8. [DOI] [PubMed] [Google Scholar]
- Romans SM, Stefanatos G, Roeltgen DP, Kushner H, Ross JL. Transition to young adulthood in Ullrich-Turner syndrome: neurodevelopmental changes. Am J Med Genet 1998; 79: 140–7. [PubMed] [Google Scholar]
- Ross JL, Zinn A. Turner syndrome: potential hormonal and genetic influences on the neurocognitive profile. In: Tager-Flusberg, editor. Neurodevelopmental disorders 1999; 251–68.
- Ross JL, Roeltgen D, Kushner H, Wei F, Zinn AR. The Turner syndrome-associated neurocognitive phenotype maps to distal Xp. Am J Hum Genet 2000; 67: 672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovet JF. Behavioral manifestations of Turner syndrome in children: a unique phenotype. In: Albertsson-Wikland KA, Ranke MB, editors. Turner syndrome in a life span perspective: research and clinical aspects. Amsterdam: Elsevier; 1995. p. 285–95.
- Rovet JF, Ehrlich RM, Czuchta D, Akler M. Psychoeducational characteristics of children with Turner syndrome. J Learn Disabil 1993; 26: 333–41. [DOI] [PubMed] [Google Scholar]
- Saenger P. Turner's Syndrome. N Engl J Med 1996; 335: 1749–54. [DOI] [PubMed] [Google Scholar]
- Skuse DH. X-linked genes and mental functioning. Hum Mol Genet 2005; 14: R27–32. [DOI] [PubMed] [Google Scholar]
- Tamm L, Menon V, Reiss AL. Abnormal prefrontal cortex function during response inhibition in Turner syndrome: functional magnetic resonance imaging evidence. Biol Psychiatry 2003; 53: 107–11. [DOI] [PubMed] [Google Scholar]
- Temple CM. Oral fluency and narrative production in children with Turner's syndrome. Neuropsychologia 2002; 40: 1419–27. [DOI] [PubMed] [Google Scholar]
- Temple CM, Carney R. Reading skills in children with Turner's syndrome: an analysis of hyperplexia. Cortex 1996; 32: 335–45. [DOI] [PubMed] [Google Scholar]
- Voyvodic JT. Real-time fMRI paradigm control, physiology, and behavior combined with near real-time statistical analysis. Neuroimage 1999; 10: 91–106. [DOI] [PubMed] [Google Scholar]
- Woodcock WR, McGrew KS, Mather N. Woodcock-Johnson III Tests of cognitive abilities. Itasca, FL: Riverside Publishing; 2001.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


