Abstract
Vascular endothelial growth factor (VEGF)165 is one of the most abundant and potent angiogenic factors in both physiological and pathological conditions. However, the function and mechanism of VEGF165 in tumors and their environment remain to be elucidated. In the present study, a lentivirus vector (LV) that contained the VEGF165-enhanced green fluorescent protein (EGFP) fusion gene was constructed and transfected into the human breast cancer cell line MCF-7. Following transfection, the expression of VEGF165 in MCF-7 cells was detected by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blotting. Further cellular localization of VEGF165 was observed through fluorescence microscopy. The titer of the recombinant lentivirus was 5.44×107 TU/ml in the LV-VEGF165-EGFP group and 5.00×108 TU/ml in the LV-EGFP negative control group. RT-qPCR and western blotting demonstrated that the expression of VEGF165 was significantly increased in the LV-VEGF165-EGFP group compared with the control group. The present study lays the foundation for in vitro and in vivo studies on tumor cell derived-VEGF165. Furthermore, the present fusion gene expression vector may provide a potential approach for gene therapy treatment of cancer and other diseases that require regulation of angiogenesis.
Keywords: breast cancer, lentivirus vector, VEGF165-EGFP fusion gene, cellular localization
Introduction
Tumors and metastases usually arise as small avascular masses that subsequently induce neovascularization in order to acquire nutrients for further growth and metastatic spread (1–3). This angiogenic switch is induced by several factors such as vascular endothelial growth factor (VEGF), which is secreted by tumor cells (4). VEGF was originally identified and isolated as an endothelial cell-specific mitogen that is able to induce physiological and pathological angiogenesis (5,6). Alternative splicing of VEGF leads to several different isoforms, which are differentially expressed in various tumor types and have distinct functions in tumor blood vessel formation (7). The angiogenic isoforms of VEGF are known as VEGF121, VEGF145, VEGF165 and VEGF189 in humans (8). VEGF165 is one of the most abundant and potent angiogenic agents among all the VEGF isoforms (9). Cellular responses to VEGF165 are mediated by two high-affinity type III tyrosine kinase receptors, VEGF receptor (VEGF-R)2 (also known as kinase insert domain receptor and fetal liver kinase 1) and VEGF-R1 (also known as Fms-related tyrosine kinase 1), and two receptors of the semaphorin receptor family, neuropilin-1 and neuropilin-2 (10). In addition to its functions in endothelial cells, the role of VEGF in tumor cells is currently an emerging area of importance; therefore, an increasing number of studies have focused on the biology of VEGF in tumor cells (11–13).
Vector-mediated gene expression is one of the most important tools for studying gene functions in vitro and in vivo (14). To date, several viral vectors have been applied in laboratory experiments or clinical trials and have achieved good results (15–17). In the majority of cases, the efficiency of gene transfer represents the most relevant obstacle, since it limits the success of gene overexpression or gene silencing (18). Compared with non-viral methods, viruses are highly-evolved, natural delivery agents for genetic materials (16,19). In addition to their remarkable transduction efficiency, previous laboratory experiments have suggested that lentivirus vectors (LVs) exhibit low immunogenicity and durable expression (14). Furthermore, one of the most striking characteristics distinguishing the lentivirus genus from gammaretroviruses is their ability to infect non-replicating cells (20). These advantages make lentiviruses a powerful tool in the studies of gene functions.
The present study intended to construct a recombinant LV containing the VEGF165-enhanced green fluorescent protein (EGFP) fusion gene, and investigated the feasibility of using LV to express the fused VEGF165-EGFP gene in the breast cancer cell line MCF-7. The present study lays the foundation for future in vitro and in vivo studies on tumor cell derived-VEGF.
Materials and methods
Cell lines and cell culture
The breast carcinoma cell line MCF-7 and the human embryonic kidney epithelial cell line 293T were obtained from Shanghai Cell Bank, Chinese Academy of Sciences (Shanghai, China). All cells were cultured in Dulbecco's modified Eagle medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin, and were maintained at 37°C under 5% CO2. Cells used in the experiments were in logarithmic growth phase.
Construction and sequencing of pLVX-VEGF165-EGFP- 3FLAG recombinant plasmid expression vector
The VEGF165 (NM_001171626) coding region was amplified by polymerase chain reaction (PCR) using the PrimeScript™ RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China) and the following cycling conditions: Pre-denaturation at 98°C for 3 min; 30 cycles at 94°C for 10 sec, 55°C for 15 sec and 72°C for 1 min; and 72°C for a final 10 min. The VEGF165 fragment DNA was extracted and purified using the QIAquick Gel Extraction kit and the QIAquick PCR Purification kit, respectively (Qiagen GmbH, Hilden, Germany). The purified VEGF165 fragment and the pLVX-EGFP-3FLAG plasmid (Shanghai Sbo-bio Biotechnology, Shanghai, China) (Fig. 1) were digested separately using EcoRI restriction endonuclease. Upon digestion, the mixture was incubated at 65°C for 10 min to stop the reaction, after which the products were separated by 1.5% agarose gel electrophoresis. The bands were visualized using a gel scanning analysis system (ChemiDoc MP imaging system; Bio-Rad Laboratories, Inc., Hercules, CA, USA). Then, the target fragment of about 7.7 kb in size was recycled by 1% agarose gel electrophoresis.
Figure 1.
Structure of the eukaryotic expression vector pLVX-EGFP-3FLAG. Image adapted from Shanghai Sbio-bio Biotechnology Co. Ltd. LV, lentivirus vector; EGFP, enhanced green fluorescent protein.
Using the In-Fusion® HD Cloning kit (Clontech Laboratories, Inc., Mountainview, CA, USA), the VEGF165 fragment was ligated into the pLVX-EGFP-3FLAG expression vector, which had been digested with EcoRI. Two control groups were used, including a blank control and a negative control group. Next, the recombinant plasmid vector was transformed into Escherichia coli DH5α competent bacteria (Takara Biotechnology Co., Ltd.) according to the manufacturer's protocol, and Luria broth (LB)-agar plates containing 100 µg/ml ampicillin were employed to select the positive clones. Upon transformation, the positive clones were identified by PCR using the PrimeScript™ RT-PCR kit and the following cycling conditions: Pre-denaturation at 94°C for 5 min; 30 cycles at 94°C for 30 sec, 55°C for 30 sec and 72°C for 1 min; and 72°C for 10 min.
The E. coli clones were cultured in LB medium at 37°C, and the plasmid was extracted using the QIAprep Spin Miniprep kit according to the manufacturer's protocol (Qiagen GmbH). The VEGF165 gene was then amplified by PCR using the Prime Script™ RT-PCR kit to identify the correct recombinant plasmids. The cycling conditions were: Pre-denaturation at 94°C for 5 min; 30 cycles at 94°C for 30 sec, 55°C for 30 sec and 72°C for 1 min; and 72°C for 10 min. The CMV-F and pEGFP-N-3 primers were used to set up the PCR reaction, and their sequences are indicated in Table I. The positive bacteria colonies were inoculated into LB medium at 37°C for 16 h, and upon adding glycerol, they were stored at −80°C. Aliquots (200 µl) of each were sent for DNA sequencing by Shanghai Sbio-bio Biotechnology Co. Ltd. (Shanghai, China).
Table I.
Primers used for VEGF165 gene fragment amplification and RT-qPCR.
| Primer | Sequence |
|---|---|
| VEGF165-EcoRI-Forward | 5′-CTCAAGCTTCGAATTCGCCACCATGAACTTTCTGCTGTCTTGG-3′ |
| VEGF165-EcoRI-Reverse | 5′-CATGGTGGCGAATTCCCGCCTCGGCTTGTCACA-3′ |
| CMV-F | 5′-CGCAAATGGGCGGTAGGCGTG-3′ |
| pEGFP-N3 | 5′-CGTCGCCGTCCAGCTCGACCAG-3′ |
| EGFP-Forward | 5′-CCTTTCCGGGACTTTCGCTTT-3′ |
| EGFP-Reverse | 5′-GCAGAATCCAGGTGGCAACA-3′ |
| β-actin-Forwarda | 5′-AAGAGAGGCATCCTCACCCT-3′ |
| β-actin-Reversea | 5′-TACATGGCTGGGGTGTTGAA-3′ |
| β-actin-forwardb | 5′-GGAGATTACTGCCCTGGCTCCTA-3′ |
| β-actin-Reverseb | 5′-GACTCATCGTACTCCTGCTTGCTG-3′ |
| VEGF165-Forward | 5′-ATCTTCAAGCCATCCTGTGTGC-3′ |
| VEGF165-Reverse | 5′-CAAGGCCCACAGGGATTTTC-3′ |
β-actin was used to detect the virus titer in 293T cells.
β-actin was used in RT-qPCR analysis of VEGF165 expression in MCF-7 cells. VEGF, vascular endothelial growth factor; EGFP, enhanced green fluorescent protein; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Transfection of the VEGF165 recombinant lentiviral plasmid in 293T cells
At 24 h prior to transfection, 293T cells growing in the logarithmic phase were selected and propagated. The cell number was adjusted to 6×105 cells/ml with 10% FBS DMEM. The cells were transfected at 60–70% confluence, and 2 h prior to transfection, the medium was changed to serum-free medium. The recombinant pLVX-VEGF165-EGFP-3FLAG vector and three packaging components (pRSV-REV, pMDLg-pRRE and pMD2.G) DNAs (Addgene, Inc., Cambridge, MA, USA) were added into a sterile centrifuge tube and mixed with an appropriate volume of Gibco™ Opti-MEM™ (Thermo Fisher Scientific, Inc.). The 293T cells were then co-transfected with the above DNAs using Lipofectamine 2000 according to the manufacturer's protocol (Thermo Fisher Scientific, Inc.). At 8 h post-transfection, the medium was replaced with complete culture medium, and the cells were continuously cultured for 48 h. Subsequently, the supernatant containing lentivirus particles was harvested and concentrated by ultracentrifugation at 4,000 × g for 20 min at 4°C to obtain a high-titer lentivirus concentration. 293T cells were used to measure the supernatant virus titer.
Western blot analysis of VEGF165 expression in 293T cells
Transfected and non-transfected 293T cells were harvested, and proteins were extracted using radioimmunoprecipitation assay lysis buffer (Pioneer Biotech, Co., Ltd., Shaanxi, China) containing protease inhibitors for 30 min on ice, and then cleared at 25,000 × g for 20 min at 4°C. The protein concentration was determined using the Bradford assay (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany). Upon denaturation by heating at 100°C for 5–10 min, equivalent amounts of total cellular protein were subjected to reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8–12%), followed by blotting on a polyvinylidene difluoride membrane. After blocking in 5% non-fat dry milk for 2 h, membranes were incubated with the mouse anti-VEGF monoclonal antibody (ab1316, 1:100; Abcam, Cambridge, UK), the mouse anti-GFP polyclonal antibody (sc-9996, 1:3,000; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and the mouse anti-β-actin monoclonal antibody (sc-69879, 1:1,000; Santa Cruz Biotechnology, Inc.) overnight at 4°C. The membranes were then washed with TBST three times for 5 min each, and incubated with a horseradish peroxidase (HRP)-conjugated secondary antibody (Santa Cruz Biotechnology, Inc.) for 2 h at room temperature and visualized by enhanced chemiluminescence (GE Healthcare Life Sciences, Chalfont, UK). Images were documented by a scanner, quantified using Pro-Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, USA) and analyzed using ImageJ 1.49 software (National Institutes of Health, Bethesda, MD, USA).
Detection of recombinant lentiviral titer by quantitative (q)PCR
Viral titers were measured by qPCR, using β-actin as an internal control. The quantification cycle (Cq) value was defined as the number of cycles when the fluorescent signal reached a specified threshold, as described previously (21). The sequences of the primers used are presented in Table I.
Briefly, 293T cells (1×105) were placed into each well of a 6-well plate for 24 h culture, and 10 µl of a virus stock solution was added into an Eppendorf tube containing 90 µl of cell culture medium, mixed and diluted 10X. Three different concentrations were added separately to the wells where the 293T cells were cultured. After 48-h culture, complete culture medium was added, and 4 days later, the fluorescence expression was examined. Total RNA was extracted from the cells with TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.). The reverse transcription (RT) reaction was then performed for complementary (c)DNA synthesis using the PrimeScript First Strand cDNA Synthesis kit (Takara Biotechnology Co., Ltd.). qPCR was performed using the SYBR Green PCR kit (Takara Biotechnology Co., Ltd.) and the following cycling conditions: Pre-denaturation at 95°C for 15 sec, followed by 40 cycles at 95°C for 5 sec, 60°C for 30 sec and 60°C for 30 sec.
Lentivirus infection of MCF-7 cells
Concentrated lentivirus solutions of LV-EGFP and LV-VEGF165-EGFP were added separately into two wells of cultured MCF-7 cells once the cells reached 40% confluence. Enhanced infection solution (Shanghai Sbo-bio Biotechnology) was then added to reach a total incubation volume of 2 ml. After 12 h of incubation, the cell culture medium was changed, and 72 h later, cells expressing EGFP were imaged under an inverted fluorescence microscope (Eclipse Ti-E; Nikon Corporation, Tokyo, Japan). Images were processed using NIS-Elements Basic Research Imaging Software (Nikon Corporation).
RT-qPCR and western blot analysis of VEGF165 expression in vitro
Stably transfected MCF-7 cells were selected by limiting dilution, and the expression levels of VEGF165 in the cells were measured by RT-qPCR and western blotting. Total RNA and total protein were extracted from MCF-7 cells transfected with LV-EGFP and LV-VEGF165-EGFP. Western blotting was performed as described above, and the target proteins were hybridized with mouse anti-VEGF monoclonal antibody (1:200, Abcam), mouse anti-GFP (1:1,000, ProteinTech Group, Inc., Chicago, IL, USA), mouse anti-β-actin antibody (1:5,000, ProteinTech Group, Inc.) and HRP-conjugated goat anti-mouse immunoglobulin G (1:10,000, CWBIO, Beijing, China). Enhanced chemiluminescent substrates (SuperSignal™ West Femto Maximum Sensitivity Substrate; Pierce; Thermo Fisher Scientific, Inc.) were used to detect the signals of targeted proteins.
Statistical analyses
All statistical analyses were performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL, USA). Data are expressed as the mean ± standard error of the mean. One way analysis of variance was used for comparisons among three groups. P<0.05 was considered to indicate a statistically significant difference.
Results
pLVX-VEGF165-EGFP-3FLAG plasmid construction
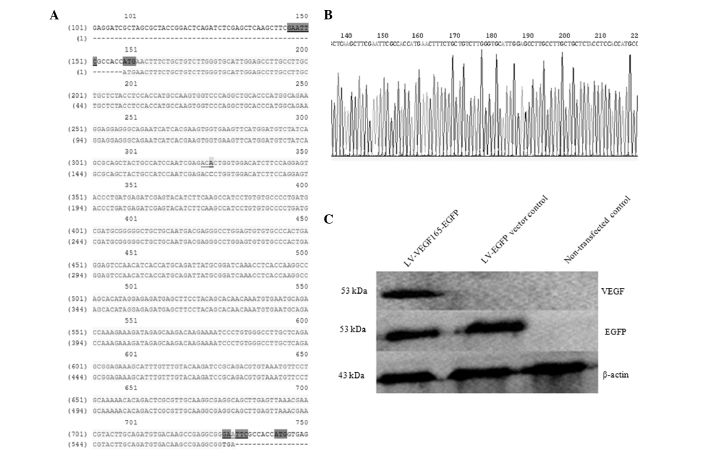
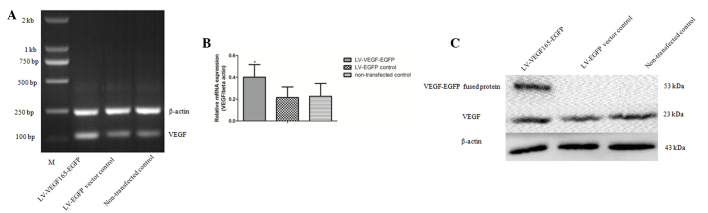
The expected PCR product size of 611 bp for the VEGF165 coding sequence was obtained (Fig. 2A), and the PCR products of VEGF165 and the pLVX-EGFP-3FLAG plasmid were then digested separately with EcoRI (Fig. 2B), followed by construction of the pLVX-VEGF-EGFP-3FLAG vector via In-Fusion™ Enzyme ligation (Clontech Laboratories, Inc.). Competent E. coli DH5α cells were transformed with the above plasmid, and three positive bacteria clones containing a fusion gene composed of the VEGF165 coding sequence and a fragment of the EGFP gene sequence were identified using PCR, which detected a product of 837 bp in size (Fig. 2C). The sequence of the recombinant plasmid pLVX-VEGF165-EGFP-3FLAG was confirmed by sequencing, and the VEGF165 sequence length was 611 bp. This sequence was consistent with that of the human VEGF165 gene published in the GenBank® database (http://www.ncbi.nlm.nih.gov/nuccore/NM_001171626.1), with the exception of a synonymous mutation from ACC to ACA, which is not expected to influence the protein synthesis (Fig. 3A and B).
Figure 2.

(A) PCR product for the VEGF165 coding sequence (611 bp). (B) Linearized pLVX-EGFP-3FLAG plasmid upon digestion with EcoRI (7.7 kb). (C) The pLVX-VEGF165-EGFP-3FLAG recombinant expression vector was identified in bacterial clones by PCR. Lane 1, blank control group; lane 2, negative control group (LV-EGFP vector, 252 bp); lane 3, DL2000 DNA Marker; lanes 4–11: eight pLVX-VEGF165-EGFP-3FLAG-transformed clones, of which, clones 6, 8 and 11 were positive clones (837-bp band). M, marker; LV, lentivirus vector; VEGF, vascular endothelial growth factor; EGFP, enhanced green fluorescent protein; PCR, polymerase chain reaction.
Figure 3.
(A) VEGF165 gene sequencing of the pLVX-VEGF165-EGFP-3FLAG vector following the selection of positive clones. (B) Sequencing waveform for the pLVX-VEGF165-EGFP-3FLAG vector. (C) Western blot analysis at 48 h post-transfection of VEGF165 recombinant lentiviral plasmid in 293T cells. The molecular weight of the fused VEG165-EGFP protein was 53 kDa; therefore, EGFP and VEGF165 were both detected in the range of 53 kDa. LV, lentivirus vector; VEGF, vascular endothelial growth factor; EGFP, enhanced green fluorescent protein.
Transfection of the VEGF165 recombinant lentiviral plasmid in 293T cells, and expression of EGFP and VEGF165
The recombinant pLVX-VEGF165-EGFP-3FLAG vector and three packaging components, pRSV-REV, pMDLg-pRRE and pMD2.G DNAs, were co-transfected in 293T cells. The expression of EGFP and VEGF165 was detected by western blotting. When incubated with an anti-VEGF antibody, the VEGF165 fusion protein at a specific band size of 53 kDa was uniquely observed in VEGF165 recombinant lentiviral-transfected 293T cells, while expression of the EGFP fusion protein (53 kDa) was observed in both the LV-VEGF165-EGFP group and the LV-EGFP group if incubated with an anti-GFP antibody. This band was consistent with the expected size of the VEGF165-EGFP fusion protein, indicating that the VEGF165 gene fused with the EGFP gene, and both could be co-expressed in 293T cells. Furthermore, the aforementioned band was not detected in non-transfected cells (Fig. 3C).
VEGF165 recombinant lentiviral packaging and determination of virus titer
To examine the virus titer of each group, qPCR was performed. The difference of Cq values was compared between the control and the test group in order to determine the titer concentration. Cq values >2.0 were considered to be significantly different. The titer of recombinant lentivirus was 5.44×107 TU/ml in the LV-VEGF165-EGFP group and 5.00×108 TU/ml in the LV-EGFP negative control group, as calculated according to the following formula: Virus titer (IU/ml) = (CxNxDx1,000)/V, where V is the volume of the diluted virus solution in µl, C is the number of virus copies integrated in each cell genome, N is the cell number and D is the dilution of the virus solution (Table II).
Table II.
Virus titers in the LV-VEGF165-EGFP and LV-EGFP groups.
| Group | V | C | N | D | Virus titera | Average titer |
|---|---|---|---|---|---|---|
| LV-VEGF165-EGFP group | ||||||
| 10 µl | 10.0 | 0.60 | 2×105 | 1 | 1.20×107 | 5.44±4.20×107 |
| 1 µl | 1.0 | 0.28 | 2×105 | 1 | 5.54×107 | |
| 10−1 µl | 0.1 | 0.05 | 2×105 | 1 | 9.60×107 | |
| LV-EGFP group | ||||||
| 10 µl | 10.0 | 15.56 | 2×105 | 1 | 3.12×108 | 5.00±1.85×108 |
| 1 µl | 1.0 | 3.41 | 2×105 | 1 | 6.82×108 | |
| 10−1 µl | 0.1 | 0.25 | 2×105 | 1 | 5.06×108 |
Virus titer (IU/ml) = (C × N × D × 1,000) / V. V, volume of the diluted virus solution (µl); C, number of virus copies integrated in each cell genome; N, cell number; D, dilution of the virus solution. LV, lentivirus vector; VEGF, vascular endothelial growth factor; EGFP, enhanced green fluorescent protein.
Observation of cellular localization of VEGF165 expression in MCF-7 cells by fluorescence microscopy
After 72 h of transfection, MCF-7 cells were observed under a fluorescence microscope to assess the expression of EGFP. Both LV-EGFP and LV-VEGF165-EGFP groups demonstrated transfection efficiencies >80%. As represented in Fig. 4A-F, EGFP was highly expressed in MCF-7 cells transfected with LV. By contrast, no EGFP signal was detected in non-transfected cells. Fluorescence microscopy was used to observe the cellular localization of VEGF165 in MCF-7 cells. As represented in Fig. 4G and H, VEGF165-EGFP was mainly expressed in the cell membrane and nucleus of MCF-7 cells, and certain expression was also noticed outside the cell membrane. This indicates that the VEGF165-EGFP fusion protein could be expressed and secreted normally.
Figure 4.
Expression of recombinant lentivirus carrying the VEGF165-EGFP fusion gene in MCF-7 cells after 72 h of transfection. Non-transfected MCF-7 cells in (A) bright-field and (B) dark-field (magnification, ×100). LV-EGFP-transfected MCF-7 cells in (C) bright-field and (D) dark-field (magnification, ×100). LV-VEGF165-EGFP-transfected MCF-7 cells in (E) bright-field and (F) dark-field (magnification, ×200). Cells transfected with LV-EGFP and LV-VEGF165-EGFP expressed high levels of EGFP, indicating successful transfection of lentivirus in cultured MCF-7 cells. (G) LV-VEGF165-EGFP transfected MCF-7 cells in dark-field (magnification, ×400). (H) Observation of cellular localization of VEGF165 expression in MCF-7 cells (magnification, ×400). LV, lentivirus vector; VEGF, vascular endothelial growth factor; EGFP, enhanced green fluorescent protein.
Detection of the VEGF165 expression in MCF-7 cell lines by RT-qPCR and western blotting
RT-qPCR and western blotting were applied to detect the RNA and protein expression levels of VEGF165-EGFP, respectively. RT-qPCR demonstrated that the expression of VEGF165 in the LV-VEGF165-EGFP group was significantly increased (relative gray value of PCR, 0.4019±0.1143 and 0.2147±0.0965 in the LV-VEGF165-EGFP and LV-EGFP groups, respectively; Fig. 5A and B). Similarly, compared with non-transfected cells, a characteristic band of 53 kDa was observed by western blotting in MCF-7 cells transfected with LV-VEGF165-EGFP, whose size was consistent with that of the VEGF165-EGFP fusion protein (Fig. 5C), indicating that the VEGF165-EGFP fusion gene in the recombinant LV could be expressed following transfection into MCF-7 cells.
Figure 5.
Detection of mRNA and protein levels of VEGF165 in MCF-7 cells following transfection. (A) Polymerase chain reaction analysis of VEGF165 mRNA levels in MCF-7 cells upon transfection. (B) Histogram of VEGF165 mRNA levels in MCF-7 cells. Compared with non-transfected cells and cells transfected with LV-EGFP, cells transfected with LV-VEGF165-EGFP contained significantly higher amounts of VEGF mRNA. Columns indicate means; bars indicate standard error. *P<0.05 vs. control. (C) Western blot analysis of VEGF165-EGFP fusion protein upon transfection. VEGF165-EGFP fusion protein could only be detected in the LV-VEGF165-EGFP group, while normal VEGF could be detected in all the three groups. LV, lentivirus vector; VEGF, vascular endothelial growth factor; EGFP, enhanced green fluorescent protein; mRNA, messenger RNA.
Discussion
It has been reported that different isoforms of VEGF are detected in individual tissues; however, VEGF165 is the most abundant one in almost all human organs with the exception of the lung (22). Several studies revealed that VEGF165 was highly expressed in breast cancer tissues compared with adjacent tissues (23,24). RT-qPCR experiments demonstrated that four isoforms of VEGF were expressed in human breast cancer tissues, including VEGF121, VEGF145, VEGF165 and VEGF189, being VEGF165 the most abundant one (25). In addition, previous studies demonstrated that VEGF165 fused to GFP at its C-terminus was secreted and biologically active (26). Therefore, in order to elucidate the function of tumor cell derived-VEGF in breast cancer biology, VEGF165 was selected as the target gene in the present study, and the VEGF165-EGFP fusion gene expression vector was constructed.
As a newly developed technology, recombinant expression through viral vectors have been used in numerous laboratory experiments and clinical trials (27–29). There are mainly five types of viral vectors that are commonly used for recombinant expression, namely adenovirus, adeno-associated virus, herpes simplex virus, retrovirus and lentivirus (14). Due to the wide range of host cells, the ability to infect both replicating and non-replicating cells, the easy integration of the exogenous gene in the host cells, and the long-lasting and stable expression of exogenous genes, lentivirus was the most suitable type of vector for our experiments (14,30). In the present study, a VEGF165-EGFP fusion gene recombinant lentiviral expression vector was first constructed. Using molecular cloning techniques such as PCR and DNA sequencing, the human VEGF165-EGFP fusion gene lentiviral expression plasmid was successfully constructed, and high-titer viral particles were obtained following plasmid transfection in packaging cells. Due to its safety and high transfection efficiency (31), a four-plasmid lentivirus packaging system was employed in the current study, including a carrier plasmid containing the VEGF165-EGFP fusion gene, a packaging plasmid pRSV-Rev encoding Rev response element (RRE), a packaging plasmid pMDLg-pRRE containing the Gag-Pol coding sequence, and a packaging plasmid pMD2.G encoding the vesicular stomatitis virus (VSV)-G envelope protein. In this packaging system, the RRE and Gag-Pol coding sequences are located in two different plasmids, which greatly reduces the possibility of autonomous viral replication (31,32). Furthermore, the human immunodeficiency virus-derived Env gene was replaced by the VSV-G gene in our packaging system, which significantly increased the range of host cell types that could be infected.
The VEGF165-EGFP expression rate was >80% in infected human breast cancer cells MCF-7. Furthermore, it was observed that VEGF165 was mainly located on the cell membrane and nucleus of MCF-7 cells by fluorescence microscopy. The constructed VEGF165 lentiviral recombinant vector will be used in future studies to evaluate the function of the target gene, following its stable transfection in tumor cell lines. This will enable a detailed examination of the mechanism of tumor cell derived-VEGF165 in tumor cells and in the tumor microenvironment. Besides, this fusion gene expression vector may also provide a potential approach for gene therapy in diseases that require regulation of angiogenesis.
Acknowledgements
The present study was supported by Specialized Research Fund for the Doctoral Program of Higher Education of China (Beijing, China; grant no. 20100201110059).
References
- 1.Lichtenberger BM, Tan PK, Niederleithner H, Ferrara N, Petzelbauer P, Sibilia M. Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. 2010;140:268–279. doi: 10.1016/j.cell.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 2.Ribatti D, Nico B, Crivellato E, Roccaro AM, Vacca A. The history of the angiogenic switch concept. Leukemia. 2006;21:44–52. doi: 10.1038/sj.leu.2404402. [DOI] [PubMed] [Google Scholar]
- 3.Roskoski R., Jr Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol. 2007;62:179–213. doi: 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacGabhann F, Popel AS. Systems biology of vascular endothelial growth factors. Microcirculation. 2008;15:715–738. doi: 10.1080/10739680802095964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vempati P, Popel AS, Mac GF. Extracellular regulation of VEGF: Isoforms, proteolysis and vascular patterning. Cytokine Growth Factor Rev. 2014;25:1–19. doi: 10.1016/j.cytogfr.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otrock ZK, Makarem JA, Shamseddine AI. Vascular endothelial growth factor family of ligands and receptors: Review. Blood Cells Mol Dis. 2007;38:258–268. doi: 10.1016/j.bcmd.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 11.Knizetova P, Ehrmann J, Hlobilkova A, Vancova I, Kalita O, Kolar Z, Bartek J. Autocrine regulation of glioblastoma cell-cycle progression, viability and radioresistance through the VEGF-VEGFR2 (KDR) interplay. Cell Cycle. 2008;7:2553–2561. doi: 10.4161/cc.7.16.6442. [DOI] [PubMed] [Google Scholar]
- 12.Chakraborty G, Jain S, Kundu GC. Osteopontin promotes vascular endothelial growth factor-dependent breast tumor growth and angiogenesis via autocrine and paracrine mechanisms. Cancer Res. 2008;68:152–161. doi: 10.1158/0008-5472.CAN-07-2126. [DOI] [PubMed] [Google Scholar]
- 13.Hamerlik P, Lathia JD, Rasmussen R, Wu Q, Bartkova J, Lee M, Moudry P, Bartek J, Jr, Fischer W, Lukas J, et al. Autocrine VEGF–VEGFR2–Neuropilin-1 signaling promotes glioma stem-like cell viability and tumor growth. J Exp Med. 2012;209:507–520. doi: 10.1084/jem.20111424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vannucci L, Lai M, Chiuppesi F, Ceccherini-Nelli L, Pistello M. Viral vectors: A look back and ahead on gene transfer technology. New Microbiol. 2013;36:1–22. [PubMed] [Google Scholar]
- 15.Nathwani AC, Tuddenham EGD, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia b. N Engl J Med. 2011;365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacca M, Zacchigna S. Virus-mediated gene delivery for human gene therapy. J Control Release. 2012;161:377–388. doi: 10.1016/j.jconrel.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Huang S, Kamihira M. Development of hybrid viral vectors for gene therapy. Biotechnol Adv. 2013;31:208–223. doi: 10.1016/j.biotechadv.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Lehto T, Simonson OE, Mager I, Ezzat K, Sork H, Copolovici D, Viola JR, Zaghloul EM, Lundin P, Moreno PM, et al. A peptide-based vector for efficient gene transfer in vitro and in vivo. Mol Ther. 2011;19:1457–1467. doi: 10.1038/mt.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: Progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 20.Heilbronn R, Weger S. Viral vectors for gene transfer: Current status of gene therapeutics. Handb Exp Pharmacol. 2010:143–170. doi: 10.1007/978-3-642-00477-3_5. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Zeng X, Liu Y, Liang C, Zhang H, Liu C, Du W, Zhang Z. Construction and characterization of a PDCD5 recombinant lentivirus vector and its expression in tumor cells. Oncol Rep. 2012;28:91–98. doi: 10.3892/or.2012.1756. [DOI] [PubMed] [Google Scholar]
- 22.Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J Cell Sci. 2001;114:853–865. doi: 10.1242/jcs.114.5.853. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh S, Sullivan CA, Zerkowski MP, Molinaro AM, Rimm DL, Camp RL, Chung GG. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum Pathol. 2008;39:1835–1843. doi: 10.1016/j.humpath.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshiji H, Gomez DE, Shibuya M, Thorgeirsson UP. Expression of vascular endothelial growth factor, its receptor, and other angiogenic factors in human breast cancer. Cancer Res. 1996;56:2013–2016. [PubMed] [Google Scholar]
- 25.Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871–882. doi: 10.1038/nrc3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzmán-Hernández ML, Potter G, Egervári K, Kiss JZ, Balla T. Secretion of VEGF-165 has unique characteristics, including shedding from the plasma membrane. Mol Biol Cell. 2014;25:1061–1072. doi: 10.1091/mbc.E13-07-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kotterman MA, Schaffer DV. Engineering adeno-associated viruses for clinical gene therapy. Nat Rev Genet. 2014;15:445–451. doi: 10.1038/nrg3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahn M, Ron S, Yizhar O. Viral vector-based techniques for optogenetic modulation in vivo. In: Brambilla R, editor. Viral Vector Approaches in Neurobiology and Brain Diseases. Humana Press; Totowa, NJ: 2014. pp. 289–310. [DOI] [Google Scholar]
- 29.Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2013;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller AD, Rosman GJ. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989;7:980–990. [PMC free article] [PubMed] [Google Scholar]
- 31.Maetzig T, Galla M, Baum C, Schambach A. Gammaretroviral vectors: Biology, technology and application. Viruses. 2011;3:677–713. doi: 10.3390/v3060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc Natl Acad Sci U S A. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]