Abstract
With the ongoing economic development, lifestyle changes and an aging population, diabetes mellitus has be come one of the most prevalent chronic diseases in the world. Rhino-orbito-cerebral (ROC) mucormycosis is a rare, acute and angioinvasive fungal infection that can be fatal. Mucormycosis occurs exclusively in immunocompromised patients with diabetes mellitus and other types of immunodeficiency and has three subtypes: Rhino-maxillary, rhino-orbital and ROC mucormycosis. The present study reported on a case of ROC mucormycosis in a patient with diabetic ketoacidosis. In the present case, the pathogen afflicted all of the above organs, including the left eye, nasal cavity, hard palate and cerebrum.
Keywords: mucormycosis, type 2 diabetes, angioinvasive fungal infection
Introduction
With the ongoing economic development, changes in lifestyle and an aging population, diabetes mellitus is currently among the most prevalent chronic diseases in the world. Apart from micro- and macro-vascular diabetic complications, the susceptibility of diabetics to infections, e.g., with bacteria or fungi, has shown yearly increases (1). Patients with diabetes mellitus are susceptible to certain pathogens, such as mucor. The present study presented a case of a type 2 diabetes patient with rhino-orbito-cerebral (ROC) mucormycosis.
Case report
The present case was approved by the Ethics Committee of the Second Hospital of Hebei Medical University (Shijiazhuang, China). Written informed consent was obtained from the patient for the publication of this study.
A 27-year-old man was admitted to the Department of Endocrinology of the Second Hospital of Hebei Medical University on June 2, 2013, complaining of a painful swelling in the left retro-orbital region and nose for five days. Since the first day of presenting with these signs, he was unable to open the left eye and his vision of the left eye and the left papillary light reflex were lost. The patient had a history of type 2 diabetes mellitus with uncontrolled blood glucose for two years as well as a family history of diabetes mellitus.
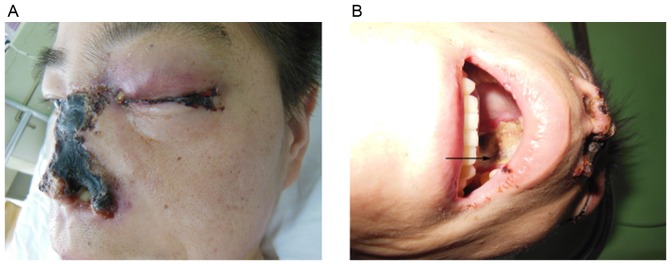
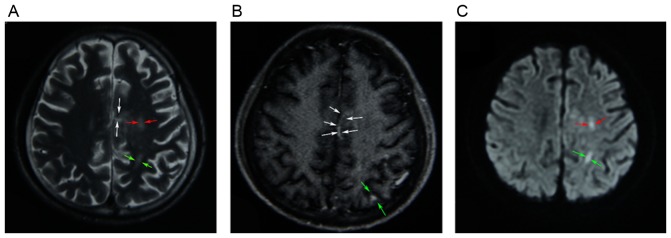
On admission, the patient was conscious and had an elevated body temperature of 38.4°C, a blood pressure of 116/78 mmHg and a pulse of 136/min. Apart from the abovementioned signs and symptoms, the physical and neurological presentation was normal. The patient's white blood cell count was 21,100 cells/µl and differential count results were as follows: Neutrophils, (80.5%; normal range, 40–75%); lymphocytes, (2.4%; normal range, 20–50%); monocytes, (17.1; normal range, 3–10%); eosinophils, (0%; normal range, 0.4–8%); basophils, (05; normal range, 0–1%). His hemoglobin was 10.8 g/dl. Other laboratory values, including liver function, renal function and electrolytes were normal. His random blood glucose level was 22.4 mmol/l and hemoglobin A1C was 15.7%. On admission, the patient's urine specimen was positive for acetone and blood gas analysis confirmed metabolic acidosis. A definite diagnosis of diabetic ketoacidosis and cheek infection was made and the patient was immediately administered an intravenous insulin infusion, an intravenous fluid replacement was performed and an insulin sliding scale therapy was commenced. The patient's blood glucose levels were strictly controlled with insulin. After one day, the patient's urine specimen was negative for acetone, but the painful swelling on the left side of the face aggravated and burst open. The vision of left eye and the left pupillary light reflex were completely lost (Fig. 1A). His left pupil was mid-dilated and his left eyeball was fixed. Fundoscopic examination of the left eye revealed that the optic disc was pale and a cherry-red spot was present in the macula (Fig. 2). The retinal arteries were narrow. The patient was diagnosed with left central retinal artery occlusion. A large amount of purulent secretion was noted in the patient's left eye and nose. Extensive regions with black necrotic lumps were present in the nasal cavity. The patient's nasal structure was completely destroyed and the hard palate was perforated (Fig. 1B). On admission, the infectious etiology was repeatedly investigated. A nasal swab and a large necrotic mass in the nasal cavity were microbiologically assessed by fungal smears and cultures. The fungal culture of the patient's purulent secretions was consistent with mucormycosis. Based on the clinical findings and fungal culture result, the definitive diagnosis of ROC mucormycosis was made. Therefore, antifungal therapy using amphotericin B was intravenously administered at 0.3 mg/kg/day at once and was thereafter gradually increased to 1 mg/kg/day. Due to the side effects of amphotericin B, the patient's serum electrolytes and renal function was monitored. After two weeks, the patient complained of a dull headache. On enhanced magnetic resonance imaging, multiple infarcts in the cerebral cortex, particularly in the parietal lobe, frontal lobe and the corpus callosum were revealed (Fig. 3). Although extensive local excision was required, the patient was not able to endure the surgery due to the patient's overall condition being poor. The necrotic tissue was too broad; therefore, surgery was not feasible. Following antifungal therapy, the patient's temperature did not obviously improve. The necrosis of local tissue was developing, which may be life threatening. After the patient's self discharge against medical advice, he was transferred to a superior hospital.
Figure 1.
Images showing the main clinical manifestations of rhino-orbito-cerebral mucormycosis in the patient. (A) Extensive regions of black necrotic lumps were present in the left eye and nose. (B) The hard palate was perforated.
Figure 2.
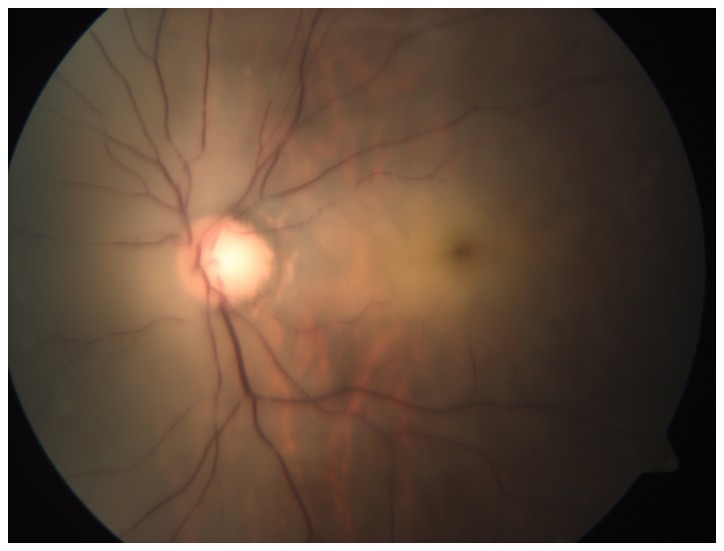
Image of the patient's left fundus showed central retinal artery occlusion. The optic disc was pale and a cherry-red spot was present in the macula.
Figure 3.
(A) T2-weighted MR image demonstrating heterogeneous high-signal intensity in the left frontal lobe (red arrows), the left parietal lobe (green arrows) and the corpus callosum (white arrows). (B) Enhanced T1-weighted MR image revealing increased signal intensity of lesions, which were mainly located in the left parietal lobe (green arrows) and the corpus callosum (white arrows). (C) MR diffusion-weighted image showing high signal intensity in the left frontal lobe (red arrows) and the left parietal lobe (green arrows). MR, magnetic resonance.
Discussion
Mucormycosis is a rare, acute and opportunistic infection caused by fungi of the order Mucorales that may be fatal (2). The infection may manifest in any part of the body and exclusively affects patients with metabolic acidosis or immune deficiency, including diabetic ketoacidosis, organ transplantation and hematological tumors. ROC mucormycosis represents an acute life-threatening disease, particularly in patients with diabetic ketoacidosis. The gold standard for diagnosing ROC mucormycosis is the histopathological examination of biopsy specimens and fungal culture. The typical fungal hyphae are broad, nonseptate and had right-angular branches. In the present case, the patient was a known diabetic and had developed diabetic ketoacidosis on admission. He presented with a painful swelling in the left retro-orbital region and nose. The infection spread to the paranasal sinuses via vessels and to the central nervous system via the eye orbit and cribriform plate within the two subsequent weeks. At present, the patient had progressed to ROC mucormycosis. In normal hosts, mucor is eliminated by mononuclear and polymorphonuclear phagocytes. Patients with diabetic ketoacidosis are known to have impaired function of these phagocytes (3) and are thus highly susceptible to mucormycosis. In addition, multiple lines of evidence supported a link between iron availability and the risk of mucormycosis (4). Due to elevated available serum iron, patients with diabetic ketoacidosis have a high risk of developing mucormycosis. The mucor invades the human host through attachment to the endothelial cell lining of the blood vessels. A hallmark of mucormycosis is the presence of extensive angioinvasion. Therefore, the major clinical manifestation of mucormycosis is vascular thrombosis and tissue necrosis (2,5). In the present case, the patient's left eye and nose had numerous black necrotic lumps, which was is in line with the clinical manifestation of mucormycosis.
At present, antifungal therapy and aggressive surgical intervention are used to treat mucormycosis. Based on retrospective clinical studies, amphotericin B is currently used as the first-line antifungal agent. Several case reports have documented that patients with rhino-cerebral mucormycosis were successfully treated by amphotericin B (6,7). Unfortunately, despite antifungal therapy and disfiguring surgical debridement, the overall mortality of mucormycosis patients remains high and approaches 40% in diabetic patients with rhino-cerebral mucormycosis (2). In this case, this patient did not receive any surgical debridement previously or at our department. Given the high mortality of mucormycosis patients, alternative efficacious therapies are being explored. A large amount of experimental evidence showed that posaconazole and ravuconazole have activity against the mucor in vitro (8,9). According to a case study, a patient with ROC mucormycosis was successfully treated with posaconazole and amphotericin (10). In addition to posaconazole, isavuconazole was recently approved by the Food and Drug Administration for the treatment of mucormycosis. Isavuconazole, which has been approved for the treatment of invasive mucormycosis, may have therapeutic advantages over its predecessors (11). Due to the central role of iron metabolism in the pathogenesis of mucormycosis, it is possible that effective iron chelators serve as adjunctive agents in combination with antifungal therapies (2). The establishment of these novel therapies for mucormycosis in the clinic depends on long-term observations and research.
References
- 1.Peleg AY, Weerarathna T, McCarthy JS, Davis TM. Common infections in diabetes: Pathogenesis, management and relationship to glycaemic control. Diabetes Metab Res Rev. 2007;23:3–13. doi: 10.1002/dmrr.682. [DOI] [PubMed] [Google Scholar]
- 2.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: Pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–569. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: Relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123–1129. doi: 10.1128/iai.38.3.1123-1129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim AS, Gebermariam T, Fu Y, Lin L, Husseiny MI, French SW, Schwartz J, Skory CD, Edwards JE, Jr, Spellberg BJ. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649–2657. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ibrahim AS. Host cell invasion in mucormycosis: Role of iron. Curr Opin Microbiol. 2011;14:406–411. doi: 10.1016/j.mib.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cagatay AA, Oncü SS, Calangu SS, Yildirmak TT, Ozsüt HH, Eraksoy HH. Rhinocerebral mucormycosis treated with 32 gram liposomal amphotericin B and incomplete surgery: A case report. BMC Infect Dis. 2001;1:22. doi: 10.1186/1471-2334-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ericsson M, Anniko M, Gustafsson H, Hjalt CA, Stenling R, Tärnvik A. A case of chronic progressive rhinocerebral mucormycosis treated with liposomal amphotericin B and surgery. Clin Infect Dis. 1993;16:585–586. doi: 10.1093/clind/16.4.585. [DOI] [PubMed] [Google Scholar]
- 8.Pfaller MA, Messer SA, Hollis RJ, Jones RN. SENTRY Participants Group: Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: Report from SENTRY Antimicrobial Surveillance Program, 2000. Antimicrob Agents Chemother. 2002;46:1032–1037. doi: 10.1128/AAC.46.4.1032-1037.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun QN, Fothergill AW, McCarthy DI, Rinaldi MG, Graybill JR. In vitro activities of posaconazole, itraconazole, voriconazole, amphotericin B, and fluconazole against 37 clinical isolates of zygomycetes. Antimicrob Agents Chemother. 2002;46:1581–1582. doi: 10.1128/AAC.46.5.1581-1582.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoon YK, Kim MJ, Chung YG, Shin IY. Successful treatment of a case with rhino-orbital-cerebral mucormycosis by the combination of neurosurgical intervention and the sequential use of amphotericin B and posaconazole. J Korean Neurosurq Soc. 2010;47:74–77. doi: 10.3340/jkns.2010.47.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rybak JM, Marx KR, Nishimoto AT, Rogers PD. Isavuconazole: Pharmacology, pharmacodynamics, and current clinical experience with a new triazole antifungal agent. Pharmacotherapy. 2015;35:1037–1051. doi: 10.1002/phar.1652. [DOI] [PubMed] [Google Scholar]