Abstract
Liposarcomas of the spermatic cord, a rare cause of an inguinal mass, may closely mimic inguinal hernias on clinical examination. However, these tumors require a different surgical approach and treatment plan; therefore, intraoperative diagnosis might complicate patient management. We report the case of a 63-year-old man who presented with a mobile mass in the inguinal canal consistent with an inguinal hernia. The patient was subsequently diagnosed with a liposarcoma of the spermatic cord and successfully treated with extensive local resection, including radical orchiectomy and en bloc resection of the mass and associated cord structures. No adjuvant therapy was deemed necessary, and the patient remained asymptomatic and disease-free 10 years after surgery. The details of this case are presented, along with a review and discussion of the currently available data regarding the diagnosis and management of this challenging condition.
Keywords: liposarcoma, spermatic cord, hernia, inguinal hernia, sarcoma
Introduction
The inguinal region is notable for the spectrum of diseases presenting in that anatomic location. Clinical distinction between these entities based on history and physical examination remains challenging. Tumors of the spermatic cord are usually benign (70–80%), with the majority being simple lipomas. Among malignant tumors of the spermatic cord, sarcomas are the most common type. Rhabdomyosarcomas are the most aggressive type and the predominant type in children. The other histological types of sarcomas, namely liposarcomas, leiomyosarcomas and fibrosarcomas, are most frequently encountered in the adult population. Liposarcomas, derived embryologically from mesodermal tissue, are the most common type of sarcoma and account for 3–7% of all spermatic cord tumors (1–3). Liposarcomas are most commonly located in the lower extremities (41%), retroperitoneum (19%) and inguinal region (12%) (4). Even within the inguinal region, liposarcomas of the spermatic cord are quite rare, with case reports constituting the majority of the published literature on this topic (5).
Sarcoma of the spermatic cord was first reported by Lesauvage in 1845 (1). Based on the case reports since then, it appears that the majority of the patients with liposarcomas of the spermatic cord present in the fifth or sixth decade of life with a painless, irregular, slow-growing inguinal or inguinoscrotal mass clearly distinct from the testis (3,6,7). As previously mentioned, diagnosing liposarcomas of the spermatic cord preoperatively may be challenging, as this clinical presentation may indicate several more common conditions, including inguinal hernia, lipoma, hydrocele, epididymal cyst, funicular cyst or testicular tumors (3–5,8,9).
On ultrasound examination, a liposarcoma of the spermatic cord usually exhibits heterogeneous echogenicity; however, these ultrasound findings lack specificity. A CT scan is suggestive of the diagnosis in approximately half of the cases and helps to determine the involvement of the anterior abdominal wall and/or retroperitoneum (1,2,4,10).
We herein report a case of liposarcoma of the spermatic cord and review available data on the pathophysiology, management and follow-up of patients with this condition.
Case report
A 63-year-old man presented in September, 2006 at the Hotel Dieu de France University Hospital with a painless, mobile mass in the right inguinal region. On clinical examination, the mass was found to descend from the inguinal canal into the scrotum with the Valsalva maneuver. The patient was diagnosed with an indirect right inguinal hernia and scheduled for surgical repair.
Intraoperatively, following dissection of the spermatic cord, a multi-lobulated, encapsulated, yellow mass was identified within the cord. The mass appeared to infiltrate surrounding soft tissue structures, such as muscle and the external tunica vaginalis. Further dissection and delivery of the right testis demonstrated an atrophic testis with no evidence of local invasion. After a thorough on-site evaluation, surgical resection was delayed to permit better assessment of the disease and to obtain patient consent for possible orchiectomy. An incisional biopsy of the mass was performed, and pathological analysis revealed a well-differentiated liposarcoma of the sclerosing type (Fig. 1).
Figure 1.
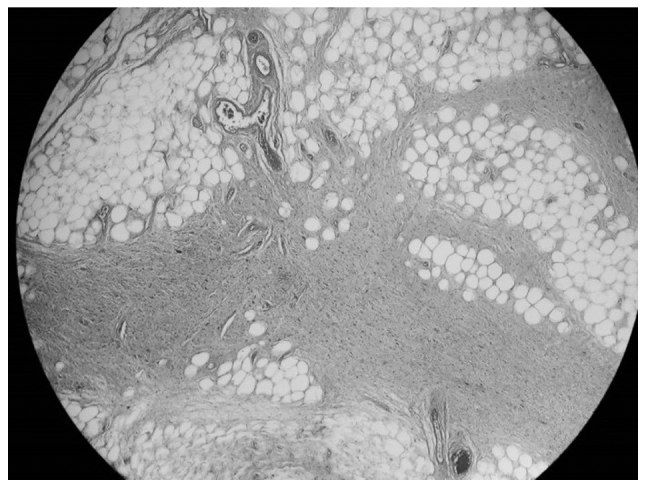
Histological examination of the spermatic cord tumor revealed mature vacuolated adipocytes with variation in cell size and fibrous septa containing atypical stromal cells, consistent with a well-differentiated liposarcoma of the sclerosing type (hematoxylin and eosin staining; magnification, ×4).
A computed tomography (CT) scan revealed a 5-cm mass with heterogeneous enhancement located inside the right inguinal canal, surrounded by local inflammation. Despite the atrophic appearance of the ipsilateral testis intraoperatively, on CT scan it appeared to be enlarged due to the thickening of the overlying layers (Fig. 2).
Figure 2.
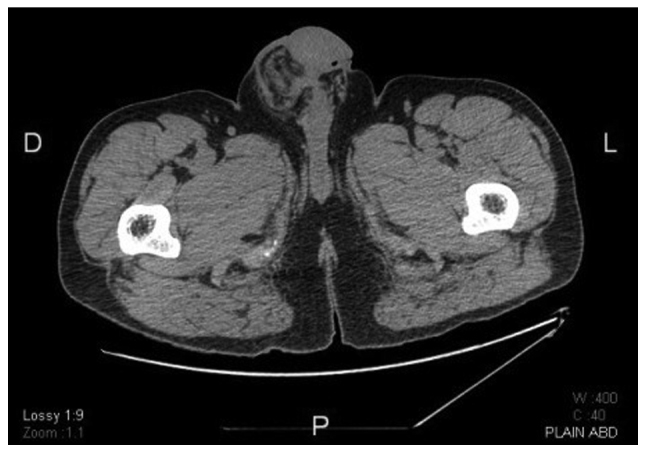
Computed tomography scan at the level of the scrotum demonstrating thickening of the layers overlying the right testis, as well as the surrounding inflammatory reaction.
The surgical team decided to proceed with a radical orchiectomy and an en bloc resection of the mass and associated cord structures. Final pathology was consistent with a well-differentiated liposarcoma, sized 7×3 cm, adherent to the testicle at the level of the tunica albuginea. There was no invasion of the testicular tissue or the epididymis, but the tumor extended close to the spermatic cord structures (Fig. 3). All the margins were negative; thus, no further treatment was recommended.
Figure 3.
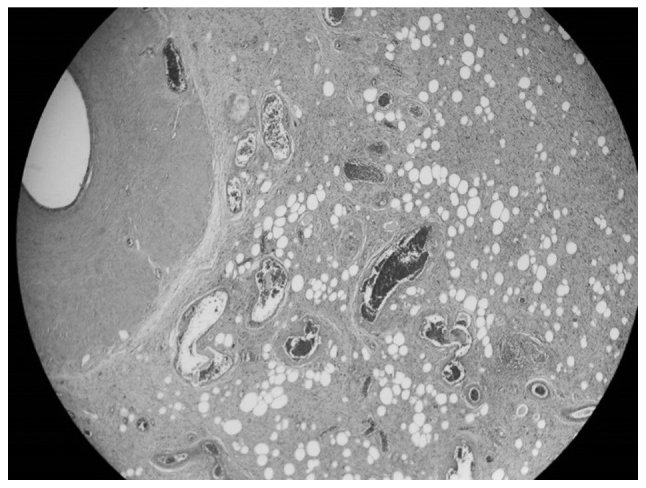
Liposarcomatous tissue infiltrating the external layers of the spermatic cord (hematoxylin and eosin staining; magnification, ×4).
Screening CT scans were obtained at 6 months and at 1, 3, 5 and 10 years. The CT scan at 6 months revealed no signs of tumor recurrence, as well as a net decrease in local inflammation. The patient remained asymptomatic throughout the follow-up period. The most recent CT scan, performed in October, 2016 (10 years after the surgery), revealed no signs of tumor recurrence.
The patient provided consent to the publication of the case details and associated images.
Discussion
Based on surgeon experience and the limited available literature, the preferred first-line treatment for spermatic cord liposarcomas is surgical resection. This is performed through a wide excision of the mass, including orchiectomy and high ligation of the spermatic cord to achieve a negative margin. A number of prospective studies on spermatic cord sarcomas (including liposarcomas) emphasize the importance of aggressive surgical management, including wide re-resection of tumor recurrence, in decreasing local recurrence and improving disease-free survival (6,11). Rates of positive margins as high as 19% have been reported following initial local excision, which underscores the challenges associated with surgical resection of these tumors. Intraoperative assessment of tumor resection margins is limited by the accuracy of frozen biopsy, making complete surgical resection difficult.
Tumor staging is based on histological examination and grading, and the presence of metastases. The World Health Organization classification of soft tissue tumors classifies liposarcomas into five categories as follows: Myxoid (most common), well-differentiated (adipocytic, sclerosing and inflammatory subtypes), dedifferentiated, round-cell and pleomorphic. Low-grade subtypes are histologically well-differentiated and have no or minimal tendency to metastasize, although they may be locally invasive. High-grade subtypes (round-cell and pleomorphic) are rarer, but they are associated with a higher rate of recurrence and hematogenous metastasis to the lungs and bone (1,3,8,9). Neither low- nor high-grade liposarcomas typically spread via lymphatic routes, which is consistent with the lack of a survival benefit from superficial inguinal or retroperitoneal lymphadenectomy (1,2,4,8). Local recurrence rates for sarcomas, including liposarcomas of the spermatic cord, have been reported to be as high as 50%. However, these numbers vary between case reports. In a recent case series of 42 patients, only 7 (17%) developed local recurrence (12). In that study, recurrence developed on average at 40.9 months after resection; two of the patients had systemic metastases and succumbed to the disease. This study reported no significant association between recurrence and margin status, tumor size, or tumor grade (P>0.05), although it was likely underpowered to detect these differences.
The use of adjuvant treatment (chemotherapy/radiotherapy) is controversial, due to the paucity of data in the literature. Some authors only recommend adjuvant radiotherapy in cases with multiple local recurrences, positive margins, and/or poor prognostic factors, such as high-grade tumors. A study of patients treated for spermatic cord tumors showed improved locoregional control and disease-free survival in patients receiving adjuvant radiation; however, the 5-year overall survival rates did not improve significantly. A more recent study confirmed that adjuvant radiation therapy improved local control in patients at high risk for local failure, but did not assess its effect on long-term survival (13). Based on this limited literature, there is no definitive role for chemotherapy in the management of localized liposarcoma of the spermatic cord (1,2,5,8,9,14,15).
As the surgical margins were negative in our case and the efficiency of adjunctive therapies in such cases is uncertain, it was decided that radiotherapy and chemotherapy were not indicated for our patient. A similar strategy was undertaken by Malizia et al, who reported two cases of well-differentiated liposaromas treated by wide excision, high ligation and ipsilateral radical orchiectomy, without any adjuvant therapy. No recurrence or distant metastases were observed after 8 years in the first case or after 20 months in the second case (2). Ikinger et al also reported one case of a combination mixoid liposarcoma and angiolipoma of the spermatic cord, which was treated surgically and had no demonstrable recurrences or metastases during the 30 months of follow-up (7).
In summary, liposarcomas of the spermatic cord are challenging to diagnose clinically. Suspicion of the possible diagnosis when evaluating an inguinal hernia should prompt imaging studies. As there is no gold standard treatment, the management of these tumors relies on the guidance of case reports in the literature. These reports suggest that a wide and complete resection with clear microscopic margins is key to the management of spermatic cord liposarcomas. If the margins are positive, re-resection should be performed (1,5,6,10,13,16). Adjuvant radiation therapy should be considered in cases at high risk for local recurrence. However, a relatively conservative approach may reduce morbidity in patients with localized disease. The indications for retroperitoneal lymph node dissection remain very limited. For patient follow-up, the consensus is to perform close and regular follow-up with imaging at 3, 6, 12 and 24 months; the benefit of longer follow-up has not been determined. As the literature on this subject grows, guidelines for treatment should be developed.
Figure 4.
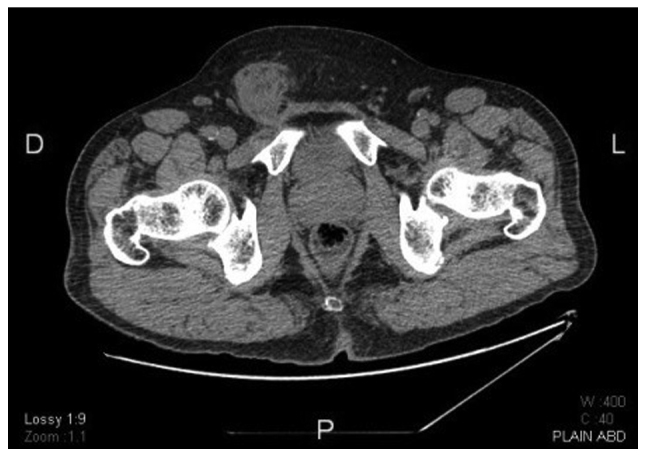
Non-contrast computed tomography scan at the level of the pelvis showing the characteristic bulky nature of the tumor and its relation to the anterior abdominal wall.
References
- 1.Chintamani Tandon M, Khandelwal R, Jain S, Narayan N, Kumar Y, Saxena S. Liposarcoma of the spermatic cord: A diagnostic dilemma. JRSM Short Rep. 2010;1:49. doi: 10.1258/shorts.2010.010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malizia MBE, Bertaccini A, Palmieri F, Vitullo G, Martorana G. Liposarcoma of the spermatic-cord: Description of two clinical cases and review of the literature. Arch Ital Urol Androl. 2005;77:115–117. [PubMed] [Google Scholar]
- 3.Papageorgiou MS, Dadakas G, Donev K. Liposarcoma of the spermatic cord: A case report. Case Rep Med. 2011;2011:197584. doi: 10.1155/2011/197584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vázquez-Lavista GL, Pérez-Pruna C, Flores-Balcázar HC, Guzmán-Valdivia G, Romero-Arredondo E, Ortiz-López BJ. Spermatic cord liposarcoma: A diagnostic challenge. Hernia. 2006;10:195–197. doi: 10.1007/s10029-005-0046-2. [DOI] [PubMed] [Google Scholar]
- 5.Bestman TJ, Populaire J, Lauwers K, Molderez C. Liposarcoma of the spermatic cord: Report of 2 cases. Acta Chir Belg. 2007;107:58–59. doi: 10.1080/00015458.2007.11680012. [DOI] [PubMed] [Google Scholar]
- 6.Ballo MT, Zagars G, Pisters PW, Feig BW, Patel SR, von Eschenbach AC. Spermatic cord sarcoma: Outcome, patterns of failure and management. J Urol. 2001;166:1306–1310. doi: 10.1016/S0022-5347(05)65758-8. [DOI] [PubMed] [Google Scholar]
- 7.Ilkinger U, Westrich M, Pietz B, Mechtersheimer G, Schmidt C. Combined myxoid liposarcoma and angiolipoma of the spermatic cord. Urology. 1997;49:635–637. doi: 10.1016/S0090-4295(96)00545-6. [DOI] [PubMed] [Google Scholar]
- 8.Bouropoulos C, Skopelitou A, Vaggos G, Papamichael C. Liposarcoma of the spermatic cord. Int Urol Nephrol. 2001;33:397–398. doi: 10.1023/A:1015234301661. [DOI] [PubMed] [Google Scholar]
- 9.Domşa I, Olinici CD, Crişan D. Spermatic cord mixed liposarcoma. Case report and review of the literature. Rom J Morphol Embryol. 2008;49:105–109. [PubMed] [Google Scholar]
- 10.Hsu YF, Chou YY, Cheng YH. Spermatic cord myxoid liposarcoma presenting as an incarcerated inguinal hernia: Report of a case and review of literatures. Hernia. 2012;16:719–722. doi: 10.1007/s10029-011-0803-3. [DOI] [PubMed] [Google Scholar]
- 11.Fagundes MAZA, Althausen AF, Coen JJ, Shipley WU. The management of spermatic cord sarcoma. Cancer. 1996;77:1873–1876. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1873::AID-CNCR17>3.3.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 12.Kryvenko ON, Rosenberg AE, Jorda M, Epstein JI. Dedifferentiated liposarcoma of the spermatic cord: A series of 42 cases. Am J Surg Pathol. 2015;39:1219–1225. doi: 10.1097/PAS.0000000000000426. [DOI] [PubMed] [Google Scholar]
- 13.Coleman J, Brennan M, Alektiar K, Russo P. Adult spermatic cord sarcomas: Management and results. Ann Surg Oncol. 2003;10:669–675. doi: 10.1245/ASO.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DE, Harris J, Ayala AG. Liposarcoma of the spermatic cord. Urology. 1978;11:190–192. doi: 10.1016/0090-4295(78)90108-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhao H, Zhou L, Chen X, Liu Y. Liposarcoma of the spermatic cord: A case report. Urologe A. 2011;50:600–602. doi: 10.1007/s00120-010-2487-1. (In German) [DOI] [PubMed] [Google Scholar]
- 16.Ugidos L, Suárez A, Cubillo A, Durán I. Mixed paratesticular liposarcoma with osteosarcoma elements. Clin Transl Oncol. 2010;12:148–149. doi: 10.1007/S12094-010-0480-1. [DOI] [PubMed] [Google Scholar]


