Abstract
The present study aimed to investigate the expression of tumor necrosis factor receptor superfamily member 8 (CD30) in extranodal natural killer/T-cell lymphoma (ENKTL) using immunohistochemistry, and to evaluate the association between CD30 and clinicopathological and prognostic significance. CD30 expression was detected using immunohistochemistry on paraffin-embedded sections obtained from 122 patients with ENKTL prior to treatment. In total, 70 of these patients with complete clinical data were collected for prognostic analysis. The level of CD30 expression, of the 122 patients with ENKTL, was grouped on the basis of a 5-tiered scale as follows: 0%, no staining; 1+, <25% positive cells; 2+, 25–50% positive cells; 3+, 50–75% positive cells; and 4+, >75% positive cells). In total, 36 (29.5%) were classified as 0; 46 (37.7%) as 1+; 22 (18.0%) as 2+; 12 (9.8%) as 3+; and 6 (4.9%) as 4+. Among the 86 patients with scores between 1+ and 4+, the membranous staining patterns of CD30 expression were sporadic (33.7%), focal (43.2%), diffuse (15.1%) and angiocentric (8.1%). When considering a score of ≥3+ as CD30 positivity (CD30+), the CD30+ group had significantly shorter overall survival rates (P=0.0023) and progression-free survival rate (P=0.0008) compared with CD30 negative group. However, no statistically significant association was found between CD30 expression and clinicopathological features (P<0.05). The present study found that the expression of CD30 (≥3+) was significantly associated with poor prognosis but was not associated with clinical and histopathological parameters in ENKTL. Therefore, CD30 may be a useful prognostic marker in ENKTL.
Keywords: extranodal natural killer/T-cell lymphoma, CD30, immunohistochemistry, prognosis
Introduction
Extranodal natural killer/T-cell lymphoma (ENKTL) is considered to be an aggressive disease with particular morphologic, immunohistochemical and clinical features (1). According to a review performed by the Pathology of Lymphoma Collaborative Group (Beijing, China), ENKTL is the second most common type of lymphoma following diffuse large B-cell lymphoma (DLBCL) in China (2). According to the anatomic site involved, ENKTL can be classified into either upper aerodigestive tract (UAT) group or non-UAT group. Subdivision of the 2 groups is necessary due to the difference in clinical characteristics and prognosis (3–5). Due to the poor prognosis of ENKTL, a great number of clinical and pathological efforts have been made to identify prognostic markers for ENKTL (6–8).
Tumor necrosis factor (TNF) receptor superfamily member 8 (CD30) is a transmembrane glycoprotein belonging to the TNF family (9). Among lymphoproliferative disorders (LPDs), CD30 expression was originally reported in classical Hodgkin's lymphoma and anaplastic large cell lymphoma (10). CD30 expression has previously been reported in a set of peripheral T-cell lymphomas (PTCL) including PTCL-not otherwise specified (NOS) (11,12), angioimmunoblastic T-cell lymphoma (13) and primary cutaneous LPDs (14). There are few studies investigating the expression of CD30 in ENKTL patients (15–23). In addition, the association between CD30 and the clinicopathological features and prognosis of ENKTL remains controversial.
In the present study, CD30 expression was determined using immunohistochemistry in 122 patients with Chinese ENKTL, to evaluate its association with clinicopathological features. The prognostic significance of CD30 expression was also studied through the analysis of selected patients with complete data.
Materials and methods
Patient selection
Biopsied tissues of patients with NK/T-cell lymphomas between May 2011 and November 2013 at the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China) were identified. All patients were diagnosed according to the World Health Organization classification criteria (1): The morphological and immunophenotypic characteristics of the tumor cells fulfilled the criteria of ENKTL, and all the cases were EBV positive. The enrolled patients had lymphomatous lesions on the upper aerodigestive tract and had not received any previous treatment for lymphoma. Reactive hyperplasia of lymph node tissue was collected as control. Written informed consent was obtained from all patients prior to starting the study. This project was approved by the First Affiliated Hospital of Zhengzhou University Clinical Research Ethics Committee (Zhengzhou, China).
Immunohistochemistry
Immunohistochemistry was performed using 10% formalin-fixed paraffin-embedded tissue blocks that were divided into 4 µm sections, followed by the modified avidin-biotin complex method (Envision method) on an automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ, USA) (24). The following antibodies were used: Membrane spanning 4-domains A1 (CD20; dilution, ready-to-use; catalogue no., 14357208; ZSGB-BIO, Beijing, China), membrane-bound immunoglobulin-associated protein (CD79a; dilution, ready-to-use; catalogue no., 15620806; Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China), cytoplasmic cluster of differentiation (CD) 3 (dilution, ready-to-use; catalogue no., CRM01420309; Shanghai Jiehao Biotech Co., Ltd., Shanghai, China), CD3 (dilution, ready-to-use; catalogue no., 130801543E; Fuzhou Maixin Biotech Co., Ltd.), CD43 (dilution, ready-to-use; catalogue no., 131006032b; Fuzhou Maixin Biotech Co., Ltd.), neural cell adhesion molecule (CD56; dilution, ready-to-use; catalogue no., cm03510419; Shanghai Jiehao Biotech Co., Ltd.), Granzyme-B (dilution, ready-to-use; catalogue no., 16691906;ZSGB-BIO), T-cell-restricted intracellular antigen 1 (TIA-1; dilution, ready-to-use; catalogue no., 160426599E; Fuzhou Maixin Biotech Co., Ltd.), CD30 (dilution, ready-to-use; catalogue no., 15331003;ZSGB-BIO) and anaplastic lymphoma kinase (ALK; dilution, ready-to-use; catalogue no., 151223281C; Fuzhou Maixin Biotech Co., Ltd.). The percentage of CD30 expression was determined by quantifying the number of positive cells with strong membrane staining, excluding all necrotic area. Those with strong, complete and uniform brown coloring at the cytoplasmic membrane were considered as CD30-positive. The results were scored on a 5-tiered scale: 0, 0% positive cells or no staining; 1+, <25% positive cells; 2+, 25–50% positive cells; 3+, >50–75% positive cells; and 4+, >75% positive cells. A score of ≥1+ was taken to represent CD30 positivity.
In situ hybridization for Epstein-Barr virus (EBV)
The presence of EBV RNA was detected with an in situ hybridization (ISH) technique, using the Epstein-Barr Virus Early RNA kit (ZSGB-BIO) according to the manufacturer's protocol. Briefly, 4–6 µm sections cut from paraffin tissues were deparaffinized, rehydrated, predigested with gastric enzyme, prehybridized, and then hybridized with a digoxigenin (DIG)-labeled RNA probe (ZSGB-BIO). Subsequent to washing, the reaction was performed using anti-DIG horseradish peroxidase conjugate antibody (dilution, ready-to-use; catalogue no., 151110; ZSGB-BIO) followed by development with diaminobenzidine (ZSGB-BIO, Beijing, China). A known case of EBV-positive nasopharyngeal carcinoma was used as a positive control.
Statistical analysis
All analyses were performed with SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). The association between CD30 expression and clinicopathological parameters was assessed using a χ2 test or Fisher's exact test. Survival rate analysis was determined using the Kaplan-Meier method with the log-rank test. Overall survival (OS) rate was defined as between the date of diagnosis and the date of last follow-up or mortality. Progression-free survival (PFS) rate was defined as between the date of diagnosis and the time of progression or mortality. P<0.05 was considered to indicate a statistically significant difference.
Results
Immunohistochemical analyses of CD30 expression
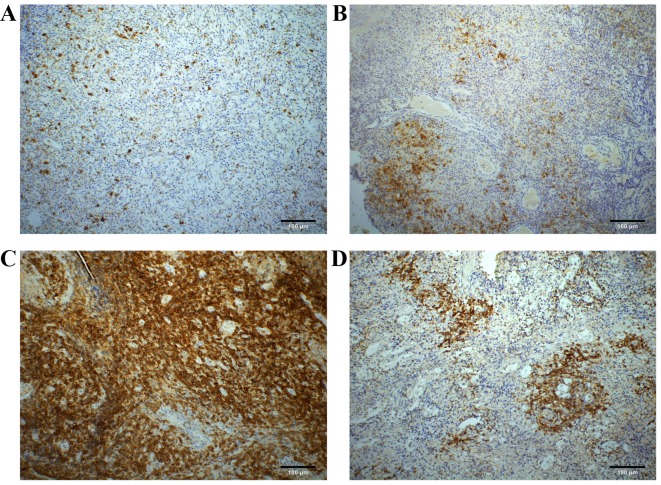
According to the scoring system aforementioned, the numbers of CD30 expression scoring 0, 1+, 2+, 3+ and 4+ accounted for 36 (29.5), 46 (37.7), 22 (18.0), 12 (9.8) and 6 (4.9%) of 122 patients with ENKTL, respectively. Overall, 14.7% (18 of 122) of the considered patients showed a CD30 expression score of ≥3+. Among the 86 patients with score 1+ to score 4+, the membranous staining patterns of CD30 expression were sporadic (33.7%; 29 of 86), focal (43.0%; 37 of 86), diffuse (15.1%; 13 of 86) and angiocentric (8.1%; 7 of 86) (Fig. 1A-D).
Figure 1.
Representative images of CD30 expression by immunohistochemistry in natural killer/T-cell lymphoma. CD30 immunostaining shows (A) sporadic positivity, (B) focal positivity, (C) diffuse positivity and (D) angiocentric positivity (magnification, ×200). Scale bar represents 100 µm.
Association between CD30 expression and clinical features
Complete clinical data was collected from 70 patients. All the patients received 1 of the following therapies: Chemotherapy alone (50%; 35 of 70); radiotherapy alone (4.3%; 3 of 70); or concurrent chemo-radiotherapy (45.7%; 32 of 70). All the patients were followed-up between 11 months and 50 months with a median follow-up length of 28 months. The Kaplan-Meier analysis of OS for the whole group (n=70) was 72.9% at 5 years. Survival rate analysis was performed between CD30+ and CD30-patients with ENKTL based on the 5-tiered scale between 0 and 4+. When considering a score of ≥3+ as CD30+, the CD30+ group had a significantly shorter OS (P=0.0023) and PFS (P=0.0008) compared with the CD30-group (Fig. 2). Thus, the criterion for CD30 positivity, ≥3+, was used for additional analysis.
Figure 2.
Kaplan-Meier curve of (A) overal survival and (B) progression-free survival according to CD30 expression. CD30, tumor necrosis factor receptor superfamily member 8.
Comparison of clinical and histopathological features according to CD30 expression
The clinicopathological features of the 122 patients with ENKTL stratified according to level of CD30 expression are listed in Table I. Overall, there were 84 men and 38 women (male/female, 2.2:1) with a median age of 45 years and a range of 15–80 years. Over half (53.3%) of the patients presented with B symptoms, which consist of: Weight loss of >10% within 6 months; night sweats; and fever of unknown origin, and ~1/3 had advanced Ann Arbor staging of stage III or IV (25). No clinical difference was reported between patients in the CD30+ group compared with the CD30-group when CD30 positivity was defined as ≥3+.
Table I.
Clinicopathological features of ENKTL stratified according to CD30 expression.
| Characteristic | Overall, n (%) | CD30+, n (%) | CD30−, n (%) | P-value |
|---|---|---|---|---|
| Gender | ||||
| Male | 84 (68.9) | 12 (66.7) | 72 (69.2) | 0.828 |
| Female | 38 (31.1) | 6 (33.3) | 32 (30.8) | |
| Age, years | ||||
| ≤60 | 97 (79.5) | 15 (83.3) | 82 (78.8) | 0.663 |
| >60 | 25 (20.5) | 3 (16.7) | 22 (21.2) | |
| B symptoms | ||||
| Absence | 57 (46.7) | 10 (55.6) | 47 (45.2) | 0.416 |
| Presence | 65 (53.3) | 8 (44.4) | 57 (54.8) | |
| Elevated serum LDH | ||||
| Yes | 49 (40.2) | 9 (50.0) | 40 (38.5) | 0.357 |
| No | 73 (59.8) | 9 (50.0) | 64 (61.5) | |
| Bone marrow involvement | ||||
| Yes | 3 (2.5) | 1 (5.6) | 2 (1.9) | 0.358 |
| No | 119 (97.5) | 17 (94.4) | 102 (98.1) | |
| Ann Arbor staging | ||||
| I, II | 80 (65.6) | 14 (77.8) | 66 (63.5) | 0.238 |
| III, IV | 42 (34.4) | 4 (22.2) | 38 (36.5) | |
| Histological types | ||||
| SC | 27 (22.1) | 4 (22.2) | 23 (22.1) | 0.540 |
| MC | 65 (53.3) | 8 (44.4) | 57 (54.8) | |
| LC | 10 (8.2) | 3 (16.7) | 7 (6.7) | |
| PC | 20 (16.4) | 3 (16.7) | 17 (16.3) | |
| Necrosis | ||||
| Yes | 105 (86.1) | 17 (94.4) | 88 (84.6) | 0.266 |
| No | 17 (13.9) | 1 (5.6) | 16 (15.4) | |
| Vascular destruction | ||||
| Yes | 45 (36.9) | 8 (44.4) | 37 (35.6) | 0.472 |
| No | 77 (63.1) | 10 (55.6) | 67 (64.4) |
P-values were calculated from a χ2 test. B symptoms are defined as weight loss of >10% within 6 months, night sweats and fever of unknown origin. CD30, tumor cell necrosis factor superfamily member 8; ENKTL, extranodal natural killer/T cell-cell lymphoma; LDH, lactic dehydrogenase; SC, small cell type; MC, medium-sized cell type; LC, large cell type; PC, pleomorphic cell type.
Based on the prevailing population size of tumor cells, ENKTL in the present study can be classified into 4 histological types: 27 patients with small cell type; 65 patients with medium-sized cell (MC) type; 10 patients with large cell type; and 20 patients with pleomorphic cell type. In total, 45 patients exhibited an angiodestructive growth pattern and 105 patients exhibited various degrees of coagulative necrosis. No statistically significant association was observed between the expression of CD30 and histologic subtypes, the association between CD30 expression and patients with vascular destruction and necrosis failed to reach statistical significance as well.
In terms of immunohistochemical findings, the neoplastic cells in all patients tested positive for cytoplasmic CD3 and CD43, but negative for CD20. In total, 95.1% (116/122) showed staining for CD3 and 91.8% (112/122) were positive for CD56. The expression of the cytotoxic markers granzyme B and TIA-1 was observed in 91.8% (112/122), and 92.6% (113/122) of all patients, respectively. All 122 patients were EBV-positive according to ISH detection.
Discussion
The rate of CD30 positivity in ENKTL that has been reported in previous studies has been sporadic (range 20–70%) (15–23). Different detection rates between the immunohistochemistry techniques used perhaps attributed to different numbers of patients and different cut-off values for CD30 being used by different researchers. Therefore, in order to figure out how many tumor cells should be defined as CD30-positive, a scoring system based on a 5-tiered scale was applied in the present study. The CD30-positive rate was 14.7% at the cut-off point of 3+.
The association of CD30 expression in the prognosis of ENKTL was analyzed first, as this has been controversial (16,17,19,26). In the present study (n=70), patients with CD30+ ENKTL showed significantly inferior OS (P=0.023) and PFS (P=0.008) compared with CD30-ENKTL. However, studies involving different groups produced contradictory results. Kuo et al (16) (n=22) reported that there was no significant statistical difference in survival rate according to CD30 expression with respect to ENKTL. Mraz-Gernhard et al (26) (n=30) reported that patients with cutaneous CD30+ NK or NK/T cell lymphomas usually demonstrated better outcomes compared withCD30 patients. By contrast, Hong et al (19) (n=22) found that CD30 expression was associated with a poor prognosis. These studies mentioned that additional studies involving larger numbers of patients were required due to limited sample size. A study involving a greater number of patients performed by Li et al (17) (n=96) demonstrated that OS and PFS rates in the CD30+ group were significantly decreased compared with those in the CD30-group, which is consistent with the findings of the present study. Another focus of the present study was figuring out the optimal cut-off for CD30+ that would be of statistical significance, as previous studies used different criteria. In the present study, survival rate analysis was performed between the CD30+ and the CD30-group based on different cut-off levels, when considering a score ≥3+ as CD30 positivity, a level of significance of P<0.001 was observed. In the present study, CD30 expression was considered to be positive when >50% of the tumor cells exhibited strong membrane staining. This criterion was used in the analyses.
In the present study, the most common type of morphological pattern was MC type, which accounted for 53.3% of the 122 patients with ENKTL. The majority of patients (86.1%) showed coagulative necrosis. The association of the morphological parameters in nasal NK/T cell lymphoma with CD30 expression has not been clear thus far. Kuo et al (16) revealed that CD30 expression appeared to correlate with vascular destruction and thrombosis, and 2 other studies conducted by Kim and Heo (4) and Gaal et al (15) reported that CD30 positivity may be associated with large pleomorphic (anaplastic) morphology. However, in these studies, no association was found between CD30 expression and other morphological parameters including histologic subtypes, vascular destruction and necrosis. In the present study, 4 types of CD30+ pattern were observed and almost half of the patients (43.2%) were CD30 focally positive. Notably, 7 patients with CD30 expression of angiocentric positivity were observed, which was perhaps associated with the angiodestructive growth pattern of the tumor cells. It should be noted that 13 patients with ENKTL exhibited strong and diffuse CD30 immunoreactivity, and this may be confused with CD30+ anaplastic large cell lymphoma. Immunostains for ALK and EBV in EBV-encoded RNA-ISH studies may be useful to distinguish ENKTL and anaplastic large cell lymphoma in difficult cases.
Certain studies have reported that CD30 expression was an independent prognostic factor in several types of malignant lymphoma. In a study on a large sample of patients with DLBCL conducted by Hu et al (27), CD30 expression defined a novel and unique subgroup of DLBCL with a favorable clinical outcome and a distinct gene expression signature. Another study investigating PTCL revealed, by gene expression programming analyses, that patients with CD30+ PTCL shared common molecular and phenotypic features, and that there were significant differences between CD30− and CD30+ PTCL-NOS (28). The 2 aforementioned observations indicate that CD30 can be used as a valuable means to define 2 distinct biological subgroups, and it was demonstrated that CD30 expression was associated with improved outcomes. The mechanism underlying the poor prognosis of CD30+ ENKTL has not been well defined. EBV affects cell proliferation via latent membrane protein (LMP) 1. CD30 and LMP1 belong to the TNF-receptor family and their expression of protein has the potential to activate nuclear transcription factor-κB (10). The authors hypothesize that LMP1 and CD30 act cooperatively to affect the prognosis of ENKTL. CD30+ ENKTL may exhibit a distinct gene expression signature.
In conclusion, CD30 expression was associated with a poor prognosis in ENKTL, but had no effect on clinical or histopathological parameters when considering a score ≥3+ as CD30 positivity. The findings of the present study suggest that CD30 should be a good prognostic maker in ENKTL.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (grant nos. 81172118, 81402380 and U1204814) and by a grant (no. 201303016) from the Programs for Science and Technology Development of Henan, China.
References
- 1.Swerdlow SH, Campo E, Harris N, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW. World Health Organization classification of tumors of hematopoietic and lymphoid tissues. 4th. IARC; Lyon, France: 2008. [Google Scholar]
- 2.Li XQ, Li GD, Gao ZF, Zhou XG, Zhu XZ. The Chinese Lymphoma Study Group: Distribution pattern of lymphoma subtypes in China: A nationwide multicenter study of 10,002 cases. J Diagn Concepts Pract. 2012;11:111–115. [Google Scholar]
- 3.Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, Vose J, Armitage JO, Liang R. Clinical differences between nasal and extranasal natural killer⁄T-cell lymphoma: A study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009;113:3931–3937. doi: 10.1182/blood-2008-10-185256. [DOI] [PubMed] [Google Scholar]
- 4.Kim TM, Heo DS. Extranodal NK/T-cell lymphoma, nasal type: New staging system and treatment strategies. Cancer Sci. 2009;100:2242–2248. doi: 10.1111/j.1349-7006.2009.01319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oshimi K, Kawa K, Nakamura S, Suzuki R, Suzumiya J, Yamaguchi M, Kameoka J, Tagawa S, Imamura N, Ohshima K, et al. NK-cell neoplasms in Japan. Hematology. 2005;10:237–245. doi: 10.1080/10245330400026162. [DOI] [PubMed] [Google Scholar]
- 6.Ye Z, Cao Q, Niu G, Liang Y, Liu Y, Jiang L, Yu X, Han A. p63 and p53 expression in extranodal NK/T cell lymphoma, nasal type. J Clin Pathol. 2013;66:676–680. doi: 10.1136/jclinpath-2013-201454. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Wang L, Liu WJ, Xia ZJ, Huang HQ, Jiang WQ, Li ZM, Lu Y. High post-treatment serum levels of soluble programmed cell death ligand 1 predict early relapse and poor prognosis in extranodal NK/T cell lymphoma patients. Oncotarget. 2016;7:3035–3345. doi: 10.18632/oncotarget.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang H, Li P, Zhang X, Xia Z, Lu Y, Huang H. Histological vascular invasion is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. Oncol Lett. 2016;12:825–836. doi: 10.3892/ol.2016.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiarle R, Podda A, Prolla G, Gong J, Thorbecke GJ, Inghirami G. CD30 in normal and neoplastic cells. Clin Immunol. 1999;90:157–164. doi: 10.1006/clim.1998.4636. [DOI] [PubMed] [Google Scholar]
- 10.Deutsch YE, Tadmor T, Podack ER, Rosenblatt JD. CD30: An important new target in hematologic malignancies. Leuk Lymphoma. 2011;52:1641–1654. doi: 10.3109/10428194.2011.574761. [DOI] [PubMed] [Google Scholar]
- 11.Sabattini E, Pizzi M, Tabanelli V, Baldin P, Sacchetti CS, Agostinelli C, Zinzani PL, Pileri SA. CD30 expression in peripheral T-cell lymphomas. Haematologica. 2013;98:e81–e82. doi: 10.3324/haematol.2013.084913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bossard C, Dobay MP, Parrens M, Lamant L, Missiaglia E, Haioun C, Martin A, Fabiani B, Delarue R, Tournilhac O, et al. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: High correlation with mRNA levels. Blood. 2014;124:2983–2986. doi: 10.1182/blood-2014-07-584953. [DOI] [PubMed] [Google Scholar]
- 13.Nicolae A, Pittaluga S, Venkataraman G, Vijnovich-Baron A, Xi L, Raffeld M, Jaffe ES. Peripheral T-cell lymphomas of follicular T-helper cell derivation with Hodgkin/Reed-Sternberg cells of B-cell lineage: Both EBV-positive and EBV-negative variants exist. Am J Surg Pathol. 2013;37:816–826. doi: 10.1097/PAS.0b013e3182785610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duvic M. CD30+ neoplasms of the skin. Curr Hematol Malig Rep. 2011;6:245–250. doi: 10.1007/s11899-011-0096-8. [DOI] [PubMed] [Google Scholar]
- 15.Gaal K, Sun NC, Hernandez AM, Arber DA. Sinonasal NK/T-cell lymphomas in the United States. Am J Surg Pathol. 2000;24:1511–1517. doi: 10.1097/00000478-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Kuo TT, Shih LY, Tsang NM. Nasal NK/T cell lymphoma in Taiwan: A clinicopathologic study of 22 cases, with analysis of histologic subtypes, Epstein-Barr virus LMP-1 gene association, and treatment modalities. Int J Surg Pathol. 2004;12:375–387. doi: 10.1177/106689690401200410. [DOI] [PubMed] [Google Scholar]
- 17.Li P, Jiang L, Zhang X, Liu J, Wang H. CD30 expression is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. BMC Cancer. 2014;14:890. doi: 10.1186/1471-2407-14-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim WY, Nam SJ, Kim S, Kim TM, Heo DS, Kim CW, Jeon YK. Prognostic implications of CD30 expression in extranodal natural killer/T-cell lymphoma according to treatment modalities. Leuk Lymphoma. 2015;56:1778–1786. doi: 10.3109/10428194.2014.974048. [DOI] [PubMed] [Google Scholar]
- 19.Hong J, Park S, Baek HL, Jung JH, Kang IG, Sym SJ, Park J, Ahn JY, Cho EK, Kim ST, et al. Tumor cell nuclear diameter and CD30 expression as potential prognostic parameter in patients with extranodal NK/T-cell lymphoma, nasal type. Int J Clin Exp Pathol. 2012;5:939–947. [PMC free article] [PubMed] [Google Scholar]
- 20.Li T, Zhang B, Ye Y, Yin H. Immunohistochemical and genetic analysis of Chinese nasal natural killer/T-cell lymphomas. Hum Pathol. 2006;37:54–60. doi: 10.1016/j.humpath.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz EJ, Molina-Kirsch H, Zhao S, Marinelli RJ, Warnke RA, Natkunam Y. Immunohistochemical characterization of nasal-type extranodal NK/T-cell lymphoma using a tissue microarray: An analysis of 84 cases. Am J Clin Pathol. 2008;130:343–351. doi: 10.1309/V561QTM6854W4WAV. [DOI] [PubMed] [Google Scholar]
- 22.Ko YH, Ree HJ, Kim WS, Choi WH, Moon WS, Kim SW. Clinicopathologic and genotypic study of extranodal nasal-type natural killer/T-cell lymphoma and natural killer precursor lymphoma among Koreans. Cancer. 2000;89:2106–2116. doi: 10.1002/1097-0142(20001115)89:10<2106::AID-CNCR11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 23.Lu D, Lin CN, Chuang SS, Hwang WS, Huang WT. T-cell and NK/T-cell lymphomas in southern Taiwan: A study of 72 cases in a single institute. Leuk Lymphoma. 2004;45:923–928. doi: 10.1080/10428190310001625881. [DOI] [PubMed] [Google Scholar]
- 24.Ramos-Vara JA, Miller MA. Comparison of two polymer-based immunohistochemical detection systems: ENVISION+ and ImmPRESS. J Microsc. 2006;224:135–139. doi: 10.1111/j.1365-2818.2006.01679.x. [DOI] [PubMed] [Google Scholar]
- 25.Oshimi K. Progress in understanding and managing natural killer-cell malignancies. Br J Haematol. 2007;139:532–544. doi: 10.1111/j.1365-2141.2007.06835.x. [DOI] [PubMed] [Google Scholar]
- 26.Mraz-Gernhard S, Natkunam Y, Hoppe RT, LeBoit P, Kohler S, Kim YH. Natural killer/natural killer-like T-cell lymphoma, CD56, presenting in the skin: An increasingly recognized entity with an aggressive course. J Clin Oncol. 2001;19:2179–2188. doi: 10.1200/JCO.2001.19.8.2179. [DOI] [PubMed] [Google Scholar]
- 27.Hu S, Xu-Monette ZY, Balasubramanyam A, Manyam GC, Visco C, Tzankov A, Liu WM, Miranda RN, Zhang L, Montes-Moreno S, et al. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: A report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2013;121:2715–2724. doi: 10.1182/blood-2012-10-460063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bisig B, de Reyniès A, Bonnet C, Sujobert P, Rickman DS, Marafioti T, Delsol G, Lamant L, Gaulard P, de Leval L. CD30-positive peripheral T-cell lymphomas share molecular and phenotypic features. Haematologica. 2013;98:1250–1258. doi: 10.3324/haematol.2012.081935. [DOI] [PMC free article] [PubMed] [Google Scholar]