Abstract
Regorafenib (Reg) is an oral multikinase inhibitor that has achieved improved overall survival in patients with metastatic colorectal cancer (mCRC) in the salvage therapy setting. However, Reg is difficult to manage and determine the optimal dose due to adverse events (AEs). The objective of this study was to retrospectively evaluate the clinical benefit and determine the optimal dose of Reg in mCRC patients. A total of 20 mCRC patients were enrolled in this retrospective study. Initially, 8 patients who received a starting dose of 160 mg Reg (160 mg group) once a day were evaluated; however, they were unable to continue with the initial dose of 160 mg due to grade 3 adverse events (AEs), such as hand-foot skin reaction (HFSR) and small intestinal hemorrhage. Furthermore, 2 of the 8 patients refused subsequent treatment due to HFSR and the remaining 6 patients received a dose reduction from 160 to 120 mg Reg. A reduced dose of 120 mg Reg was also assessed with our dose modification method in 12 patients (120 mg group). The optimal response of the 160 and 120 mg group patients was 0.0 and 8.3% (1/12), respectively. In the 160 mg group, 3 patients exhibited stable disease (SD). Surprisingly, among the the 120 mg group patients 1 exhibited partial response (PR) and 6 had SD. The PR case displayed shrinkage of the local recurrence and morphological changes. One of the SD cases exhibited formation of a cavity in the lung metastasis, with intralesional morphological changes of the liver metastasis. The duration of the treatment in the PR case and the SD case with the cavitation was 6.5 months (9 cycles) and 5 months (6 cycles), respectively. The median progression-free survival (PFS) was 77 days (range, 30–230+ days) and the median overall survival (OS) was 204 days (range, 53–511+ days). The final date of the follow-up period was July 31, 2016. The 160 mg group was associated with a 25% (3/8) incidence of HFSR, 12.5% (1/8) of small intestinal hemorrhage and 12.5% (1/8) of anemia and thrombocytopenia; the AEs were grade >3. The 120 mg group was associated with an incidence of only 8.3% (1/12) of grade >3 hypertension. Thus, the 120 mg group experienced lower treatment-related toxicity compared with the 160 mg group. Despite a reduced initial dose of Reg, a significant effect was observed, with 1 PR and 6 favorable SD cases, with good tolerability. Therefore, an initial dose modification of 120 mg Reg is recommended as an alternative strategy for the treatment of mCRC in the salvage setting.
Keywords: initial dose, regorafenib, metastatic colorectal cancer
Introduction
The pattern of cause of death and the annual odds of death in metastatic colorectal cancer (mCRC) are similar between Japan and the global standards (1,2). Approximately 20–25% of patients with CRC have metastatic disease at diagnosis and the majority of mCRC patients require intensive or palliative chemotherapy (3,4). Patients with mCRC generally receive a combination of 5-fluorouracil (5-FU)/leucovorin and either oxaliplatin (L-OHP) (FOLFOX) or irinotecan (CPT-11) (FOLFIRI) with molecular-targeted therapy as first-line chemotherapy, and the overall survival (OS) of patients with mCRC has improved. Previous studies reported the median OS of mCRC patients to be >30 months. However, the progression-free survival (PFS) of first-line chemotherapy in mCRC has not improved over the past decade (5–12). Therefore, treatments after first-line chemotherapy are important and further therapeutic agents are required. Regorafenib (Reg) is an oral multikinase inhibitor targeting multiple tumor pathways, such as proliferation (KIT, BRAF, RAF-1 and RET), tumor microenvironment signaling (platelet-derived growth factor receptor-β and fibroblast growth factor receptor) and neoangiogenesis [vascular endothelial growth factor receptor (VEGFR)1–3 and angiopoietin-1 receptor] (13). An international phase III study (CORRECT) was conducted: A total of 760 patients with mCRC, who were refractory or intolerant to standard chemotherapy including 5-FU, L-OHP, CPT-11 and molecular-targeted drugs, such as anti-VEGF antibody and anti-epidermal growth factor receptor (EGFR) antibody, were randomized in a 2:1 ratio to receive Reg or placebo. The primary endpoint was OS and this endpoint met the planned interim analysis, with a hazard ratio of 0.77 [95% confidence interval (CI): 0.64–0.94; one-sided P=0.0052]). The most common grade ≥3 Reg-related adverse events (AEs) were fatigue, hand-foot skin reaction (HFSR), diarrhea and anorexia (14). A total of 100 Japanese patients were enrolled in the CORRECT study. The efficacy of Reg was similar between Japanese and non-Japanese patients. However, AEs such as HFSR, hypertension, thrombocytopenia and proteinuria occurred at a higher incidence among Japanese patients, and it was also reported that 1 Japanese patient succumbed to liver dysfunction due to Reg. In addition, dose modifications due to AEs were more frequent and the dose intensity of Reg was lower in Japanese compared with that in non-Japanese patients (15). Therefore, although Reg achieved clinical benefits with tolerable AEs according to previous studies (14,16), its efficacy and safety in Japanese patients should be verified in the clinical setting. The objective of the present study was to retrospectively evaluate the clinical benefit and determine the optimal dose of Reg in mCRC patients who experienced treatment failure with standard chemotherapy, such as fluoropyrimidines (FUs), L-OHP and CPT-11 with molecular-targeted antibodies.
Patients and methods
Patients
Patients with confirmed colorectal adenocarcinoma who met the following inclusion criteria were included in the present study: i) No age restriction; ii) major organ function preserved [leukocyte count ≥4,000/mm3; platelet count ≥100,000/mm3; total bilirubin ≤1.5 mg/dl; aspartate aminotransferase (AST) and alanine aminotransferase (ALT) <2.5 times upper limit of normal; and creatinine clearance ≥60 ml/min]; iii) Eastern Cooperative Oncology Group (ECOG) performance status (PS) score 0–2; iv) no active multiple primary cancers; v) confirmed KRAS or RAS mutational status; vi) no serious complications (intestinal obstruction, hypertension, proteinuria or thrombosis); and vii) written informed consent. The administration dosage and the schedule of Reg, best therapeutic effects and AEs were investigated. The PFS was defined as the period from Reg initiation to the date of tumor progression or death from any cause and the OS was defined as the period from Reg initiation to the date of death from any cause. This study was performed according to the regulations of the local Ethics Committee of our hospital and according to the principles of the Declaration of Helsinki.
Treatment and methods
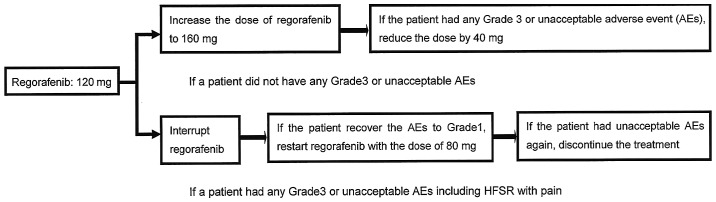
All the patients had experienced treatment failure with all the available standard chemotherapies, such as FUs, L-OHP and CPT-11 with molecular-targeted antibodies. In this study, treatment efficacy and AEs were first evaluated in 8 patients following standard-dose Reg and reduced dosage was discussed for the next patient group. The dose of Reg in the first 8 patients was 160 mg/day orally on days 1–21 every 28 days, and the dose was modified according to the dose modification/interruption protocol of the CORRECT study after treatment initiation (14). In the next 12 patients, the initial dose of Reg was modified to 120 mg/day orally on days 1–21 every 28 days; our original dose modification was used, as efficacy and AEs were evaluated in the 160 mg group patients (Fig. 1).
Figure 1.
Treatment procedure illustrating our original dose modification method.
Evaluation
The incidence of AEs and their severity were evaluated to determine treatment safety. In addition, the OS, PFS and best objective response were assessed to determine treatment efficacy. Evaluation was performed by computed tomography or magnetic resonance imaging (MRI) at the start of Reg treatment and after every 2 cycles. AEs were evaluated based on the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 (https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf). Reg was administered to a total of 20 patients between May, 2013 and June, 2016; the final date of the follow-up period was July 31, 2016.
Results
Patient characteristics
This study enrolled 20 patients, including 12 men and 8 women. The median age was 65.5 years (range, 40–73 years). The median ECOG PS was 1 (PS0, n=3; PS1, n=14; and PS2, n=3) and the median number of prior therapy cycles was 4 (range, 3–7). All the patients had received treatment with L-OHP, CPT-11, FUs and bevacizumab (Bmab), and all KRAS or RAS wild-type patients were treated with anti-EGFR antibody. The median number of Reg cycles was 2 (range, 1–9). The treatment refusal rate was 15% (3/20) (Table I).
Table I.
Patient characteristics according to the dose of regorafenib (n=20).
| Dose groups | |||
|---|---|---|---|
| Characteristics | Total (n=20) | 160 mg (n=8) | 120 mg (n=12) |
| Gender, n | |||
| Male | 14 | 6 | 8 |
| Female | 6 | 2 | 4 |
| Age, years | |||
| Median (range) | 65.5 (40–76) | 64 (40–73) | 65.5 (51–76) |
| Primary, n | |||
| Colon | 13 | 5 | 8 |
| Rectum | 7 | 3 | 4 |
| PS, n | |||
| 0 | 3 | 1 | 2 |
| 1 | 14 | 6 | 8 |
| 2 | 3 | 1 | 2 |
| BSA, m2 | |||
| Median (range) | 1.535 (1.27–1.75) | 1.54 (1.40–1.73) | 1.52 (1.27–1.75) |
| Metastatic sites, n | |||
| Lung | 10 | 3 | 7 |
| Liver | 11 | 5 | 6 |
| Peritoneum | 5 | 1 | 4 |
| Local | 4 | 2 | 2 |
| KRAS or RAS, n | |||
| Wildtype | 13 | 5 | 8 |
| Mutant | 7 | 3 | 4 |
| Prior therapy, n | |||
| Median (range) | 4 (2–7) | 4 (3–6) | 4.5 (3–7) |
| Prior drugs, n | |||
| Oxaliplatin | 20 | 8 | 12 |
| Irinotecan | 20 | 8 | 12 |
| FUs | 20 | 8 | 12 |
| Bevacizumab | 20 | 8 | 12 |
| Anti-EGFR | 13 | 5 | 8 |
PS, performance status; BSA, body surface area; FUs, fluoropyrimidines; EGFR, epithelial growth factor receptor.
Treatment outcomes
A total of 3 patients refused treatment during the first cycle in the 160 mg group due to HFSR and small intestinal hemorrhage. The dose reduction rate was 62.5% (5/8) in the 160 mg group and 25% (3/12) in the 120 mg group, mainly due to HFSR, stomatitis and thrombocytopenia. The median relative dose intensity (RDI) was 63.5% (range, 48.4–94.3%) and there was no difference between the 160 and 120 mg doses of Reg (63.6 and 62.5%, respectively). A total of 15 patients received further treatments [10 patients received TAS-102 and 4 were re-challenged with cetuximab (Cmab) monotherapy] (Table II).
Table II.
Treatment characteristics (total number of regorafenib administrations was 58).
| Dose groups | |||
|---|---|---|---|
| Characteristics | Total (n=20) | 160 mg (n=8) | 120 mg (n=12) |
| Number of cycles [median (range)] | 2 (1–9) | 2 (1–7) | 2 (2–9) |
| RDI, % [median (range)] | 63.5 (48.494.3) | 63.6 (48.4–81.4) | 62.5 (53.2–94.3) |
| Treatment refusal, n (%) | 3 (15.0) | 3 (37.5) | 0 (0.0) |
| Dose reduction, n (%) | |||
| Adverse events | 11 (55.0) | 5 (62.5) | 4 (33.3) |
| HFSR, n | 7 | 6 | 1 |
| Stomatitis, n | 3 | 1 | 1 |
| Thrombocytopenia, n | 1 | 0 | 1 |
| Hypertension, n | 1 | 0 | 1 |
| Further treatment, n (%) | 15 (75.0) | 5 (62.5) | 10 (83.3) |
| TAS-102 | 14 | 5 | 9 |
| Cetuximab alone | 1 | 0 | 1 |
RDI, relative dose intensity; HFSR, hand-foot skin reaction.
Efficacy
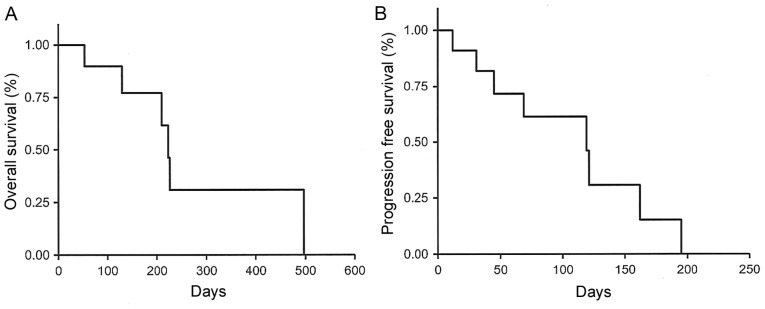
None of the patients achieved complete response; however, 1 patient achieved partial response (PR) and 9 had stable disease (SD). The objective response rate was 5.6% and the disease control rate (DCR) was 55.6% (Table III). Two patients who were treated with the dose of 120 mg were able to receive Reg for >6 cycles: One was a 66-year-old woman with local recurrence who received five prior chemotherapies with Bmab, Cmab, panitumumab, L-OHP, CPT-11 and FUs. The patient was started on Reg as sixth-line chemotherapy, and after three courses of Reg, the local recurrence exhibited a PR and the radiological tumor density decreased, with cystic changes of the lesion. The patient was eventually treated with Reg until the ninth cycle (6.5 months) (Fig. 3). The other patient was a 64-year-old man with multiple lung and liver metastases who received five prior chemotherapies; he was also started on Reg as sixth- line chemotherapy and, after two courses of Reg, formation of a cavity in a lung metastasis with intralesional cystic change of the liver metastases were observed. The patient received treatment with Reg until the sixth cycle (5 months) (Fig. 4). The median OS was 204 days (Fig. 2A) and the PFS was 77 days (Fig. 2B) in all the patients.
Table III.
Treatment efficacy (n=20).
| Best objective response | Total (n=20) | 160 g (n=8) | 120 mg (n=12) |
|---|---|---|---|
| CR | 0 | 0 | 0 |
| PR | 1 | 0 | 1 |
| SD | 9 | 3 | 6 |
| PD | 7 | 2 | 5 |
| NE | 3 | 3 | 0 |
| Response rate | 5.6% | 0% | 8.3% |
| Disease control rate | 55.6% | 60% | 58.3% |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; NE, no evaluation.
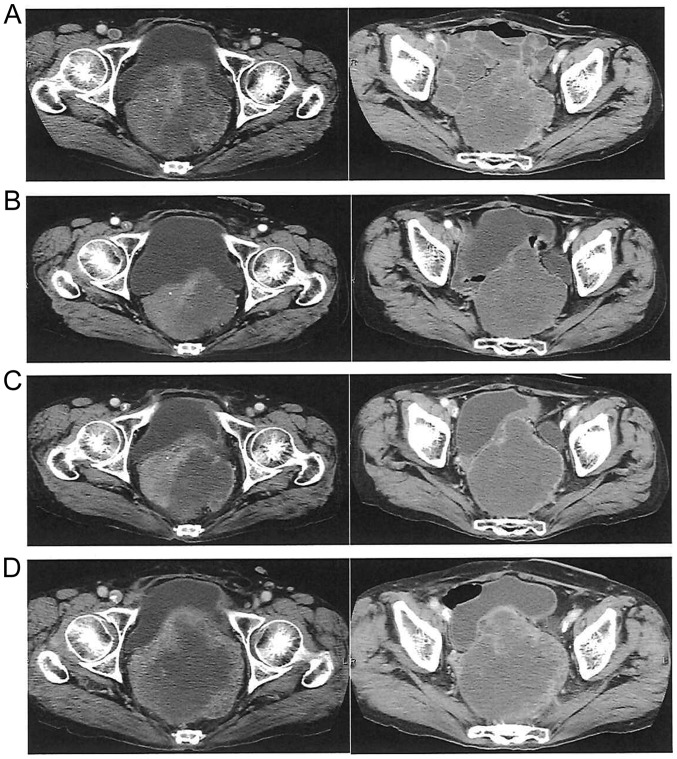
Figure 3.
Efficacy of regorafenib at 120 mg/day in a female patient please confirm who received 9 cycles of treatment and achieved partial response. (A) Pretreatment computed tomography scan. (B) Shrinkage of local recurrence and decrease of internal density after 3 cycles. (C) Change in size and internal density after 6 cycles. (D) Increased size and internal density after 9 cycles, indicating progressive disease.
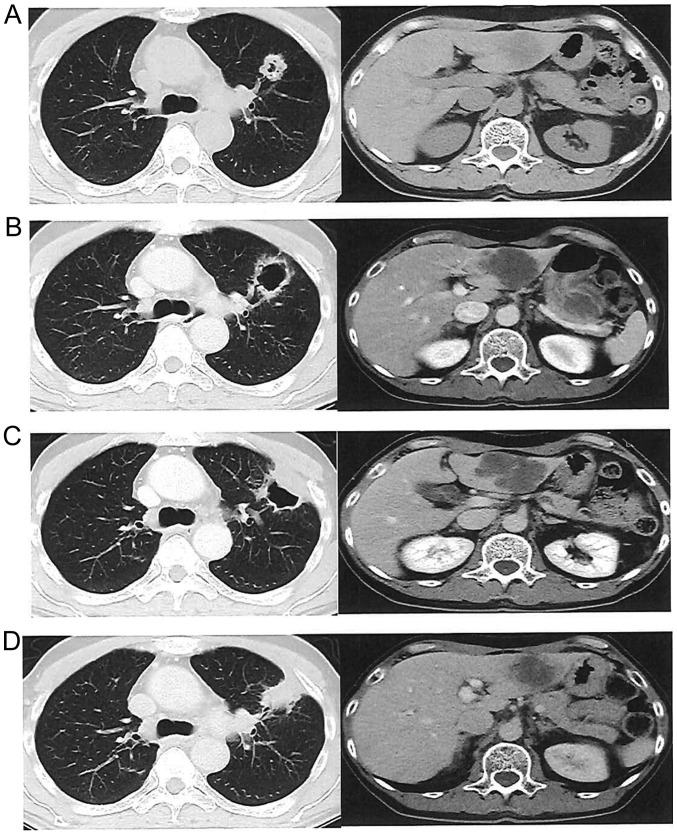
Figure 4.
Efficacy of regorafenib at 120 mg/day in a male patient with lung and liver metastases who received 6 cycles of regorafenib. (A) Pretreatment computed tomography scan. (B) Cavity formation in the lung metastasis, with cystic change of the internal structure in the liver metastases after 2 cycles. (C) Change in size and internal density after 4 cycles. (D) Increased size and internal density of lung and liver metastases after 6 cycles, indicating progressive disease.
Figure 2.
The overall survival (OS) and progression-free survival (PFS) curves of the entire investigated patient cohort (n=20). (A) The Kaplan-Meier estimate for OS was 204 days (range, 53–511+ days). (B) The Kaplan-Meier estimate for PFS was 77 days (range, 30–230+ days).
Safety
Reg was administered a total of 58 times and the AEs were assessed in 20 patients. In regards to hematological AEs, leukopenia was observed in 30%, neutropenia in 20%, anemia in 25% and thrombocytopenia in 10% of the cases. As other abnormal laboratory test values, ALT was elevated in 30%, AST in 25%, lactate dehydrogenase in 25%, γ-glutamyl transpeptidase in 25% and alkaline phosphatase in 10% of the cases. As regards non-hematological AEs, HFSR was observed in all the patients, whereas gastrointestinal disorders, including nausea, anorexia and stomatitis, were observed in 75% of the cases. In particular, a patient presented with melena due to small intestinal hemorrhage. Fatigue was also observed in 50% of the patients. These AEs tended to occur at the dose of 160 mg rather than that of 120 mg. The incidence of grade 3 HFSR was 75% and all the cases were included in the 160 mg group; 3 of those cases refused subsequent treatment with Reg. As other grade 3 AEs, neutropenia and hypertension were each observed in 5% of the cases. Grade 3 neutropenia also occurred at the dose of 160 mg, but hypertension occurred at the dose of 120 mg. There were no reported grade 4 AEs or chemotherapy-related deaths (Table IV).
Table IV.
Hematological and non-hematological adverse events (total number of regorafenib administrations was 58).
| Dose groups | ||||||
|---|---|---|---|---|---|---|
| Adverse events | Total (n=20) | 160 mg (n=8) | 120 mg (n=12) | |||
| Hematological | Any | Grade 3 | Any | Grade 3 | Any | Grade 3 |
| Leukocytopenia | 6 (30.0) | 2 (25.0) | 4 (33.3) | |||
| Neutropenia | 4 (20.0) | 1 (5.0) | 2 (25.0) | 1 (25.0) | 2 (16.7) | |
| Anemia | 5 (25.0) | 3 (37.5) | 2 (16.7) | |||
| Thrombocytopenia | 2 (10.0) | 1 (12.5) | 1 (8.3) | |||
| Increased AST | 5 (25.0) | 2 (25.0) | 3 (25.0) | |||
| Increased ALT | 6 (30.0) | 3 (75.0) | 3 (25.0) | |||
| Increased γ-GT | 5 (25.0) | 3 (75.0) | 2 (16.7) | |||
| Hyperbilirubinemia | 2 (10.0) | 1 (12.5) | 1 (8.3) | |||
| Increased LDH | 5 (25.0) | 3 (37.5) | 2 (16.7) | |||
| Increased ALP | 2 (10.0) | 1 (12.5) | 1 (8.3) | |||
| Electrolyte disorders | ||||||
| Hyponatremia | 2 (10.0) | 1 (12.5) | 1 (8.3) | |||
| Hypokalemia | 1 (5.0) | 1 (8.3) | ||||
| Non-hematological | ||||||
| Gastrointestinal disorder | ||||||
| Nausea/vomiting | 3 (15.0) | 2 (25.0) | 1 (8.3) | |||
| Anorexia | 4 (20.0) | 3 (37.5) | 1 (8.3) | |||
| Diarrhea | 1 (5.0) | 1 (12.5) | ||||
| Stomatitis | 6 (30.0) | 3 (37.5) | 3 (25.0) | |||
| Small intestinal hemorrhage | 1 (12.5) | 1 (12.5) | 1 (12.5) | |||
| General disorders | ||||||
| General fatigue | 9 (45.0) | 6 (75.0) | 3 (25.0) | |||
| Alopecia | 3 (15.0) | 2 (25.0) | 1 (8.3) | |||
| Skin and subcutaneous disorders | ||||||
| HFSR | 18 (90.0) | 8 (40.0) | 8 (100.0) | 6 (75.0) | 10 (83.3) | 2 (16.7) |
| Nervous system disorders | ||||||
| Peripheral sensory neuropathy | 2 (10.0) | 1 (12.5) | 1 (8.3) | |||
| Hypertension | 4 (20.0) | 1 (5.0) | 2 (25.0) | 2 (16.7) | 1 (8.3) | |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, γ-glutamyl transpeptidase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; HFSR, hand-foot skin reaction.
Discussion
The CORRECT study demonstrated that Reg prolonged the OS of patients with mCRC in the salvage setting compared with best supportive care (14). OS, the primary endpoint, was similar between Japanese and non-Japanese patients, whereas PFS and DCR also improved significantly in both groups. However, the AE profiles of Reg were different, and AEs such as HFSR, hypertension, thrombocytopenia, proteinuria and lipase elevation were observed at a higher rate among Japanese patients. The incidence of grade ≥3 AEs was also higher in the Japanese group, and 1 Japanese patient developed fatal liver dysfunction. The dose intensity of Reg was lower in Japanese compared with that in non-Japanese patients, as dose modifications due to AEs were more frequent in the Japanese group. The rate of treatment discontinuation due to Reg-associated AEs was also higher in the Japanese group (15). When Reg was launched in May, 2013, treatment was initiated at the dose of 160 mg. However, management and determination of the optimal dose was difficult due to AEs. Although the first 8 patients received local skin management, such as controlling the formation of calluses, providing comfort with cushions, and applying local creams, 6 patients developed severe HFSR and 1 patient developed small intestinal hemorrhage. The patients were unable to continue with the subsequent cycles, and they all required hospitalization. Following that, 2 patients deferred Reg and the remaining patients were forced to change the chemotherapy regimen. Reg treatment was then reconsidered, and dosage and Reg-related AEs were investigated. In the CORRECT study, the most frequent grade ≥3 AEs were HFSR, fatigue, diarrhea, hypertension and rash/desquamation. It was reported that these AEs occurred shortly after treatment initiation and quickly increased in severity. For example, the medium time to first occurrence and maximum severity of HFSR was 15 and 22 days in the first cycle, respectively. In this analysis, close monitoring of AEs, particularly during the first several days following the initiation of Reg, and prompt intervention, including dose modification, were strongly recommended in order to manage Reg-related AEs (17). However, as patients with Reg-related HFSR develop painful erythema and tense blisters evolving into callus-like hyperkeratoses (18), they are likely to refuse subsequent treatment if they experience severe symptoms, similar to our patients. Additionally, as there are several patients with poor PS, multiple liver metastases and a number of complications (e.g., diabetes mellitus and hypertension) in clinical practice, a reduced initial dose of Reg was considered. In a phase I study of Reg, the grade ≥3 AEs were fewer with the dose of 120 mg compared with that of 160 mg (43 and 67%, respectively). In the pharmacokinetics analysis of the study, the mean 24-h area under the concentration-time curve after multiple doses of Reg did not exhibit a dose-proportional numerical increase by increasing the administered dose from 120 to 220 mg. On pharmacodynamics analysis, tumor perfusion properties were measured by non-invasive angiogenic imaging using dynamic contrast-enhanced MRI and a ≥40% decrease in tumor perfusion was observed at the dose of 120–220 mg (19). Therefore, the initial dose of 120 mg Reg was selected and increased by 40 mg if the patients did not have any grade 3 or unacceptable AEs. Additionally, in our experience, patients with Reg-related HFSR recover soon if the treatment is interrupted prior to the symptoms becoming severe. Thus, the treatment was interrupted as soon as patients developed any grade 3 or unacceptable AEs, including HFSR with pain, and the dose was reduced by 40 mg (Fig. 1). A total of 12 patients were treated at the dose of 120 mg Reg and only 1 patient developed a grade 3 adverse event. The median RDI in the 120 mg group was similar to that in the 160 mg group. One PR and 6 SD cases were observed in the 120 mg group, with 2 exhibiting morphological changes in their metastatic lesions. It was reported that the onset of cavitation in lung metastases and low tumor density at baseline tended to be correlated with better PFS and OS (20); the duration of the treatment in our 2 cases was 5 and 6.5 months. Based on the results, the morphological changes in the metastases have the potential to become a predictive factor for Reg. Recently, certain investigators recommended a starting Reg dose of 80 mg; however, it remains unclear whether Reg-related AEs develop in a dose- or time-dependent manner. At present, the starting dose of 120 mg Reg is used to clearly evaluate dose- or time-dependent development of AEs. In conclusion, the dose of 160 mg of Reg for patients with mCRC was not fully manageable in the salvage therapy setting. However, an initial dose of 120 mg Reg, with our dose modification methods, was not only effective and tolerable, but also maintained the RDI of Reg. Future investigation is required to confirm the clinical benefit of this treatment, as the present study was retrospective and only included a small number of patients. In addition, patients often experience unexpected AEs in the salvage setting; thus, oncologists must closely monitor the patients' condition.
References
- 1.Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, Nishimoto H. Japan Cancer Surveillance Research Grou: Cancer incidence and incidence rates in Japan in 2008: A study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol. 2014;44:388–396. doi: 10.1093/jjco/hyu003. [DOI] [PubMed] [Google Scholar]
- 2.Haggar FA, Boushey RP. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg. 2009;22:191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold D, Stein A. New developments in the second-line treatment of metastatic colorectal cancer: Potential place in therapy. Drugs. 2013;73:883–891. doi: 10.1007/s40265-013-0076-5. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. ESMO Guidelines Working Group: Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii1–iii9. doi: 10.1093/annonc/mdu260. (Suppl 3) [DOI] [PubMed] [Google Scholar]
- 5.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 6.Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 7.Bokemeyer C, Bondarenko I, Hartmann JT, de Braud F, Schuch G, Zubel A, Celik I, Schlichting M, Koralewski P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann Oncol. 2011;22:1535–1546. doi: 10.1093/annonc/mdq632. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejper S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 9.Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 10.Modest DP, Stintzing S, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmuller C, Kahl C, et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: First-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol. 2015;33:3718–3726. doi: 10.1200/JCO.2015.61.2887. [DOI] [PubMed] [Google Scholar]
- 11.Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Mahoney MR, O'Neil BH, Shaw JM, Polite BN, Hochster HS, Atkins JN, et al. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC) J Clin Oncol. 2014;32:5s. (Suppl; abstr LBA3) [Google Scholar]
- 12.Yamazaki K, Nagase M, Tamagawa H, Ueda S, Murata K Tamura, Tsuda T, Baba E, Tsuda M, Moriwaki T, Esaki T, et al. A randomized phase III trial of mFOLFOX6 plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment for metastatic colorectal cancer: West Japan Oncology Group study 4407G (WJOG4407G) J Clin Oncol. 2014;32:5s. (Suppl; abstr 3534) [Google Scholar]
- 13.Schmieder R, Hoffmann J, Becker M, Bhargava A, Müller T, Kahmann N, Ellinghaus P, Adams R, Rosenthal A, Thierauch KH, et al. Regorafenib (BAY 73–4506): Antitumor and antimetastatic activities in preclinical models of colorectal cancer. Int J Cancer. 2014;15:1487–1496. doi: 10.1002/ijc.28669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–312. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 15.Yoshino T, Komatsu Y, Yamada Y, Yamazaki K, Tsuji A, Ura T, Grothey A, Van Cutsem E, Wagner A, Cihon F, et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: Analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs. 2015;33:740–750. doi: 10.1007/s10637-014-0154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu J, Bai Y, Chi Y, Wang L, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–629. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 17.Grothey A, Sobrero AF, Siena S, Falcone A, Ychou M, Humblet Y, Bouche O, Mineur L, Barone C, Adenis A, et al. Time profile of adverse events (AEs) from regorafenib (REG) treatment for metastatic colorectal cancer (mCRC) in the phase III CORRECT study. J Clin Oncol. 2013;31 (Suppl; abstr 3637) [Google Scholar]
- 18.McLellan B, Ciardiello F, Lacouture ME, Segaert S, Van Cutsem E. Regorafenib-associated hand-foot skin reaction: Practical advice on diagnosis, prevention, and management. Ann Oncol. 2015;26:2017–2026. doi: 10.1093/annonc/mdv244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mross K, Frost A, Steinbild S, Hedbom S, Büchert M, Fasol U, Unger C, Krätzschmar J, Heinig R, Boix O, et al. A phase I dose-escalation study of regorafenib (BAY 73–4506), an inhibitor of oncogenic, angiogenic, and stromal kinases, in patients with advanced solid tumors. Clin Cancer Res. 2012;18:2658–2667. doi: 10.1158/1078-0432.CCR-11-1900. [DOI] [PubMed] [Google Scholar]
- 20.Ricotta R, Sartore-Bianchi A, Verrioli A, Vanzulli A, Siena S. Regorafenib for metastatic colorectal cancer. Lancet. 2013;381:1537. doi: 10.1016/S0140-6736(13)60977-0. [DOI] [PubMed] [Google Scholar]