Abstract
Melatonin, which is synthesized by the pineal gland and released into the blood, exhibits antitumor properties. However, the mechanisms underlying these effects, particularly in stomach cancer, remain unknown. In the present study, the effect of melatonin on the nuclear factor (NF)-κB signaling pathway and the mitogen-activated protein kinase signaling pathway, involving p38 and c-Jun-N-terminal kinase (JNK), were investigated in SGC7901 gastric cancer cells. In addition, the effect of melatonin on the survival, migration and apoptosis of these cells was investigated in vitro in order to evaluate the use of melatonin for the treatment of gastric cancer. The results of the present study revealed that melatonin decreased the viability and migration of SGC7901 cells. Furthermore, melatonin induced apoptosis. Melatonin was identified to elevate the expression levels of phosphorylated (p)-p38 and p-JNK protein, and decrease the expression level of nucleic p-p65. These results suggest that the protein levels of p65, p38 and JNK are associated with the survival of SGC7901 cells following treatment with melatonin. The optimal concentration of melatonin was demonstrated to be 2 mM, which significantly induced apoptosis following a 24 h treatment period. These findings suggest that conflicting growth signals in cells may inhibit the efficacy of melatonin in the treatment of gastric cancer. Therefore, adjunct therapy would be required to improve the efficacy of melatonin in the treatment of cancer.
Keywords: melatonin, nuclear factor-κB, p38, c-Jun-N-terminal kinase, viability, apoptosis
Introduction
Gastric carcinoma is one of the most common types of malignancy, worldwide (1). In Asia, patients tend to be diagnosed at an average age of 66.8 years, and exhibit poor rates of survival (1). Gastric cancer is a refractory cancer and the third-leading cause of cancer-associated mortality in China (2). Gastric adenocarcinoma is the predominant type of gastric cancer and has limited treatment options (3). The typical treatments for gastric cancer, surgery and chemotherapy, only partially improve the overall survival of patients with this disease (4,5). Therefore, more effective drugs or comprehensive therapies are required (6).
Melatonin is an indole hormone secreted by the pineal gland and was first identified in 1958 (7). Melatonin serves a significant role in a wide variety of biological processes, including sleep, immunomodulation, anti-inflammation and antioxidative stress (8–11). In addition, melatonin is an anticancer hormone and a potential target for cancer treatments (12). Several studies have revealed that melatonin is useful for the prevention of cancer (13,14). The antitumor ability of melatonin may be mediated by numerous mechanisms, including the activation of antioxidative stress, inhibition of migration and induction of apoptosis (15–17). Melatonin has also been demonstrated to suppress the growth, migration and invasion of SGC7901 gastric cancer cells in vitro (18), and to exert an apoptotic effect in the hepatocellular carcinoma HepG2 cell line (19). Additionally, the use of melatonin has not been associated with significant side effects (20). Therefore, the present study hypothesized that melatonin may exhibit efficacy in the prevention and treatment of numerous types of cancer, likely as a supplement to adjunct therapies.
Melatonin, which acts as an inhibitor of activated nuclear factor (NF)-κB signaling in prostate cancer cells, may serve as a potential therapeutic molecule to inhibit the proliferation and metastasis of LNCaP and 22Rv1 prostate cancer cells in subsequent clinical stages of cancer progression (21). NF-κB, a transcription factor, stimulates the expression of numerous genes associated with oxidative stress, immune responses, cytokine production and apoptosis (22,23). NF-κB belongs to the Rel family, which consists of p50, p52, c-Rel, p65 and RelB. Typically, NF-κB dimers reside in the cytosol bound to NF-κB inhibitor (IκB) proteins and are rapidly activated by stimuli capable of inducing the phosphorylation and proteolysis of IκB. Upon the removal of IκB, NF-κBp65, which belongs to the Rcl family, enters the nucleus to induce the expression of target genes, thereby controlling immunity, inflammation, cell growth and survival (24).
In the present study, the effect of pharmacological concentrations of melatonin on the proliferation, migration and apoptosis of SGC7901 gastric cancer cells was evaluated in vitro to examine the efficacy of melatonin for the treatment of cancer. With respect to nuclear transcription factors, the NF-κB-mediated suppression of apoptosis involves the inhibition of the c-Jun N-terminal kinase (JNK) signaling cascade (24). The key mammalian mitogen-activated protein kinases (MAPKs), JNK and p38, may be associated with cell growth and apoptosis (25). JNK and p38 have been suggested to serve a role in cancer development and have been demonstrated to be affected by melatonin (26). In the present study, the effect of melatonin on the viability, invasion and apoptosis of gastric cancer cells was evaluated in association with the expression of NF-κB-p65, p38 and JNK.
Materials and methods
Cell culture and melatonin treatment
The human gastric cancer cell line SGC7901 was obtained from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). The human gastric mucosal cell line GES-1 was obtained from the American Type Culture Collection (Manassas, VA, USA) and used as the control. The cells were cultured in a complete culture medium of RPMI-1640 (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) supplemented with 10% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich; Merck Millipore) and 1% penicillin/streptomycin at 37°C with 5% CO2 in a humidified incubator. Melatonin (Sigma-Aldrich; Merck Millipore) was dissolved in 0.2% dimethyl sulfoxide (DMSO) and the cells were treated with 0–2 mM melatonin for 0–48 h. The vehicle control group of only DMSO was administered 0.2% DMSO alone. Control group were cultured in the absence of melatonin and DMSO. In all experiments, the cells were maintained in RPMI-1640 medium supplemented with 10% FBS at 37°C for 24 h prior to the melatonin treatment.
Colony formation assays
The SGC7901 cells were seeded into 6-well plates at a concentration of 5×105 cells/well and cultured at 37°C in a humidified atmosphere containing 5% CO2. On the following day, the cells were treated with 2 mM melatonin once and subsequently incubated at 37°C for 8 days. Each well was subsequently washed twice with PBS and stained with crystal violet (10 mg/ml). During the 8-day incubation, the medium was replaced every 72 h in all wells.
Cell viability assay
The Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto, Japan) assay was used to assess the effect of melatonin on cell viability. For the CCK-8 assay, SGC7901 and GES-1 cells were seeded into 96-well plates at a density of 4×103 cells/well, in a total volume of 200 µl complete medium. The medium was replaced 24 h following seeding. Various concentrations of melatonin (0–2 mM) were subsequently added and the cells were cultured at 37°C for 0–48 h. Each treatment group had 3 parallel wells. Subsequent to the exposure of the cells to melatonin, cell viability was evaluated using the CCK-8 assay, according to the manufacturer's instructions. The absorbance at a wavelength of 450 nm was determined for each well using an Infinite 200 PRO NanoQuant plate reader (Tecan Austria GmbH, Grödig, Austria).
Wound healing assay
SGC7901 cells were plated into 12-well plates at a density of 3×104 cells/well in complete medium and cultured at 37°C with 5% CO2. When the cells formed a monolayer, a scratch was made in the middle of the well with a P100 pipette tip. Subsequently, the debris was washed away with PBS and the wells were cultured with fresh media supplemented with 1% FBS and various concentrations of melatonin (0–2 mM). Subsequent to an incubation of 0, 24 or 48 h, images of the cells were captured using a phase-contrast microscope. Each experiment was performed in triplicate. The migration distance was calculated as follows: Area in the field of view/length devoid of cells. This analysis was performed using Image-Pro Plus software 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). The results are expressed as the difference in migration distance between 0 and 48 h of treatment.
Apoptosis assay
Due to the detection of the extent of early apoptosis and necrosis, SGC7901 cells were stained with fluorescein isothiocyanate (FITC)-conjugated Annexin V and propidium iodide (PI; Annexin V-FITC/PI apoptosis kit; Multi Sciences Biotech Co., Ltd., Hangzhou, China). In total, 4×105 cells were plated into 6-well plates and treated with 0, 0.5, 1 and 2 mM melatonin. Following a 24-h incubation, the cells were harvested by trypsinization and washed with PBS. The cells were then centrifuged at 10.8 × g at 37°C for 5 min and the supernatant was discarded. A total of 2×105-1×106 cells/ml cells were resuspended in 1X binding buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 1 mM MgCl2, 5 mM KCl, 2.5 mM CaCl2). Subsequently, 5 µl FITC-conjugated Annexin-V and 10 µl PI were added to 500 µl of this solution for 5 min at room temperature in the dark. Apoptosis was detected using the BD FACSCalibur (BD Biosciences, San Jose, CA, USA) and quantified using FlowJo software 10.0 (Tree Star, Inc., Ashland, OR, USA).
Immunofluorescence staining
To investigate the association between melatonin and NF-κB activation, the localization of NF-κB protein in SGC7901 cells was investigated using an immunofluorescence staining assay. A total of 4×105 cells were seeded onto glass coverslips in 6-well plates and treated with 2 mM melatonin for 24 h. Subsequently, the glass disks were removed from the wells, washed 3 times with PBS and fixed with 4% paraformaldehyde at room temperature for 20 min. The disks were washed with PBS and 1% Triton X-100 was added for 5 min in order to permeabilize the cell membranes. Following blocking with 1% bovine serum albumin (BD Biosciences), the cells were incubated with anti-NF-κB-p65 antibody (D14E12 rabbit mAb; #8242; dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA) followed by incubation with a goat anti-rabbit secondary IgG horseradish peroxidase (HRP) conjugated antibody (#BS13278; dilution, 1:10,000; Bioworld Technology Inc., St. Louis Park, MN, USA). The cells were then stained with DAPI for 5 min in the dark at room temperature. Images were captured using a Leica Spectral confocal laser microscope.
Western blot analysis
Cells were plated into 100 mm dishes at a density of 5×105 cells/dish in complete medium and cultured at 37°C with 5% CO2 for 48 h. Following 2 mM melatonin treatment the cells were washed twice with PBS and lysed by adding ice-cold radioimmunoprecipitation lysis buffer (Beyotime Biotechnology, Shanghai, China) and PhosSTOP (dilution, 1:10; Roche Diagnostics, Basel, Switzerland) for 15 min on ice. The cells were then scraped off the plate, and the extracts transferred into a microfuge tube and centrifuged at 12,000 × g at 4°C for 10 min. Protein concentration was determined using the BCA Assay kit (Beyotime Institute of Biotechnology, Haimen, China). Equal amounts (40 µg) of the supernatant protein were separately subjected to SDS-PAGE on a 10% gel and transferred to a polyvinylidene fluoride membrane (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The membrane was incubated with antibodies directed against the following proteins: Phosphorylated (p)-JNK (Thr183/Tyr185 rabbit mAb; #4668), JNK (#9252), p-p38 (Thr180/Tyr182 rabbit mAb; #9215), p38 (D13E1 rabbit mAb; #8690), p-p65 (Ser536 rabbit mAb; #3033), p65 (D14E12 rabbit mAb; #8242; dilutions for all 1:1,000; all Cell Signaling Technology, Inc.) and GAPDH (1:1,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Following washing with TBS/Tween-20, the membranes were incubated for 1 h at room temperature with a goat anti-rabbit secondary IgG HRP conjugated anti-rabbit secondary fluorescent antibody (#BS13278; dilution, 1:10,000; Bioworld Technology Inc.) and visualized using a WesternBright Enhanced Chemiluminescence Substrate kit (Advansta Inc., Menlo Park, CA, USA). Images of the blots were captured using the ChemiDoc MP system and the density of the specific bands was quantified using Image Lab software version 5.0 (both Bio-Rad Laboratories, Inc.).
Statistical analysis
Results are expressed as the mean ± standard error of the mean. Data were compared by one-way analysis of variance. When the analysis indicated the presence of a significant difference, the means were compared with using the Newmann-Keuls method. All statistical analyses were performed using SPSS software (version 13; SPSS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
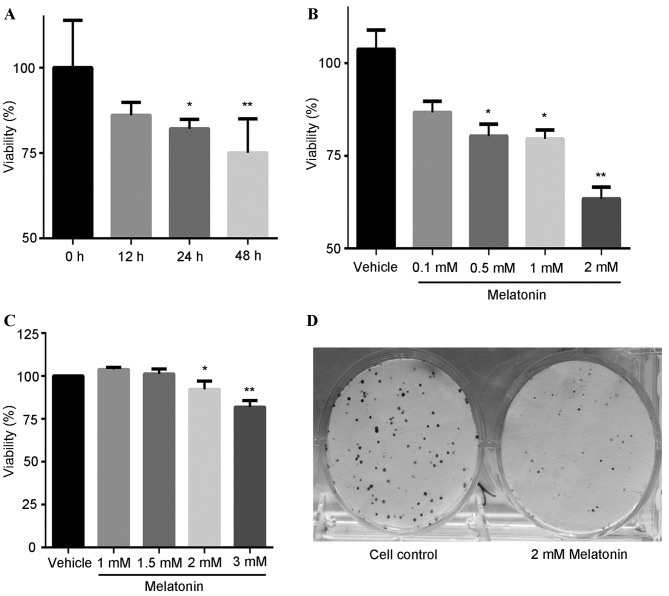
The effect of melatonin on SGC7901 cell viability was investigated using the CCK-8 assay. The cells were treated with 1 mM melatonin for different lengths of time (Fig. 1A) or at different concentrations of melatonin (0.1-2 mM) for 24 h (Fig. 1B). As shown in Fig. 1, melatonin decreased cell viability in a concentration- and time-dependent manner. At a concentration of 1 mM, melatonin significantly decreased the viability of SGC7901 cells compared with the control group when exposure was 24 (P<0.05) or 48 (P<0.01) h (Fig. 1A). In addition, treatment with 2 mM melatonin for 24 h significantly reduced the viability of SGC7901 cells to 61% compared with the control group (P<0.01; Fig. 1B), while in human gastric mucosal GES-1 cells the same treatment was less cytotoxic (Fig. 1C). Colony formation assays revealed that the colony-forming ability of SGC7901 cells was markedly reduced following treatment with 2 mM melatonin for 8 days (Fig. 1D). From these results, 2 mM melatonin was identified as the optimal concentration for reducing the viability and colony-forming ability of SGC7901 cells compared with the control treatment.
Figure 1.
Effect of melatonin on decreases the viability and colony-forming ability of SGC7901 cells. (A) SGC7901 cells treated with 1 mM melatonin for 0–48 h. (B) SGC7901 cells treated with 0.1-2 mM melatonin for 24 h. (C) GES-1 cells treated with 1–3 mM melatonin for 24 h. (D) SGC7901 cells incubated in the absence (control) or presence of 2 mM melatonin for 8 days. *P<0.05, **P<0.01 vs. the control group.
To evaluate the effect of melatonin on cell migration, a wound healing assay was conducted, where the SGC7901 cells were treated with 0.1, 0.5, 1 and 2 mM melatonin for 48 h, with cell migration measured at 0, 24 ad 48 h. As presented in Fig. 2A, melatonin inhibited cell migration in a dose-dependent manner. A total of 48 h exposure to 2 mM melatonin significantly reduced SGC7901 cell migration (29% of control; P<0.0001; Fig. 2B).
Figure 2.
Melatonin inhibits the migration of SGC7901 cells. (A) Cells treated with 0–2 mM melatonin for 0, 24 and 48 h (the red lines are the width of the 0 h group and used to compare with the other groups). (B) Analysis of the relative migration of cells following treatment with 0.1-2 mM melatonin 48 h compared with the 0 h control group. *P<0.005, **P<0.01, ***P<0.0001 vs. the control group.
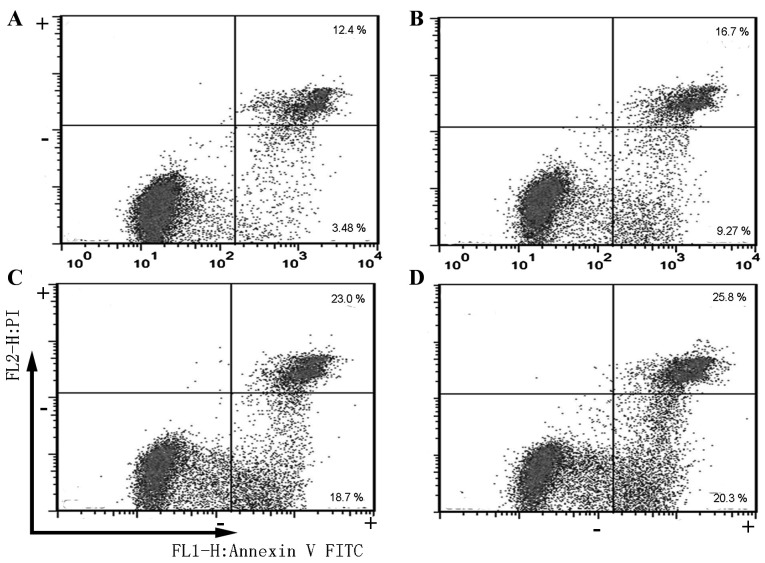
An apoptosis assay was subsequently performed using FITC-conjugated Annexin V and PI staining, which facilitate the early detection of apoptotic cells through flow cytometry and fluorescence microscopy. The positioning of quadrants on the FITC/PI dot plots was used to distinguish living cells (FITC−/PI−), early apoptotic cells (FITC+/PI−) and late apoptotic/secondary necrotic cells (FITC+/PI+). The apoptosis of SGC7901 cells following to exposure to 0, 0.5, 1 and 2 mM melatonin for 24 h was measured (Fig. 3). The percentage of early apoptotic cells in SGC7901 cells treated with 0.5, 1 and 2 mM melatonin for 24 h were ~2.6-, ~5.3- and ~5.8-fold of that of the control group, respectively (0 mM; data not shown). At the concentrations of 0.5, 1 and 2 mM melatonin (Fig. 3), late apoptotic/secondary necrotic and early apoptotic cells increased by 26.0, 41.7 and 46.1%, respectively, compared with the control group (0 mM; data not shown).
Figure 3.
Melatonin induces SGC7901 cell apoptosis. Cells were (A) untreated or treated with (B) 0.5, (C) 1 mM or (D) 2 mM melatonin for 24 h.
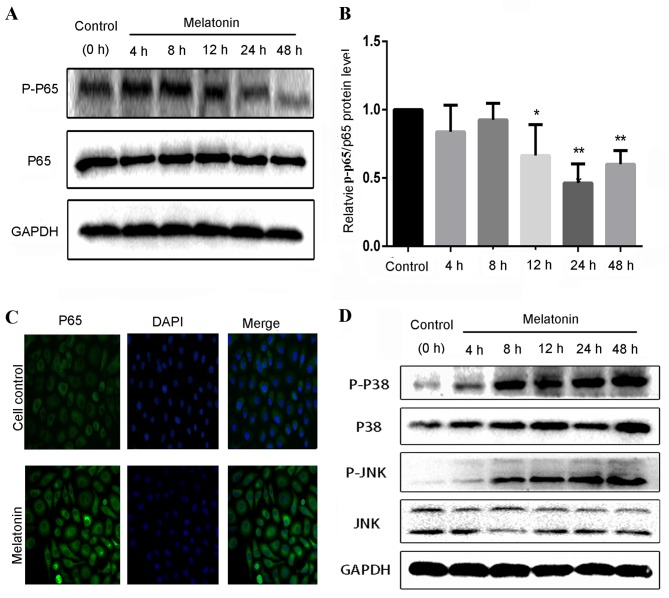
The aforementioned findings suggest that melatonin inhibits the viability and migration, and induces the apoptosis of SGC7901 cells. The NF-κB and MAPK signaling pathways serve an essential role in the proliferation, migration and apoptosis of cancer cells. Therefore, to determine whether melatonin regulates the NF-κB and MAPK signaling pathways, the effect of melatonin on the expression and activation of proteins in these signaling pathways was detected by western blotting (Fig. 4). As presented in Fig. 4A, the expression level of the nuclear protein p-p65 decreased in SGC7901 cells with melatonin treatment. A decrease in the level of the p-p65/p65 protein in SGC7901 cells treated with 2 mM melatonin was observed at 12 h (P<0.05), with a maximal significantly decreased level at 24 h of ~0.46 fold compared with that of the control cells (P<0.01; Fig. 4B). The changes in the protein levels of p65 and p-p65 detected via western blotting subsequent to the administration of melatonin were reflected in the results of the immunofluorescent staining of SGC7901 cells (Fig. 4C). Melatonin-stimulated cells exhibited intensive and clear cytoplasmic staining for p65. These results support the theory that melatonin inhibits the translocation of NF-κB p65 from the cytoplasm to the nucleus.
Figure 4.
Effect of melatonin treatment on the expression of p65, p38 and JNK/MAPK proteins in SGC7901 cells. (A) Western blot analysis for p65 and p-p65 following treatment with 2 mM melatonin for 0–48 h. (B) The expression levels of p-p65/p65 proteins were quantified by densitometric analysis using the control as 100%. (C) Altered nuclear translocation of NF-κB p65 following 2 mM melatonin treatment for 24 h. Following cell fixation, nuclei were visualized by blue DAPI staining, and nuclear NF-κB translocation was observed by overlaying with anti-p65 green immunofluorescence microscopy. (D) Western blot analysis for p38, p-p38, JNK and p-JNK following melatonin treatment. *P<0.05, **P<0.01 vs. the control group. p-, phosphorylated; JNK, c-Jun-N-terminal kinase.
In the MAPK signaling pathway, melatonin was demonstrated to increase the active phosphorylated forms of JNK and p38, p-JNK and p-p38 (Fig. 4D), in a time-dependent manner. Following a 48 h treatment with 2 mM melatonin, the levels of p-p38 exhibited a 4.6-fold increase, whereas the levels of p-JNK increased by 9.8-fold compared with the control group (data not shown). The aforementioned results are consistent with the changes in cell viability, migration and apoptosis observed following treatment with melatonin.
Discussion
The present study investigated the effect of pharmacological concentrations of melatonin on human gastric cancer cell survival and viability in vitro, and evaluated the role of melatonin in the NF-κB and MAPK signaling pathways, which are known to be involved in the development of cancer (27). Treatment with melatonin at a concentration of 2 mM was demonstrated to increase expression of p-p38 and p-JNK proteins. Previous immunofluorescence studies of p65-stained cells in addition to results of the present study, have suggested that 1–2 mM melatonin inhibits the translocation of NF-κB-p65 from the cytoplasm to the nucleus (28–30). These results correspond with the findings of the current study that melatonin markedly decreased cell viability and induced apoptosis in SGC7901 cells. These findings suggest that p65, p-p38 and p-JNK are associated with the survival of SGC7901 cells following melatonin treatment, and that at a concentration of 2 mM, a pharmacological concentration, melatonin can induce cell death.
Melatonin possesses a wide variety of physiological functions, ranging from the regulation of the circadian rhythm (31) to acting as a potent antioxidant (32). A number of cellular membrane receptors are regulated by melatonin, including nuclear and extracellular membrane receptors (33), which may partially account for the differential responses to melatonin observed in normal and tumor cells. For example, a previous study found that treatment with 1 mM melatonin for 24 h inhibited activation of NF-κB in the human prostate cancer LNCaP cell line (21). In addition, several prior reports have revealed that melatonin inhibits NF-κB activity (34,35), supporting the hypothesis that melatonin is a proteasome inhibitor and a natural NF-κB inhibitor. NF-κB inhibition serves an important role in the treatment of leukemia and lymphoma (36). Melatonin has also been reported to exhibit a protective role against diabetic neuropathy via the NF-κB signaling pathway (37). However, another study demonstrated that melatonin significantly suppressed interleukin-1β-induced NF-κB translocation and transcriptional activity in human hepatocellular HepG2 cells (17). The inhibitory action of melatonin on NF-κB leads to an anti-inflammatory and pro-apoptotic effect on cancer cell lines (38,39). Additionally, several meta-analyses support the theory that melatonin administration in patients with cancer is a safe and effective treatment (40,41). These findings support the hypothesis that the mechanism by which melatonin regulates cell growth is affected by the cell type and the concentration of melatonin.
In addition, previous studies indicate that melatonin may be regulated by melatonin receptor 1A (MT1) and melatonin receptor 1B (MT2), which are the target receptors of melatonin and exhibit a high affinity to the plasma membrane (42,43). Although the mechanism of melatonin with respect to the downregulation of cell proliferation in cancer is not fully understood, it has been demonstrated that in ovarian CHO cells the ERK1/2 pathway can be activated by melatonin treatment through the MT1 receptor (44). Melatonin may affect the phosphorylation of JNK and p38 in hepatocellular carcinoma cells (45). Notably, melatonin induced apoptosis and increased levels of the apoptosis-associated proteins B-cell lymphoma 2 (Bcl-2)-associated X protein and cytochrome c, which are associated with the JNK and p38 signaling pathways, but not the ERK signaling pathway (26). In the present study, the p38 and JNK pathways appeared to be activated at therapeutic concentrations of melatonin in a gastric adenocarcinoma cell line. However, it has been shown that melatonin significantly inhibits the migration of A549 cells, which may be associated with the downregulation of the JNK/MAPK signaling pathway (46). The results of the current study indicate that the activation of p38 and JNK by melatonin initiates a molecular proliferative response in SGC7901 cells.
Although the proliferation of SGC7901 cells was not measured, treatment with 2 mM melatonin decreased cell viability and induced apoptosis. A number of previous studies have reported that the primary mechanism of antitumor activity is cell apoptosis (47,48). Melatonin has been shown to reduce the expression of the apoptosis-associated protein Bcl-2 and increased pro-apoptotic activity in gastric cancer cells in vitro and in vivo (16). These results suggest that numerous mechanisms comprising distinct molecular signaling pathways may be involved in the effects of melatonin treatment.
In conclusion, melatonin may be useful for the treatment of several types of cancer, including gastric cancer. The results of the present study suggest the requirement of additional or adjunct therapies in combination with melatonin treatment to fully inhibit the progression of cancer. The potential to target the mechanisms that inhibit NF-κB-p65, and promote p38 and JNK, may provide a strategy to investigate the efficacy of a number of types of chemotherapy. Similar studies in other types of tumor may provide useful data on the cellular response to melatonin that inhibits cell survival (26,28). The results of the present and previous studies lead to the hypothesize that melatonin induces apoptosis and oncostasis via the modulation of the JNK, p38 and NF-κB-p65 signaling pathways. Additional studies investigating these signaling pathways may be useful in the development of chemotherapeutics that function synergistically with melatonin, a non-toxic natural product with apoptotic properties, to induce cancer-specific cell death.
Acknowledgements
The authors of the present study would like to thank Mrs. Lihui Yin at the Institute of Translational Medicine (The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China) for technical support with respect to the cell culture and Dr. Gaoxiang Huang at the Department of Pathophysiology of the Second Military Medical University (Shanghai, China) for reviewing the English of this manuscript. The present study was supported by a grant from the Science and Technology Bureau of Wenzhou (grant no. 2014S0192).
References
- 1.Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between caucasian and asian patients treated in the united states: Results from the surveillance epidemiology and end results (SEER) database. Ann Surg Oncol. 2015;22:2965–2971. doi: 10.1245/s10434-015-4388-4. [DOI] [PubMed] [Google Scholar]
- 2.Hou R, Cao B, Chen Z, Li Y, Ning T, Li C, Xu C, Chen Z. Association of cytotoxic T lymphocyte-associated antigen-4 gene haplotype with the susceptibility to gastric cancer. Mol Biol Rep. 2010;37:515–520. doi: 10.1007/s11033-009-9705-1. [DOI] [PubMed] [Google Scholar]
- 3.Wesolowski R, Lee C, Kim R. Is there a role for second-line chemotherapy in advanced gastric cancer? Lancet Oncol. 2009;10:903–912. doi: 10.1016/S1470-2045(09)70136-6. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Allum WH, Stenning SP, Thompson JN, van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ, Falk SJ, Iveson TJ, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 5.Kampschöer GH, Maruyama K, Sasako M, Kinoshita T, van de Velde CJ. The effects of blood transfusion on the prognosis of patients with gastric cancer. World J Surg. 1989;13:637–643. doi: 10.1007/BF01658891. [DOI] [PubMed] [Google Scholar]
- 6.Lissoni P, Brivio F, Ardizzoia A, Tancini G, Barni S. Subcutaneous therapy with low-dose interleukin-2 plus the neurohormone melatonin in metastatic gastric cancer patients with low performance status. Tumori. 1993;79:401–404. doi: 10.1177/030089169307900606. [DOI] [PubMed] [Google Scholar]
- 7.Lerner AB, Case JD, Takahashi Y, Lee TH, Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocyteS1. J Am Chem Soc. 1958;80:2587. doi: 10.1021/ja01543a060. [DOI] [Google Scholar]
- 8.Berra B, Rizzo AM. Melatonin: Circadian rhythm regulator, chronobiotic, antioxidant and beyond. Clin Dermatol. 2009;27:202–209. doi: 10.1016/j.clindermatol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Cardinali DP, Esquifino AI, Srinivasan V, Pandi-Perumal SR. Melatonin and the immune system in aging. Neuroimmunomodulation. 2007;15:272–278. doi: 10.1159/000156470. [DOI] [PubMed] [Google Scholar]
- 10.Ambriz-Tututi M, Rocha-González HI, Cruz SL, Granados-Soto V. Melatonin: A hormone that modulates pain. Life Sci. 2009;84:489–498. doi: 10.1016/j.lfs.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 11.Fischer TW, Kleszczyński K, Hardkop LH, Kruse N, Zillikens D. Melatonin enhances antioxidative enzyme gene expression (CAT, GPx, SOD), prevents their UVR-induced depletion, and protects against the formation of DNA damage (8-hydroxy-2′-deoxyguanosine) in ex vivo human skin. J Pineal Res. 2013;54:303–312. doi: 10.1111/jpi.12018. [DOI] [PubMed] [Google Scholar]
- 12.Cutando A, López-Valverde A, Arias-Santiago S, DE Vicente J, DE Diego RG. Role of melatonin in cancer treatment. Anticancer Res. 2012;32:2747–2753. [PubMed] [Google Scholar]
- 13.Shiu SY, Li L, Xu JN, Pang CS, Wong JT, Pang SF. Melatonin-induced inhibition of proliferation and G1/S cell cycle transition delay of human choriocarcinoma JAr cells: Possible involvement of MT2 (MEL1B) receptor. J Pineal Res. 1999;27:183–192. doi: 10.1111/j.1600-079X.1999.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 14.Bush SH, Lacaze-Masmonteil N, McNamara-Kilian MT, MacDonald AR, Tierney S, Momoli F, Agar M, Currow DC, Lawlor PG. The preventative role of exogenous melatonin administration to patients with advanced cancer who are at risk of delirium: Study protocol for a randomized controlled trial. Trials. 2016;17:399. doi: 10.1186/s13063-016-1525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu L, Liu H, Zhang H, Wang RX, Song J, Zhou RX. Growth-inhibitory activity of melatonin on murine foregastric carcinoma cells in vitro and the underlying molecular mechanism. Anat Rec (Hoboken) 2013;296:914–920. doi: 10.1002/ar.22689. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Jin QD, Gong X, Liu H, Zhou RX. Anti-gastric cancer effect of melatonin and Bcl-2, Bax, p21 and p53 expression changes. Sheng Li Xue Bao. 2014;66:723–729. (In Chinese) [PubMed] [Google Scholar]
- 17.Ordoñez R, Carbajo-Pescador S, Prieto-Dominguez N, García-Palomo A, González-Gallego J, Mauriz JL. Inhibition of matrix metalloproteinase-9 and nuclear factor kappa B contribute to melatonin prevention of motility and invasiveness in HepG2 liver cancer cells. J Pineal Res. 2014;56:20–30. doi: 10.1111/jpi.12092. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S, Qi Y, Zhang H, He W, Zhou Q, Gui S, Wang Y. Melatonin inhibits cell growth and migration, but promotes apoptosis in gastric cancer cell line, SGC7901. Biotech Histochem. 2013;88:281–289. doi: 10.3109/10520295.2013.769633. [DOI] [PubMed] [Google Scholar]
- 19.Martín-Renedo J, Mauriz JL, Jorquera F, Ruiz-Andrés O, González P, González-Gallego J. Melatonin induces cell cycle arrest and apoptosis in hepatocarcinoma HepG2 cell line. J Pineal Res. 2008;45:532–540. doi: 10.1111/j.1600-079X.2008.00641.x. [DOI] [PubMed] [Google Scholar]
- 20.Vijayalaxmi, Reiter RJ, Tan DX, Herman TS, CR Jr Thomas. Melatonin as a radioprotective agent: A review. Int J Radiat Oncol Biol Phys. 2004;59:639–653. doi: 10.1016/j.ijrobp.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 21.Shiu SY, Leung WY, Tam CW, Liu VW, Yao KM. Melatonin MT1 receptor-induced transcriptional up-regulation of p27(Kip1) in prostate cancer antiproliferation is mediated via inhibition of constitutively active nuclear factor kappa B (NF-κB): Potential implications on prostate cancer chemoprevention and therapy. J Pineal Res. 2013;54:69–79. doi: 10.1111/j.1600-079X.2012.01026.x. [DOI] [PubMed] [Google Scholar]
- 22.Crawford LJ, Walker B, Irvine AE. Proteasome inhibitors in cancer therapy. J Cell Commun Signal. 2011;5:101–110. doi: 10.1007/s12079-011-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kucharczak J, Simmons MJ, Fan Y, Gélinas C. To be, or not to be: NF-kappaB is the answer-role of Rel/NF-kappaB in the regulation of apoptosis. Oncogene. 2003;22:8961–8982. doi: 10.1038/sj.onc.1207230. [DOI] [PubMed] [Google Scholar]
- 24.Bubici C, Papa S, Pham CG, Zazzeroni F, Franzoso G. NF-kappaB and JNK An intricate affair. Cell Cycle. 2004;3:1524–1529. doi: 10.4161/cc.3.12.1321. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh MH, Nguyen HT. Molecular mechanism of apoptosis induced by mechanical forces. Int Rev Cytol. 2005;245:45–90. doi: 10.1016/S0074-7696(05)45003-2. [DOI] [PubMed] [Google Scholar]
- 26.Joo SS, Yoo YM. Melatonin induces apoptotic death in LNCaP cells via p38 and JNK pathways: Therapeutic implications for prostate cancer. J Pineal Res. 2009;47:8–14. doi: 10.1111/j.1600-079X.2009.00682.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang M, Huang CZ. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J Gastroenterol. 2015;21:11673–11679. doi: 10.3748/wjg.v21.i41.11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin YW, Lee LM, Lee WJ, Chu CY, Tan P, Yang YC, Chen WY, Yang SF, Hsiao M, Chien MH. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J Pineal Res. 2016;60:277–290. doi: 10.1111/jpi.12308. [DOI] [PubMed] [Google Scholar]
- 29.León J, Casado J, Jiménez Ruiz SM, Zurita MS, González-Puga C, Rejón JD, Gila A, Muñoz de Rueda P, Pavón EJ, Reiter RJ, et al. Melatonin reduces endothelin-1 expression and secretion in colon cancer cells through the inactivation of FoxO-1 and NF-κβ. J Pineal Res. 2014;56:415–426. doi: 10.1111/jpi.12131. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Hao H, Yao L, Zhang X, Zhao S, Ling EA, Hao A, Li G. Melatonin suppresses migration and invasion via inhibition of oxidative stress pathway in glioma cells. J Pineal Res. 2012;53:180–187. doi: 10.1111/j.1600-079X.2012.00985.x. [DOI] [PubMed] [Google Scholar]
- 31.Cajochen C, Kräuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol. 2003;15:432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 32.Chakravarty S, Rizvi SI. Circadian modulation of human erythrocyte plasma membrane redox system by melatonin. Neurosci Lett. 2012;518:32–35. doi: 10.1016/j.neulet.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Hidalgo M, Guerrero JM, Villegas I, Packham G, de la Lastra CA. Melatonin, a natural programmed cell death inducer in cancer. Curr Med Chem. 2012;19:3805–3821. doi: 10.2174/092986712801661013. [DOI] [PubMed] [Google Scholar]
- 34.Bekyarova G, Apostolova M, Kotzev I. Melatonin protection against burn-induced hepatic injury by down-regulation of nuclear factor kappa B activation. Int J Immunopathol Pharmacol. 2012;25:591–596. doi: 10.1177/039463201202500305. [DOI] [PubMed] [Google Scholar]
- 35.Min KJ, Jang JH, Kwon TK. Inhibitory effects of melatonin on the lipopolysaccharide-induced CC chemokine expression in BV2 murine microglial cells are mediated by suppression of Akt-induced NF-κB and STAT/GAS activity. J Pineal Res. 2012;52:296–304. doi: 10.1111/j.1600-079X.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- 36.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Negi G, Kumar A, Sharma SS. Melatonin modulates neuroinflammation and oxidative stress in experimental diabetic neuropathy: Effects on NF-κB and Nrf2 cascades. J Pineal Res. 2011;50:124–131. doi: 10.1111/j.1600-079X.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 38.Uguz AC, Cig B, Espino J, Bejarano I, Naziroglu M, Rodríguez AB, Pariente JA. Melatonin potentiates chemotherapy-induced cytotoxicity and apoptosis in rat pancreatic tumor cells. J Pineal Res. 2012;53:91–98. doi: 10.1111/j.1600-079X.2012.00974.x. [DOI] [PubMed] [Google Scholar]
- 39.Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González-Gallego J. A review of the molecular aspects of melatonin's anti-inflammatory actions: Recent insights and new perspectives. J Pineal Res. 2013;54:1–14. doi: 10.1111/j.1600-079X.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang YM, Jin BZ, Ai F, Duan CH, Lu YZ, Dong TF, Fu QL. The efficacy and safety of melatonin in concurrent chemotherapy or radiotherapy for solid tumors: A meta-analysis of randomized controlled trials. Cancer Chemother Pharmacol. 2012;69:1213–1220. doi: 10.1007/s00280-012-1828-8. [DOI] [PubMed] [Google Scholar]
- 41.Seely D, Wu P, Fritz H, Kennedy DA, Tsui T, Seely AJ, Mills E. Melatonin as adjuvant cancer care with and without chemotherapy: A systematic review and meta-analysis of randomized trials. Integr Cancer Ther. 2012;11:293–303. doi: 10.1177/1534735411425484. [DOI] [PubMed] [Google Scholar]
- 42.Vanecek J. Cellular mechanisms of melatonin action. Physiol Rev. 1998;78:687–721. doi: 10.1152/physrev.1998.78.3.687. [DOI] [PubMed] [Google Scholar]
- 43.González-Arto M, Vicente-Carrillo A, Martínez-Pastor F, Fernández-Alegre E, Roca J, Miró J, Rigau T, Rodríguez-Gil JE, Pérez-Pé R, Muiño-Blanco T, et al. Melatonin receptors MT1 and MT2 are expressed in spermatozoa from several seasonal and nonseasonal breeder species. Theriogenology. 2016;86:1958–1968. doi: 10.1016/j.Theriogenology.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 44.Bondi CD, McKeon RM, Bennett JM, Ignatius PF, Brydon L, Jockers R, Melan MA, Witt-Enderby PA. MT1 melatonin receptor internalization underlies melatonin-induced morphologic changes in Chinese hamster ovary cells and these processes are dependent on Gi proteins, MEK 1/2 and microtubule modulation. J Pineal Res. 2008;44:288–298. doi: 10.1111/j.1600-079X.2007.00525.x. [DOI] [PubMed] [Google Scholar]
- 45.Carbajo-Pescador S, García-Palomo A, Martín-Renedo J, Piva M, González-Gallego J, Mauriz JL. Melatonin modulation of intracellular signaling pathways in hepatocarcinoma HepG2 cell line: Role of the MT1 receptor. J Pineal Res. 2011;51:463–471. doi: 10.1111/j.1600-079X.2011.00910.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Q, Gui S, Zhou Q, Wang Y. Melatonin inhibits the migration of human lung adenocarcinoma A549 cell lines involving JNK/MAPK pathway. PLoS One. 2014;9:e101132. doi: 10.1371/journal.pone.0101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elumalai P, Arunakaran J. Review on molecular and chemopreventive potential of nimbolide in cancer. Genomics Inform. 2014;12:156–164. doi: 10.5808/GI.2014.12.4.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohammad RM, Muqbil I, Lowe L, Yedjou C, Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol. 2015;35(Suppl):S78–S103. doi: 10.1016/j.semcancer.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]