Abstract
The present multicentre, prospective, open-label, single treatment arm study (Val-Perfect) examined the efficacy and tolerability of once-daily valsartan monotherapy (80 mg for two weeks, followed by 160 mg for eight weeks) in 195 Chinese patients with mild to moderate hypertension, using office, home, and ambulatory blood pressure (BP) monitoring. Significant mean reductions (P<0.0001) were observed in office BP from baseline to week 10, with mean sitting systolic BP (MSSBP) and mean sitting diastolic BP (MSDBP) values of 15.6±12.3 and 11.1±8.6 mmHg, respectively. The office BP control rate at week 10 was 56.9% (target MSSBP/MSDBP <130/80 mmHg for patients with type 2 diabetes or chronic kidney disease, <140/90 mmHg for others). Valsartan treatment significantly reduced mean 24-h SBP/DBP (−6.1/−4.4 mmHg; both P<0.0001) and mean home-monitored SBP/DBP (−13.3/−9.1 mmHg; both P<0.0001) at week 10. The incidence of adverse events (AEs) leading to discontinuation (1.5%) or drug-related AEs (3.1%) was low, with no instances of mortality or drug-related serious AEs. These results indicate that 160 mg valsartan is safe and effective at lowering BP in Chinese patients with mild to moderate hypertension. The significant reductions in office-based and out-of-office BP measures support the clinical relevance of moderate-dose valsartan monotherapy for effective 24-h BP control.
Keywords: Val-Perfect, hypertension, valsartan, office blood pressure, home blood pressure, ambulatory blood pressure
Introduction
Hypertension is one of the most common and important risk factors for cardiovascular disease worldwide and it has a high prevalence in Asia (1). Despite the availability and widespread use of antihypertensive drugs, control rates of hypertension remain low (2). The most recent Chinese national survey of blood pressure (BP) control reported a control rate of 30.6% among hypertensive outpatients (3). Pharmacological treatment for hypertension is conventionally initiated with monotherapy. If BP control is not achieved, this may be followed by up-titration or combination therapy with another pharmacological agent. Although early introduction of combination therapy is an increasingly favoured treatment approach (4), the use of multiple-drug combinations may not be appropriate for all patients. For patients with less severe forms of the disease, monotherapy with angiotensin II receptor blockers such as valsartan, which has placebo-like tolerability (5), remains a viable option. Valsartan is widely used alone and in combination with other antihypertensive drugs (6). Dose-dependent antihypertensive efficacy has been demonstrated for valsartan at doses up to 320 mg, with 80 or 160 mg as the recommended starting dose in Europe and North America (7,8). The antihypertensive efficacy of 160 mg valsartan has been demonstrated in several large controlled clinical trials, including VALUE and NAVIGATOR (9,10). However, clinicians in China typically use a once-daily dose of 80 mg to initiate valsartan therapy. Efficacy and safety data for 160 mg daily dosage of valsartan in Chinese hypertensive patients remain insufficient (11,12). Therefore, the present study was conducted to investigate the potential beneficial effects of 160 mg valsartan, thereby providing more evidence for its utilization in China.
Screening, diagnosis, and management of hypertension are conventionally based on office BP measurements, although the clinical relevance of out-of-office BP monitoring is also well established (13). Out-of-office BP monitoring, using home or ambulatory BP monitoring (HBPM or ABPM), is recognised as an important adjunct to office BP for assessing true BP status (4). There is extensive evidence out-of-office BP, particularly ambulatory BP, has a superior predictive value for cardiovascular outcomes and hypertension-induced organ damage than office BP (14–16). The objective of the Val-Perfect study was to evaluate the efficacy and tolerability of 160 mg valsartan for treatment of mild to moderate hypertension in Chinese patients. In parallel with office-based BP measurements, the present study also evaluated the impact of valsartan on ambulatory and home BP parameters.
Patients and methods
Study design
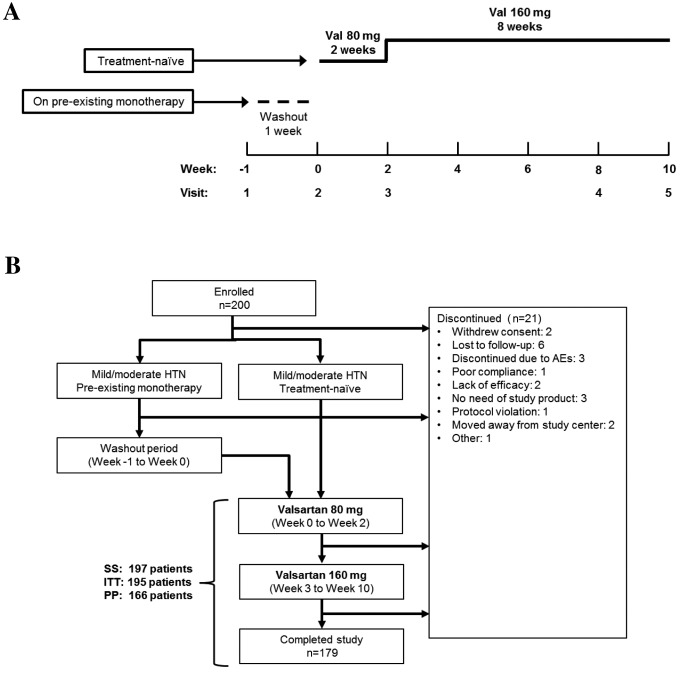
Val-Perfect was a multi-centre, prospective, open-label, single treatment arm study conducted in the outpatient clinics of 10 tertiary hospitals in China, including the Peking University People's Hospital, Peking Union Medical College Hospital, Peking University First Hospital, Beijing Chaoyang Hospital, Chinese PLA General Hospital (all Beijing, China), Ruijin Hospital, Shanghai Jiaotong University School of Medicine (Shanghai, China), The First Affiliated Hospital of Nanjing Medical University (Nanjing, China), First Affiliated Hospital of Sun Yat-sen University, Guangdong Province People's Hospital (both Guangzhou, China) and West China Hospital, Sichuan University (Nanchong, China). The study consisted of a one-week washout period for patients on pre-existing antihypertensive monotherapy, followed by a 10-week valsartan treatment period. During the 10-week treatment period, all patients received 80 mg valsartan (Beijing Novartis Pharma Ltd., Beijing, China) once daily for the first two weeks, followed by 160 mg valsartan once daily for a further eight weeks (Fig. 1A). Treatment was discontinued if a patient withdrew informed consent, or if continuation was judged by investigators to be detrimental to the patient's well being. The present study was designed, conducted and written-up in accordance with the International Conference on Harmonisation (ICH) guidelines for good clinical practice (GCP), with the applicable laws and regulations governing clinical research in China, and with the ethical principles outlined in the Declaration of Helsinki (clinicaltrials.gov; NCT01541189). The study protocol was approved by the Ethics Committees of the participating institutions.
Figure 1.
(A) Study design and (B) patient flow-chart. The present study consisted of a 10-week treatment period (weeks 0–2, once-daily 80 mg valsartan; weeks 3–10, once-daily 160 mg Val). For patients on pre-existing monotherapy, antihypertensive medication was gradually removed over a one-week washout period (week-1 to 0). Newly diagnosed (treatment-naïve) patients entered the study at the treatment phase (week 0, baseline). Val, valsartan; HTN, hypertension; AE, adverse event; ITT, intent-to-treat; SS, safety set; PP, per-protocol.
Patients
Patients eligible for inclusion in the present study had to meet the following criteria: Aged 18–75 year exhibiting mild to moderate primary hypertension; naïve to antihypertensive therapy or on monotherapy; and able and willing to give informed consent to participate in the study. Patients on pre-existing monotherapy were required to have a mean sitting systolic BP/mean sitting diastolic BP (MSSBP/MSDBP) of <160/100 mmHg at the beginning of the washout phase (week 1). At the beginning of the open-label treatment phase (week 0), all patients were required to have an MSSBP of 140-<180 mmHg and an MSDBP of 90-<110 mmHg.
Key exclusion criteria were: Severe hypertension (MSSBP ≥180 mmHg or MSDBP ≥110 mmHg) at the beginning of the washout period, malignant hypertension, secondary hypertension, type 1 diabetes mellitus, renal function impairment (serum creatinine >2.0 mg/dl or 176.8 µmol/l), a history of significant cardiovascular disease within the 6-month period prior to screening and a known allergy to the study product.
Study product administration and BP monitoring
The study product (valsartan) was supplied as an 80 mg film-coated tablet and was taken daily at 8:00 a.m. Patients ingested one tablet per day in weeks 0–2 and two tablets per day in weeks 3–10. At each study visit (weeks-1, 0, 1, 2, 8 and 10), office BP was measured using an electronic sphygmomanometer (HEM-7112; Omron Corp., Kyoto, Japan). This measurement was taken ~23 h after the last dose of the study product. BP was measured with the patient in a seated position, with the cuff at heart level. At the initial visit, BP was measured on both arms, and the arm with the higher BP reading was used for all visits. The mean of three BP readings (2/3 readings differing by <10 mmHg), taken at 2-min intervals, was used for analysis. Sitting heart rate was also recorded.
Home BP measurements were obtained by patients using a similar procedure and BP monitor (HEM-7112; Omron Corp.). BP was measured in the morning (before ingestion of the study product) and evening (12 h post-morning dose). HBPM was performed on the day prior to the week 0 (baseline) visit, and on five consecutive days before each follow-up visit (weeks 2, 6 and 10).
Ambulatory BP monitoring was performed over a 24-h period prior to study visits at baseline and week 10, using a validated ABP monitor (SpaceLabs 90207; SpaceLabs Healthcare, Snoqualmie, WA, USA) worn on the non-dominant arm. BP was recorded at 30-min intervals. For quality control, the monitoring time was required to be ≥20 h, with a minimum of 14 valid awake-period (8:00–22:00) readings and at least 10 valid sleep-period (22:00–8:00) readings.
Efficacy and safety evaluation
Primary endpoints were the changes in office MSSBP and MSDBP at week 10, relative to week 2 or 0 (baseline). Secondary endpoints included changes in home BP and 24-h ambulatory BP at weeks 2 and 10 relative to baseline, as well as the office BP and 24-h ambulatory BP control rates at week 10. The control rate for home BP at week 10 was also determined. BP control rates were determined according to the targets for office, home and ambulatory BP published in the 2010 guidelines for the management of hypertension in China (17). Two sets of office BP goal definitions were used: i) MSSBP/MSDBP <140/90 mmHg for all patientand ii) MSSBP/MSDBP <130/80 mmHg for patients with type 2 diabetes mellitus (T2DM) or chronic kidney disease (CKD), <140/90 mmHg for all other patients. Home BP targets were SBP/DBP <135/85 mmHg for all patients. ABP targets were 24-h mean SBP/DBP <130/80 mmHg, daytime SBP/DBP <135/85 mmHg, and nighttime SBP/DBP <120/70 mmHg, for all patients.
Efficacy analyses were performed for the intent-to-treat (ITT) population, which included all enrolled patients who received at least one dose of the study product, undergone a baseline evaluation and at least one subsequent primary or secondary efficacy evaluation. Analyses were repeated for the per-protocol (PP) population, which included all patients who completed the study without major deviations from the study protocol.
ABPM analyses included only patients who exhibited valid 24-h ABP recordings at baseline and at week 10, and whose sleep-wake schedules were in line with that of the majority of the study population. Nocturnal BP dipper status was determined from 24-h ABPM data. Patients whose nocturnal SBP showed a decrease of <10% of mean daytime SBP [(mean daytime SBP-mean nighttime SBP)/daytime SBP<10%] were classified as non-dippers (17).
Safety and compliance
Safety was evaluated in the safety set (SS; patients who received at least one dose of the study product). Adverse events (AEs) reported by patients or observed by investigators were recorded, along with their severity and possible relationship to the study product. Laboratory test results, including haematology, blood chemistry and renal function, were recorded at each visit. These were assessed by investigators for a possible relationship to the study product and for clinical significance, based on local laboratory reference ranges. Safety was assessed using AE frequency and on the numbers of patients with laboratory values that were outside normal ranges. Treatment compliance was assessed using records of actual vs. prescribed numbers of tablets ingested by patients.
Statistical methods
Sample size calculation was based on the change in MSDBP from weeks 2–10. Assuming a standard deviation of 8 mmHg for MSDBP and a dropout rate of 10%, it was calculated that a sample size of 200 was required to detect a 2-mmHg change in MSDBP from weeks 2–10, with 90% power and a significance level of 0.05 (two-sided). Paired t-tests were used to evaluate the significance of BP changes at different time-points, relative to week 2 or baseline, as applicable. Differences of paired samples were analyzed using the McNemar paired samples non-parametric test with an α=0.01. All significance tests were two-sided unless otherwise stated. Analyses were performed using the SAS software package (version 9.2; SAS Institute, Inc., Cary, NC, USA). Data are presented as the mean ± standard deviation, unless indicated otherwise.
Results
Characteristics of the study population
The present study enrolled 200 patients diagnosed with mild to moderate primary hypertension who were either naïve to treatment or on antihypertensive monotherapy (Fig. 1A). Of these, 197 patients initiated treatment with valsartan and were included in the SS (Fig. 1B). A total of 179 patients completed the study, with a discontinuation rate of 10.5% (n=21) and 13 patients were excluded from the PP analysis due to protocol violations. The SS, ITT and PP groups consisted of 197, 195 and 166 patients, respectively.
Demographics and baseline characteristics of the study population are summarised in Table I. A total of 115 males (59.0%) and 80 females (41.0%) were included, and the mean age was 52.9±10.2 years. At baseline, the mean SBP was 147.3±10.4 mmHg and DBP was 94.7±6.8 mmHg. Of the total study population, 98 patients were newly diagnosed (treatment-naïve) and 97 were on pre-existing antihypertensive monotherapy (Table I). Median disease duration was three years (range, 0–43 years).
Table I.
ITT Patient demographics and baseline characteristics.
| Characteristic | All (n=195) |
|---|---|
| Age, years | 52.9±10.2 |
| Gender | |
| Male, n (%) | 115 (59.0) |
| Female, n (%) | 80 (41.0) |
| BMI (kg/m2) | 25.6±3.3 |
| Ethnic group | |
| Han Chinese, n (%) | 192 (98.5) |
| Other, n (%) | 3 (1.5) |
| SBP (mmHg) | 147.3±10.4 |
| DBP (mmHg) | 94.7±6.8 |
| Heart rate (beats/min) | 71.5±8.3 |
| ALT (U/l) | 26.2±14.5 |
| AST (U/l) | 24.5±8.9 |
| BUN (mmol/l) | 5.2±1.5 |
| Cr (µmol/l) | 74.0±17.4 |
| TC (mmol/l) | 5.0±1.0 |
| TG (mmol/l) | 2.0±1.7 |
| HDL-C (mmol/l) | 1.2±0.3 |
| LDL-C (mmol/l) | 3.0±0.7 |
| Newly diagnosed hypertension | |
| Yes, n (%) | 98 (50.3) |
| No, n (%) | 97 (49.7) |
| Disease duration (years) | |
| Median (range) | 3.0 (0–43) |
| Mean ± SD | 6.3±7.5 |
| Concomitant illnessa | 75 (38.5) |
| Yes, n (%) | 75 (38.5) |
| No, n (%) | 120 (61.5) |
| Present cardiovascular risk factors/medical history, n (%) | |
| Dyslipidemia | 55 (28.2) |
| Diabetes | 16 (8.2) |
| Kidney disease | 3 (1.5) |
| Clinically significant laboratory findings (SS)b, n (%) | |
| Triglycerides | 55 (27.9) |
| Total cholesterol | 28 (14.2) |
| LDL-C | 26 (13.2) |
| Uric acid | 23 (11.7) |
| HDL-C | 20 (10.2) |
| Previous antihypertensive drug classes, n (%) | |
| β-blockers | 3 (1.5) |
| CCBs | 39 (20.0) |
| ACEIs | 8 (4.1) |
| ARBs | 38 (19.5) |
| Other | 9 (4.6) |
Data are presented as the mean ± standard deviation unless otherwise indicated.
During the one-month period prior to study entry.
Abnormal with clinical significance in >5% of patients in the SS population (n=197). ITT, intent-to-treat; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; SD, standard deviation; SS, safety set; ALT, alanine transaminase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatine; TC, total cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; CCBs, calcium channel blockers; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin-II receptor blockers.
Reductions in office and home BP
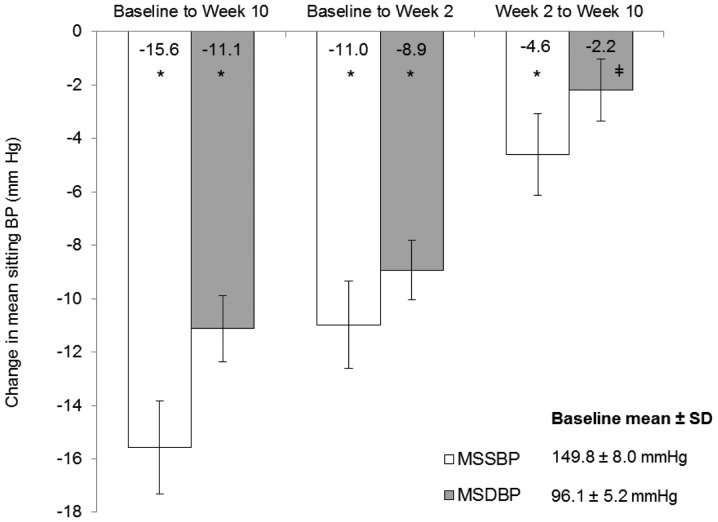
In the ITT population, mean reductions in office MSSBP and MSDBP from baseline to week 10 were statistically significant: 15.6 mmHg and 11.1 mmHg, respectively (both P<0.0001; Fig. 2). Notably, reductions in MSSBP and MSDBP from weeks 2–10, during the 160 mg valsartan phase, were also statistically significant (MSSBP: 4.6 mmHg, P<0.0001; MSDBP: 2.2 mmHg, P=0.0003). Mean reductions in office MSSBP and MSDBP from baseline to week 2 were 11.0 and 8.9 mmHg, respectively (both P<0.0001). Similar results were obtained for the PP analyses (data not shown).
Figure 2.
Reduction in office BP following 10-week valsartan treatment. *P<0.0001 vs. baselin; ‡P=0.0003 vs. week 2. Error bars represent 95% confidence intervals of the mean. BP, blood pressur; and MSSBP, mean sitting systolic BP; MSDBP, mean sitting diastolic BP; baseline, week 0; SD, standard deviation.
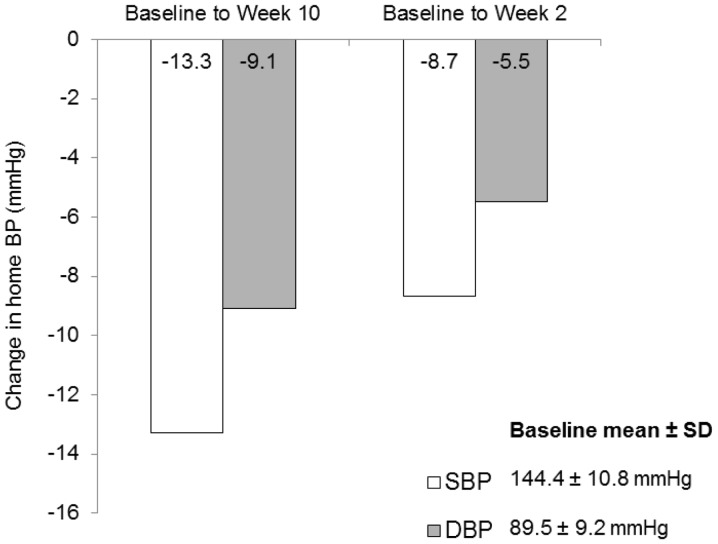
Home BP also decreased significantly following 10-week treatment. Mean overall reductions in SBP and DBP from baseline to week 2 were 8.7 and 5.5 mmHg, respectively (both P<0.0001; Fig. 3). Mean SBP and DBP reductions from baseline to week 10 were 13.3 and 9.1 mmHg, respectively (both P<0.0001). Similar results were obtained in the PP analysis (data not shown).
Figure 3.
Reduction in ambulatory BP following 10-week valsartan treatment. All changes from baseline in home-monitored SBP and DBP were significant (P<0.0001). BP, blood pressur; and SBP, systolic BP; DBP, diastolic BP; baseline, week 0; SD, standard deviation.
Changes in ambulatory BP parameters
At baseline, 24-h mean ambulatory SBP/DBP was 136.3/88.0 mmHg. ABPM revealed significant BP reductions at week 10, relative to baseline. Reductions in overall (24-h), daytime, and nighttime mean SBP/DBP were all significant: 6.1/4.4, 5.6/4.0 and 7.8/5.4 mmHg, respectively (all P<0.0001; Table II). Significant BP reduction was also observed during the final four h of the 24-h dosing interval (SBP/DBP, −6.5/−4.3 mmHg; P<0.0001). The proportion of patients with a nocturnal ‘dipper’ BP profile increased from 37.0% (n=57) at baseline to 48.1% (n=74) at week 10. In addition, a significant proportion (41.2%; n=40) of patients with a ‘non-dipper’ profile at baseline exhibited a ‘dipper’ profile after 10 weeks of treatment (P=0.0322; McNemar's test for paired samples).
Table II.
Changes in ambulatory BP monitoring parameters following 10 weeks of valsartan treatment.
| Ambulatory BP parameters (mmHg) | SBP | DBP | P-value |
|---|---|---|---|
| 24-h average ABP | |||
| Baseline (Week 0) | 136.3±11.8 | 88.0±10.0 | – |
| Change from baseline to week 10 −6.1±11.4 | −4.4±7.9 | <0.0001 | |
| Daytime average ABP | |||
| Baseline (Week 0) | 139.4±12.7 | 90.5±11.1 | – |
| Change from baseline to week 10 | −5.6±12.8 | −4.0±9.2 | <0.0001 |
| Nighttime average ABP | |||
| Baseline (Week 0) | 129.0±13.5 | 82.3±10.0 | – |
| Change from baseline to week 10 | −7.8±12.8 | −5.4±9.2 | <0.0001 |
| Change in final 4 h of dosing interval (baseline to week 10) | −6.5±12.5 | −4.3±8.6 | <0.0001 |
| Patients with nocturnal ‘dipper’ profile | |||
| At baseline, n (%) | 57 (37.0) | ||
| At Week 10, n (%) | 74 (48.1) |
Data are presented as the mean ± standard deviation unless otherwise indicated. P<0.05 was considered to indicate a statistically significant result. BP, blood pressure; SBP, systolic BP; DBP, diastolic BP; ABP, ambulatory BP; dipper, patients whose nocturnal SBP showed a decrease of at least 10% of mean daytime SBP.
Improvements in office, home, and ambulatory BP control rates
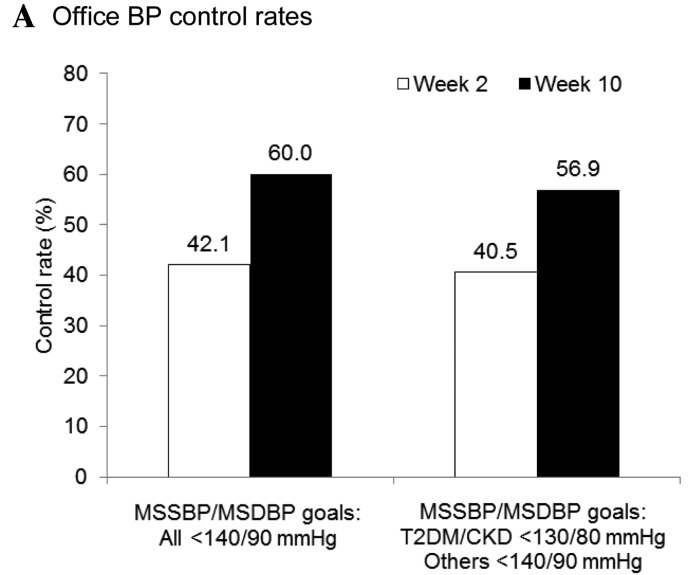
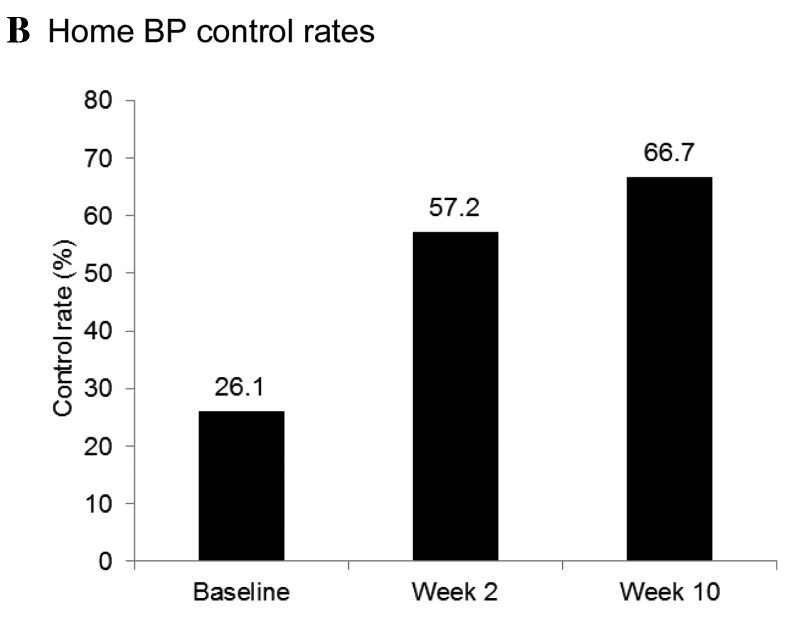
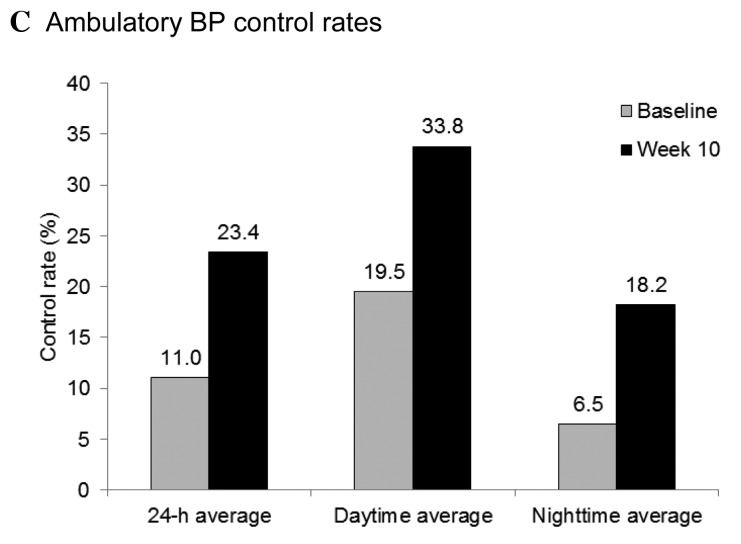
Office and home BP control rates were markedly increased at the end of the treatment period. Following eight weeks of treatment with once-daily 160 mg valsartan, office BP control rates increased from 42.1% at week 2 to 60.0% at week 10 (BP goal definition 1, MSSBP/MSDBP<140/90 mmHg for all patient; and Fig. 4A). A similar increase in control rate, from 40.5% at week 2 to 56.9% at week 10, was observed using BP goal definition 2 (MSSBP/MSDBP <130/80 mmHg for patients with T2DM or CKD, <140/90 mmHg for others). The baseline home BP control rate was 26.1%. Consistent with the improvement in office BP control, the home BP control rate (target SBP/DBP <135/85 mmHg for all patients) increased from 57.2% at week 2 to 66.7% at week 10 (Fig. 4B). Among patients who attained their office BP goals (SBP/DBP <130/80 mmHg for patients with T2DM or CKD, SBP/DBP <140/90 mmHg for all other patients) by the completion of the study, the home BP control rate was 80.4%. Overall control rates for 24-h ambulatory BP markedly increased following 10 weeks of valsartan treatment, from 11.0 to 23.4% (Fig. 4C).
Figure 4.
Attainment of (A) office, (B) home, and (C) ambulatory BP goals. Office BP targets: Definition 1, MSSBP/MSDBP<140/90 mmHg for all patient; and definition 2, MSSBP/MSDBP<130/80 mmHg for patients with T2DM or CKD, <140/90 mmHg for all other patients. Home BP target: SBP/DBP<135/85 mmHg for all patients. Ambulatory BP targets: 24-h mean SBP/DBP<130/80 mmHg, daytime SBP/DBP<135/85 mmHg, and nighttime SBP/DBP<120/70 mmHg for all patients. BP, blood pressur; and MSSBP, mean sitting systolic BP; MSDBP, mean sitting diastolic BP; baseline, week 0; T2DM, type 2 diabetes mellitu; CKD, chronic kidney disease.
Safety and compliance
Of the 197 patients who received at least one dose of the study product (the SS), 44 (22.3%) reported one or more AEs, the majority of which were mild in severity. The incidence of both AEs leading to discontinuation (1.5%; n=3) and study product-related AEs (3.1%; n=6) was low. Table III presents the types of study product-related AEs reported; of these, mild dizziness was the most frequent (1.5%). There were no instances of mortality or study product-related severe AEs. The number of patients with clinically significant abnormalities in laboratory parameters (blood lipids and uric acid) was similar at the beginning (Table I) and the end of the study period (Table IV). Within the SS (n=197), 177 (98.3%) patients exhibited good treatment compliance (80–120%), as assessed by pill counts.
Table III.
Incidence of AEs.
| Variable | No. of patients (%) SS: n=197 |
|---|---|
| Total AEs | 44 (22.3) |
| Study product-related AEs | 6 (3.1) |
| Severe AEs | 2 (1.0) |
| AEs leading to discontinuation | 3 (1.5) |
| Study product-related AEs by type | |
| Amaurosis | 1 (0.5) |
| Dizziness | 3 (1.5) |
| Headache | 1 (0.5) |
| Hypotension | 1 (0.5) |
| Pruritis | 1 (0.5) |
| Mucosal and skin rash | 1 (0.5) |
| Rash | 1 (0.5) |
SS, safety set; AE, adverse effect.
Table IV.
Clinically significant laboratory findings following 10 weeks of valsartan treatment.
| Variable | No. of patients (%) SS: n=197 |
|---|---|
| Clinically significant laboratory findingsa (%) | |
| Triglycerides | 40 (20.3) |
| Total cholesterol | 21 (10.7) |
| LDL-C | 19 (9.6) |
| Uric acid | 15 (7.6) |
| HDL-C | 11 (5.6) |
Abnormal with clinical significance in >5% of patients. SS, safety set; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol.
Discussion
Antihypertensive efficacy of valsartan has been established over a range of doses, up to 320 mg/day, in North American and European hypertensive patient populations (7,8,18). The results of the present study demonstrated the antihypertensive efficacy of once-daily 160 mg valsartan in Chinese patients with mild to moderate hypertension. The blood pressure reductions observed in the current study (15.6/11.1 mmHg for office SBP/DBP following 10 weeks treatment) were comparable to those obtained in previous studies (7,11,19–23), which examined single-drug or combination valsartan therapy in patients with mild to moderate hypertension over a similar time frame. In the latter studies, mean SBP reductions between 10.2 and 16.5 mmHg were observed, whereas mean DBP reductions were between 5.3 and 10.3 mmHg. In the present study, beyond the significant BP reduction observed following the initial two-week treatment with valsartan 80 mg, there was an additional, significant BP reduction following up-titration to 160 mg for a further eight weeks.
Use of ABPM and HBPM facilitates assessment of overall BP control and may contribute to improved BP management. Studies have shown that out-of-office (ambulatory and home) BP is able to predict cardiovascular events and hypertension-induced organ damage more effectively than office BP (14–16). In the present study, a significant antihypertensive effect of valsartan was detected, regardless of the type of BP measurement (office, home, or ambulatory BPM), indicating its effectiveness in reducing out-of-office and office BP. In addition to reductions in 24-h mean SBP/DBP, significant BP reductions were also observed in the final 4 h of the 24-h dosing interval, indicating that the effects were sustained throughout the 24-h period.
Following 10 weeks of treatment, ~60% of patients were able to achieve their office BP goal. This level is similar to control rates achieved in a previous study that compared 80 and 160 mg valsartan monotherapy (up to 59%) with a single-pill combination of 5/160 mg amlodipine/valsartan (~70%), in a group of patients who had not responded to treatment with 80 mg valsartan (21). Therefore, although certain groups of patients may require combination therapy, a substantial proportion of patients are likely to be able to achieve adequate BP control on higher-dose valsartan monotherapy (160 mg vs. the 80 mg dose currently used in China).
One important limitation of the present study is the open-label non-comparative design. A possible placebo effect cannot be excluded without a comparative control group, which ultimately weakens the reliability of the present conclusions. However, ABPM is generally considered to reflect blood pressure levels more objectively, thus potentially limiting the placebo effect. Significant BP reductions were confirmed by ABPM analyses following 10-week valsartan treatment. In addition, the present design corresponds more closely to real-world assessments of the 160 mg dose, which does not permit formal evaluation of the efficacy of this dose. Furthermore, the present results are consistent with the known dose-dependent efficacy and safety profile of valsartan in other patient populations (8,24). Although the relatively short follow-up period did not permit direct assessment of long-term effectiveness, the pattern of BP reduction over the course of the study is consistent with previous investigations of valsartan (18,20); maximal reduction is typically detected within four weeks and persists throughout long-term therapy. Consistent with existing safety data (25), valsartan exhibited a favourable safety profile in the present study, with a low incidence of study product-related AEs and discontinuation due to AEs (both <5%). There were no instances of mortality or study product-related SAEs.
The present results provide further evidence of a positive benefit-risk balance for the use of the 160 mg dose of valsartan, compared with the 80 mg dose, in Chinese patients with mild to moderate hypertension. Given the proven dose-dependent efficacy of valsartan across a wide dose range and its favourable safety profile, treatment with the higher dose of 160 mg may be a reasonable therapeutic option, particularly for patients with less severe hypertension.
Acknowledgements
The authors would like to thank Hongzhi Xie (Peking Union Medical College Hospital, Beijing, China), Fang Zhou (The First Affiliated Hospital, Nanjing Medical University), Hao Xue (Academy of Military Medical Sciences, Beijing, China), and Tao Tao (Novartis Pharmaceuticals, China) for their valuable contributions to this study, as well as Patrick Brunel (Worldwide medical affairs, Novartis Pharma AG), Rosemarie Kelly (Worldwide medical affairs, Novartis Pharma AG) and Ashwani Kumar (Worldwide medical affairs, Novartis Pharma AG) for critical review of the manuscript. This study was sponsored by Novartis Pharmaceuticals (China).
References
- 1.Chiang CE, Chen CH. Hypertension in the Asia-Pacific region. J Hum Hypertens. 2008;22:441–443. doi: 10.1038/jhh.2008.17. [DOI] [PubMed] [Google Scholar]
- 2.Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: A systematic review. J Hypertens. 2004;22:11–19. doi: 10.1097/00004872-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Hu DY, Liu LS, Yu JM, Yao CH. China STATUS Study Group: National survey of blood pressure control rate in Chinese hypertensive outpatients-China STATUS. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:230–238. (In Chinese) [PubMed] [Google Scholar]
- 4.Mancia G, Fagard R, Narkiewicz K, Redón J, Zanchetti A, Böhm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, et al. 2013 ESH/ESC Practice Guidelines for the Management of Arterial Hypertension. Blood Press. 2014;23:3–16. doi: 10.3109/08037051.2014.868629. [DOI] [PubMed] [Google Scholar]
- 5.Verdecchia P, Angeli F. Assessment of the optimal daily dose of valsartan in patients with hypertension, heart failure, or both. Clin Ther. 2004;26:460–472. doi: 10.1016/S0149-2918(04)90049-5. [DOI] [PubMed] [Google Scholar]
- 6.Markham A, Goa KL. Valsartan. A review of its pharmacology and therapeutic use in essential hypertension. Drugs. 1997;54:299–311. doi: 10.2165/00003495-199754020-00009. [DOI] [PubMed] [Google Scholar]
- 7.Parati G, Asmar R, Bilo G, Kandra A, Di Giovanni R, Mengden T. Effectiveness and safety of high-dose valsartan monotherapy in hypertension treatment: The ValTop study. Hypertens Res. 2010;33:986–994. doi: 10.1038/hr.2010.120. [DOI] [PubMed] [Google Scholar]
- 8.Pool JL, Glazer R, Chiang YT, Gatlin M. Dose-response efficacy of valsartan, a new angiotensin II receptor blocker. J Hum Hypertens. 1999;13:275–281. doi: 10.1038/sj.jhh.1000788. [DOI] [PubMed] [Google Scholar]
- 9.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 10.Study Group NAVIGATOR, McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, Belenkov Y, Boolell M, Buse JB, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362:1477–1490. doi: 10.1056/NEJMoa1001121. [DOI] [PubMed] [Google Scholar]
- 11.Ke YN, Dong YG, Ma SP, Yuan H, Ihm SH, Baek SH. ADVISE study group: Improved blood pressure control with nifedipine GITS/valsartan combination versus high-dose valsartan monotherapy in mild-to-moderate hypertensive patients from Asia: Results from the ADVISE study, a randomized trial. Cardiovasc Ther. 2012;30:326–332. doi: 10.1111/1755-5922.12003. [DOI] [PubMed] [Google Scholar]
- 12.Zhu D, Yang K, Sun N, Gao P, Wang R, Grosso A, Zhang Y. trial investigators: Amlodipine/valsartan 5/160 mg versus valsartan 160 mg in Chinese hypertensives. Int J Cardiol. 2013;167:2024–2030. doi: 10.1016/j.ijcard.2012.05.039. [DOI] [PubMed] [Google Scholar]
- 13.Stergiou GS, Nasothimiou E, Giovas P, Kapoyiannis A, Vazeou A. Diagnosis of hypertension in children and adolescents based on home versus ambulatory blood pressure monitoring. J Hypertens. 2008;26:1556–1562. doi: 10.1097/HJH.0b013e328301c411. [DOI] [PubMed] [Google Scholar]
- 14.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, et al. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 15.Perloff D, Sokolow M, Cowan R. The prognostic value of ambulatory blood pressures. JAMA. 1983;249:2792–2798. doi: 10.1001/jama.249.20.2792. [DOI] [PubMed] [Google Scholar]
- 16.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.HYP.24.6.793. [DOI] [PubMed] [Google Scholar]
- 17.National Revision Committee for the Guidelines on Prevention and Control of Hypertension in China, corp-author. Guidelines On Prevention And Control Of Hypertension In China 2010. Chinese Journal of Hypertension. 2011;19:701–741. [Google Scholar]
- 18.Oparil S, Dyke S, Harris F, Kief J, James D, Hester A, Fitzsimmons S. The efficacy and safety of valsartan compared with placebo in the treatment of patients with essential hypertension. Clin Ther. 1996;18:797–810. doi: 10.1016/S0149-2918(96)80040-3. [DOI] [PubMed] [Google Scholar]
- 19.Benz JR, Black HR, Graff A, Reed A, Fitzsimmons S, Shi Y. Valsartan and hydrochlorothiazide in patients with essential hypertension. A multiple dose, double-blind, placebo controlled trial comparing combination therapy with monotherapy. J Hum Hypertens. 1998;12:861–866. doi: 10.1038/sj.jhh.1000718. [DOI] [PubMed] [Google Scholar]
- 20.Black HR, Graff A, Shute D, Stoltz R, Ruff D, Levine J, Shi Y, Mallows S. Valsartan, a new angiotensin II antagonist for the treatment of essential hypertension: Efficacy, tolerability and safety compared to an angiotensin-converting enzyme inhibitor, lisinopril. J Hum Hypertens. 1997;11:483–489. doi: 10.1038/sj.jhh.1000482. [DOI] [PubMed] [Google Scholar]
- 21.Huang J, Sun NL, Hao YM, Zhu JR, Tu Y, Curt V, Zhang Y. Trial Investigators: Efficacy and tolerability of a single-pill combination of amlodipine/valsartan in Asian hypertensive patients not adequately controlled with valsartan monotherapy. Clin Exp Hypertens. 2011;33:179–186. doi: 10.3109/10641963.2010.531849. [DOI] [PubMed] [Google Scholar]
- 22.Nash DT, Crikelair N, Zappe D. Achieving BP goals with valsartan and HCTZ alone and in combination: Pooled analysis of two randomized, double-blind, placebo-controlled studies. Curr Med Res Opin. 2008;24:2617–2626. doi: 10.1185/03007990802333282. [DOI] [PubMed] [Google Scholar]
- 23.Pool J, Oparil S, Hedner T, Glazer R, Oddou-Stock P, Hester A. Dose-responsive antihypertensive efficacy of valsartan, a new angiotensin II-receptor blocker. Clin Ther. 1998;20:1106–1114. doi: 10.1016/S0149-2918(98)80107-0. [DOI] [PubMed] [Google Scholar]
- 24.Weir MR, Levy D, Crikelair N, Rocha R, Meng X, Glazer R. Time to achieve blood-pressure goal: Influence of dose of valsartan monotherapy and valsartan and hydrochlorothiazide combination therapy. Am J Hypertens. 2007;20:807–815. doi: 10.1016/j.amjhyper.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 25.Fogari R, Zoppi A. A drug safety evaluation of valsartan. Expert Opin Drug Saf. 2011;10:295–303. doi: 10.1517/14740338.2011.543416. [DOI] [PubMed] [Google Scholar]