Abstract
To study the effects of miR-22 on the proliferation and the apoptosis of osteosarcoma MG-63 cell line and to explore the potential molecular mechanism that miR-22 regulates this biological process. Quantitive real-time polymerase chain reaction (RT-qPCR) was performed to explore the miRNA level of miR-22. The MG-63 cell line was infected with miR-22 mimics for establishment of miR-22 overexpression. Non-infected cells were in blank group and cells infected with empty vector were served as negative control (NC group). MTT assay was conducted to measure cell viability. The cell cycle and apoptosis were explored using flow cytometry and the apoptosis-related markers were detected by western blotting. RT-qPCR results revealed that the miR-22 miRNA level in the MG-63 cells was significantly lower than that in osteoblasts (P<0.05). MTT assay showed that the MG-63 cells infected with miR-22 mimics exhibited markedly decreased proliferation ability compared with blank and empty vector (NC) groups. Next, we found that overexpression of miR-22 remarkably increased the apoptosis of the MG-63 cells, evidenced from the flow cytometry results and elevated Bax and reduced Bcl-2. Furthermore, results revealed that percentage of the cells at G0/G1 phase in miR-22 mimic group (66.75±3.67%) was significantly higher than blank (52.9±2.58%) and NC (50.5±2.45%) groups. miR-22 attenuated the proliferation and induced the apoptosis of the MG-63 cells via promoting G0/G1 cell cycle arrest. Thus, miR-22 may have the potential to be a novel therapeutic in treatment of osteosarcoma.
Keywords: osteosarcoma, miR-22, cell apoptosis, cell proliferation, cell cycle arrest
Introduction
As a primary tumor of bone, osteosarcoma (OS) is believed to be derived from the malignant mesenchymal stem cells (1,2). The tumor usually develops in the metaphyses of the long bones, such as the proximal humerus, the distal femur, and the proximal tibia (3). Although in the past decades, the neoadjuvant chemotherapy combined with the local control have been applied to ameliorate the outcomes of patients with OS, the rate of 5-year survival rises to approximately 65%, and after metastasis or recurrence the rate is significantly lowered to approximately 30% (4–6). In addition, patients with OS metastasis to some other tissues such as lung mostly decease in about 6–12 months (7).
miRNAs, approximately 22–25 nt in length, are a kind of non-coding small RNAs and are crucial mediators on post-transcriptional gene expression (8,9). Mature miRNAs regulate the gene expression through binding 3′-UTR of their target genes, resulting in either decreased protein translation or miRNA degradation (10,11). Abnormal level of miRNAs was found to contribute to various malignant tumors, including OS (12–14).
Zhu et al found that miR-29b can exert its tumor-suppressive function in OS via targeting CDK6 (15). In another study, miR-140 was showed to be associated with the chemoresistance by reducing cell proliferation through G1/G2 phase arrest mediated partly through the suppression of HDAC4 (16).
Recent studies have demonstrated the important role of miR-22 in the occurrence and development of OS. Xin et al suggested that miR-22 could significantly attenuate tumor growth and metastasis by targeting ATP citrate lyase (17). However, how miR-22 exerts its antitumor effects remains unclear.
The aim of our present study was to explore the miR-22 effects on the proliferation and the apoptosis in the osteosarcoma MG-63 cell line, and to investigate its potential underlying mechanism.
Materials and methods
Cell culture
The human MG-63 cell line was purchased from Shanghai Institute of Cell Biology (Shanghai, China). Cells were cultivated in the DMEM coupled with 10% fetal bovine serum (FBS), 100 U/ml penicillin, 100 itg/ml streptomycin, and 2 mM glutamine (BioSharp, Hefei, China).
Cell transfection
miR-22 mimic and the negative control were supplied by GenePharma (Shanghai, China). For transfection, the MG-63 cell line was planted in a 12-well plate at a 5×104/ml density and were transfected with 100 nM of miR-22 mimic or NC using Lipofectamine 3000 (Life Technologies, Gaithersburg, MD, USA) and then incubated for 6 h. Following transfection, medium were changed to 2% FBS-DMEM without antibiotics.
Real-time qPCR
Isolation of the total cellular RNA was performed following the manufacturers instructions of miRNeasy Mini kit (Qiagen, Hilden, Germany) and the levels of miRNA were quantified using the miRNA assay (TaqMan; Applied Biosystems). The RT-qPCR experiment was carried on a real-time quantified PCR system (ABI StepOne and StepOnePlus; Applied Biosystems) with LightCycler 480 (Roche). The miRNA levels were detected from the relative threshold cycle, which were determined using the method of arithmetic formulas 2−ΔΔcp normalizated to level of the U6 small nuclear RNA.
MTT assay
The cells viability was detected following the introduction of the MTT assay. At various time-points after transfection (6, 12, 24 and 48 h), cultivated medium of cells was removed, washed cells twice with PBS and then incubated with 0.5 mg/ml MTT at 37°C. After 4-h incubation, removing the supernatant and the isopropanol was administrated to cells to dissolve formazan. The formazan concentration was detected by calculating the relative absorbance at wavelengh of 570 nm with the SpectraMax 360 pc microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
FACS analysis
After transfection for 24 h, cells were first fixed in the ice-cold 70% ethanol and then stained by PBS containing propidium iodide (50 µg/ml)/Annexin V and RNase A (100 µg/ml) for DNA analysis using the flow cytometry analysis on a BD FACSCalibur system (FACScan; BD Biosciences, San Diego, CA, USA). Percentage of the cells in the different phases of cellular cycle was measured using the CellQuest software version 3.3 (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA).
Western blotting
Denatured cell lysates 40 µg were run on 10% gels and transferred to the polyphorylated difluoride membranes. Then, membranes were incubated with 5% BSA dissolved in TBST (Tris-buffered saline + 0.05% Tween) for 60 min and then incubated with the primary antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) in 0.5% TBS-T overnight. Primary rabbit anti-Bcl-2 antibody (dilution, 1/1,000; cat. no. ab32124), and primary rabbit anti-Bax antibody (dilution, 1/1,000; cat. no. ab32503) were all purchased from Abcam (Cambridge, MA, USA). After washing for 3 times with TBST, secondary horseradish peroxidase (HRP)-conjugated antibodies were incubated with membranes for 1 h with TBST. The secondary antibodies were detected using the ECL Plus (Amersham, Arlington Heights, IL, USA) and imaged with the GelDoc XR System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The relative band densities were normalized to β-actin.
Statistical analysis
Statistical analysis was performed using SPSS 20.0 software (IBM, Armonk, NY, USA). Group data were expressed as mean ± standard deviation. Comparison between groups was done using one-way ANOVA test followed by post hoc test (least significant difference). Percentage (%) was used to express the enumeration data and Chi-square test was used for data analysis. P values <0.05 were considered statistically significant.
Results
miR-22 is low-expressed in the OS cells
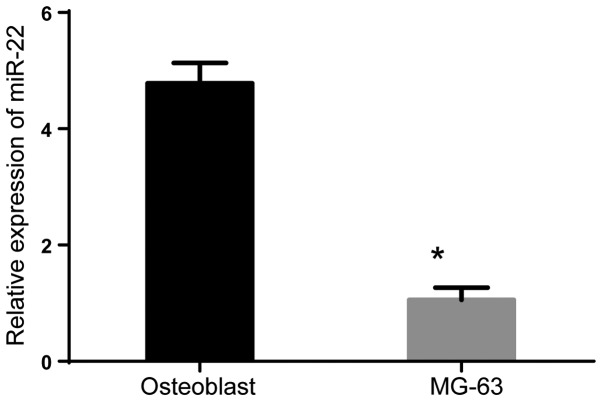
First, we explored the miR-22 level in the OS cells (MG-63) and osteoclasts. RT-PCR results revealed that the miR-22 level in the MG-63 cells was significantly lower than that in osteoclasts (Fig. 1) (P<0.05).
Figure 1.
miR-22 was low-expressed in osteosarcoma MG-63 cells. Compared with osteoblast, the expression of miR-22 in MG-63 significantly decreased. *P<0.05.
miR-22 inhibits proliferation of the MG-63 cells
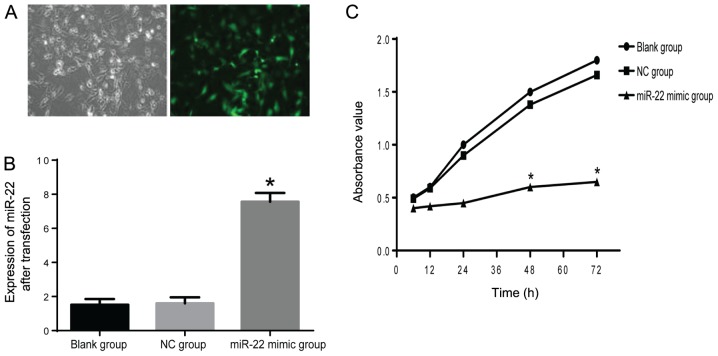
To investigate the miR-22 effects on proliferation of the MG-63 cells, miR-22 mimics were used to infect the cells. After infection for 24 h, 70% cells were successfully infected with mimics, evidenced by detected fluorescence under the fluorescence microscope (Nikon Eclipse E800; Nikon, Tokyo, Japan) (Fig. 2A). To verify the efficiency of transfection, RT-qPCR was performed. Results suggested that the level of miR-22 was nearly 8-fold of that in NC group (Fig. 2B). Next MTT assay was performed to explore cell proliferation. We found that cells infected with miR-22 mimics exhibited significant decreased cell viability at various time-points (12, 24, 48 and 72 h) (P<0.05) (Fig. 2C). These findings showed that miR-22 has inhibitory effects on MG-63 cell proliferation.
Figure 2.
miR-22 inhibits proliferation of MG-63 cells. (A) Transfection efficiency was detected by fluorescent microscopy; (B) miR-22 mimic infection induced a significant elevation of miR-22; (C) miR-22 inhibits proliferation of MG-63 cells (*compared with NC group, P<0.05). NC group, negative control group.
miR-22 promotes apoptosis of the MG-63 cells
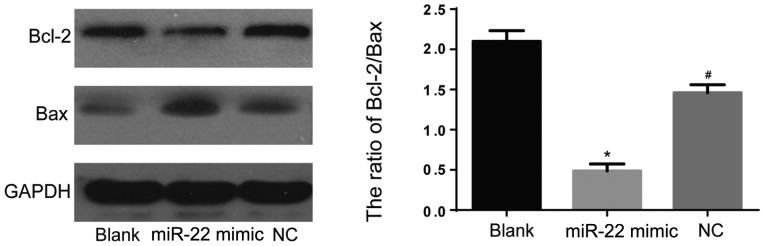
To further study the effects of miR-22 on the MG-63 cells apoptosis. We conducted the flow cytometry experiment and found that cells infected with miR-22 mimics exhibited markedly increased apoptosis compared with blank and NC group (P<0.05) (Fig. 3). To confirm these findings, we also performed western blotting to detect levels of Bcl-2 and Bax, which were classical apoptosis-related markers. As expected, infection with miR-22 mimics remarkably elevated level of Bax and downregulated Bcl-2 (Fig. 4). These results demonstrated apoptosis-promoting effects of miR-22 in MG-63 cells.
Figure 3.
Flow cytometry was performed to detect apoptosis of MG-63 cells. Cells infected with miR-22 mimics exhibited markedly increased apoptosis compared with blank and NC group (P<0.05). NC group, negative control group.
Figure 4.
Western blot analysis was performed to detect levels of Bcl-2 and Bax. Infection with miR-22 mimics remarkably elevated level of Bax and downregulated Bcl-2 (*compared with NC group, P<0.05; #compared with blank group, P<0.05). NC group, negative control group.
miR-22 induces G0/G1 cell cycle arrest
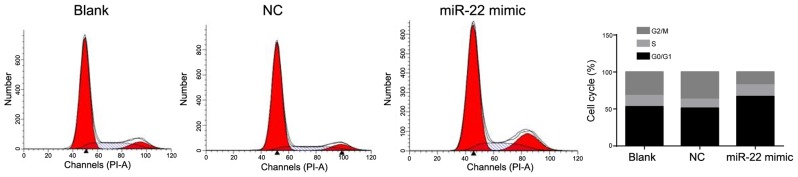
To investigate the mechanism that miR-22 increased the MG-63 cells apoptosis, we further detected the cellular cycle of the MG-63 cells using the flow cytometry experiment among 3 groups. Results showed that percentage of cells at phase of G0/G1 in miR-22 mimic group (66.75±3.67%) were significantly higher than that in blank (52.9±2.58%) or NC group (50.5±2.45%) (P<0.05) (Fig. 5). All above results indicated that miR-22 promotes MG-63 cells apoptosis via inducing G0/G1 cell cycle arrest.
Figure 5.
miR-22 induced G0/G1 cell cycle arrest. The number of cells at G0/G1 phase in mimic group was significantly bigger than that in other groups.
Discussion
OS is a kind of primary bone cancer and is most common among children and adolescents, however its pathogenesis remains unclear and elusive due to its well-known heterogeneous character (18). Despite of the technological progresses in OS diagnosis and therapy, the mortality rate of the disease remains at a high level (5).
Accumulating studies demonstrate that miRNAs play a more and more important role in regulating the apoptosis and proliferation of tumor cells (12–14). Recent researches have elucidated the miRNAs contribution in the occurrence and progression development of OS (15–17). For example, miR-27a was found to overexpress in serum of OS patients, indicating its potential to be a diagnostic marker for OS (19). Another study found that the elevated miR-130b in OS tissues was related to the aggressive progression of OS patients, thus affecting the rate of survival (20). There were also downregulated miRNAs detected in OS tumor cells, such as miR-29b, miR-181b, miR-142-5p and miR-16 (21–23) which may play a significant role in osteosarcomagenesis.
miR-22 was demonstrated to be an important regulator in various tumors by much evidence. Li et al showed that miR-22 has negative effects on cell migration and invasion and was identified as a potential metastasis-inhibitor in ovarian cancer (24). Ling et al found that miR-22 exhibited dramatically the activity of anti-lung cancer in vivo and in vitro via targeting the ErbB3 (25).
To our knowledge, this was the first attempt to figure out the potential mechanism that miR-22 regulates the OS cellular proliferation and apoptosis. Our present study firstly explored the miR-22 level in OS cells. Results revealed that miR-22 in MG-63 cells was significantly lower than that in osteoblasts, which was consistent with previous studies (17,26,27). Next, we found that cell proliferation was significantly inhibited by the overexpression of miR-22, indicating that miR-22 has inhibitory effects on MG-63 cells proliferation. Additionally, our results also suggested that the overexpression of miR-22 can remarkably promote cell apoptosis, suggesting that miR-22 has promotive effects on MG-63 cells apoptosis. Apoptosis-related proteins Bax also increased after infecting with miR-22 mimics. Further, we explored the potential mechanism by which miR-22 regulated the process of MG-63 cells apoptosis. As we know, cell cycle participates in cellular proliferation and apoptosis. Cell cycle arrest can induce cell apoptosis (28–30). Given this, we employed flow cytometry to explore the percentage of cells at different phases after transfected with miR-22 mimics. Results showed that overexpression of miR-22 induced a significant G0/G1 cell cycle arrest. These findings suggested that the G0/G1 cellular cycle arrest may be the potential biological mechanism of which miR-22 promotes apoptosis of MG-63 cells.
There are still some limitations in our study. Our findings have not been confirmed by in vivo experiments. In addition, we did not predict the target gene for miR-22 to delineate the molecular mechanism underlying this process. In our further study, we plan to employ miR-22 lentivirus to construct stable overexpression model of miR-22 in animals for verification of our findings and also to find its target.
In conclusion, our study showed that miR-22 induced G0/G1 cellular cycle arrest, thus leading to apoptosis of OS cells. These findings identified the novel tumor suppressive role of miR-22 in the development of OS. Our present study provides a new, potential therapeutic target to OS.
References
- 1.Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am. 2016;47:283–292. doi: 10.1016/j.ocl.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari S, Serra M. An update on chemotherapy for osteosarcoma. Expert Opin Pharmacother. 2015;16:2727–2736. doi: 10.1517/14656566.2015.1102226. [DOI] [PubMed] [Google Scholar]
- 3.Jaffe N. Osteosarcoma: Review of the past, impact on the future. The American experience. Cancer Treat Res. 2009;152:239–262. doi: 10.1007/978-1-4419-0284-9_12. [DOI] [PubMed] [Google Scholar]
- 4.Friebele JC, Peck J, Pan X, Abdel-Rasoul M, Mayerson JL. Osteosarcoma: A meta-analysis and review of the literature. Am J Orthop (Belle Mead NJ) 2015;44:547–553. [PubMed] [Google Scholar]
- 5.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 6.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the surveillance, epidemiology, and end results program. Cancer. 2009;115:1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma: Where do we go from here? Paediatr Drugs. 2008;10:315–327. doi: 10.2165/00148581-200810050-00005. [DOI] [PubMed] [Google Scholar]
- 8.Atkins CA, Smith PM, Rodriguez-Medina C. Macromolecules in phloem exudates - A review. Protoplasma. 2011;248:165–172. doi: 10.1007/s00709-010-0236-3. [DOI] [PubMed] [Google Scholar]
- 9.Irwandi RA, Vacharaksa A. The role of microRNA in periodontal tissue: A review of the literature. Arch Oral Biol. 2016;72:66–74. doi: 10.1016/j.archoralbio.2016.08.014. [DOI] [PubMed] [Google Scholar]
- 10.Hamidi-Asl E, Palchetti I, Hasheminejad E, Mascini M. A review on the electrochemical biosensors for determination of microRNAs. Talanta. 2013;115:74–83. doi: 10.1016/j.talanta.2013.03.061. [DOI] [PubMed] [Google Scholar]
- 11.Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ. Biological functions of microRNAs: A review. J Physiol Biochem. 2011;67:129–139. doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- 12.Iorio MV, Croce CM. MicroRNAs in cancer: Small molecules with a huge impact. J Clin Oncol. 2009;27:5848–5856. doi: 10.1200/JCO.2009.24.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garzon R, Marcucci G, Croce CM. Targeting microRNAs in cancer: Rationale, strategies and challenges. Nat Rev Drug Discov. 2010;9:775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tutar L, Tutar E, Tutar Y. MicroRNAs and cancer; An overview. Curr Pharm Biotechnol. 2014;15:430–437. doi: 10.2174/1389201015666140519095304. [DOI] [PubMed] [Google Scholar]
- 15.Zhu K, Liu L, Zhang J, Wang Y, Liang H, Fan G, Jiang Z, Zhang CY, Chen X, Zhou G. MiR-29b suppresses the proliferation and migration of osteosarcoma cells by targeting CDK6. Protein Cell. 2016;7:434–444. doi: 10.1007/s13238-016-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song B, Wang Y, Xi Y, Kudo K, Bruheim S, Botchkina GI, Gavin E, Wan Y, Formentini A, Kornmann M, et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene. 2009;28:4065–4074. doi: 10.1038/onc.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xin M, Qiao Z, Li J, Liu J, Song S, Zhao X, Miao P, Tang T, Wang L, Liu W, et al. miR-22 inhibits tumor growth and metastasis by targeting ATP citrate lyase: Evidence in osteosarcoma, prostate cancer, cervical cancer and lung cancer. Oncotarget. 2016;7:44252–44265. doi: 10.18632/oncotarget.10020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endo-Munoz L, Cumming A, Sommerville S, Dickinson I, Saunders NA. Osteosarcoma is characterised by reduced expression of markers of osteoclastogenesis and antigen presentation compared with normal bone. Br J Cancer. 2010;103:73–81. doi: 10.1038/sj.bjc.6605723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salah Z, Arafeh R, Maximov V, Galasso M, Khawaled S, Abou-Sharieha S, Volinia S, Jones KB, Croce CM, Aqeilan RI. miR-27a and miR-27a* contribute to metastatic properties of osteosarcoma cells. Oncotarget. 2015;6:4920–4935. doi: 10.18632/oncotarget.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu LD, Jin RL, Gu PC, Ling ZH, Lin XJ, Du JY. Clinical significance of microRNA-130b in osteosarcoma and in cell growth and invasion. Asian Pac J Trop Med. 2015;8:752–756. doi: 10.1016/j.apjtm.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 21.Jones KB, Salah Z, Del Mare S, Galasso M, Gaudio E, Nuovo GJ, Lovat F, LeBlanc K, Palatini J, Randall RL, et al. miRNA signatures associate with pathogenesis and progression of osteosarcoma. Cancer Res. 2012;72:1865–1877. doi: 10.1158/0008-5472.CAN-11-2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Shi W, Wu C, Ju J, Jiang J. miR-181b as a key regulator of the oncogenic process and its clinical implications in cancer (Review) Biomed Rep. 2014;2:7–11. doi: 10.3892/br.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Zhang K, Liu LH, Ouyang Y, Guo HB, Zhang H, Bu J, Xiao T. MicroRNA screening identifies circulating microRNAs as potential biomarkers for osteosarcoma. Oncol Lett. 2015;10:1662–1668. doi: 10.3892/ol.2015.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Liang S, Yu H, Zhang J, Ma D, Lu X. An inhibitory effect of miR-22 on cell migration and invasion in ovarian cancer. Gynecol Oncol. 2010;119:543–548. doi: 10.1016/j.ygyno.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 25.Ling B, Wang GX, Long G, Qiu JH, Hu ZL. Tumor suppressor miR-22 suppresses lung cancer cell progression through post-transcriptional regulation of ErbB3. J Cancer Res Clin Oncol. 2012;138:1355–1361. doi: 10.1007/s00432-012-1194-2. [DOI] [PubMed] [Google Scholar]
- 26.Li X, Wang S, Chen Y, Liu G, Yang X. miR-22 targets the 3′UTR of HMGB1 and inhibits the HMGB1-associated autophagy in osteosarcoma cells during chemotherapy. Tumour Biol. 2014;35:6021–6028. doi: 10.1007/s13277-014-1797-0. [DOI] [PubMed] [Google Scholar]
- 27.Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang Y, Wang Y, Zhao W, Wang W. miR-22 inhibits osteosarcoma cell proliferation and migration by targeting HMGB1 and inhibiting HMGB1-mediated autophagy. Tumour Biol. 2014;35:7025–7034. doi: 10.1007/s13277-014-1965-2. [DOI] [PubMed] [Google Scholar]
- 28.Moalic S, Liagre B, Corbière C, Bianchi A, Dauça M, Bordji K, Beneytout JL. A plant steroid, diosgenin, induces apoptosis, cell cycle arrest and COX activity in osteosarcoma cells. FEBS Lett. 2001;506:225–230. doi: 10.1016/S0014-5793(01)02924-6. [DOI] [PubMed] [Google Scholar]
- 29.Zhao W, Zhou SF, Zhang ZP, Xu GP, Li XB, Yan JL. Gambogic acid inhibits the growth of osteosarcoma cells in vitro by inducing apoptosis and cell cycle arrest. Oncol Rep. 2011;25:1289–1295. doi: 10.3892/or.2011.1189. [DOI] [PubMed] [Google Scholar]
- 30.Lee DS, Lee MK, Kim JH. Curcumin induces cell cycle arrest and apoptosis in human osteosarcoma (HOS) cells. Anticancer Res. 2009;29:5039–5044. [PubMed] [Google Scholar]