Abstract
Accumulating evidence suggests that autophagy is closely related to the pathogenesis of osteoarthritis (OA). The aim of this study was to determine the changes in autophagy during the progression of OA and to elucidate the specific role of autophagy in OA. For this purpose, a cellular model of OA was generated by stimulating SW1353 cells with interleukin (IL)-1β and a rabbit model of OA was also established by an intra-articular injection of collagenase, followed by treatment with the autophagy specific inhibitor, 3-methyladenine (3-MA). Cell viability was analyzed by MTS assay, and the mRNA expression levels of matrix metalloproteinases (MMP)-13 and tissue inhibitor of metalloproteinase (TIMP)-1 were determined by RT-qPCR. Cartilage degeneration was examined under a light microscope, and autophagosome and chondrocyte degeneration was observed by transmission electron microscopy. The protein expression of Beclin-1 and light chain 3 (LC3)B was evaluated by western blot analysis and immunofluorescence staining. We found that the autophagy was enhanced during the early stages and was weakened during the late stages of experimental OA. The inhibition of autophagy by 3-MA significantly aggravated the degeneration of chondrocytes and cartilage in experimental OA. Our results thus determine the changes in autophagy during different stages of OA, as well as the role of impaired autophagy in the development of OA. Our data suggest that the regulation of autophagy may be a potential therapeutic strategy with which to attenuate OA.
Keywords: autophagy, osteoarthritis, cartilage degeneration, interleukin-1β, collagenase, 3-methyladenine
Introduction
Osteoarthritis (OA) is a degenerative joint disease that severly affects the quality of life of patients (1). Traditional treatments only temporally attenuate the clinical symptoms, but do not effectively inhibit the pathological progression of OA. It is therefore important to elucidate the mechanisms responsible for OA and to identify safe and effective treatments for OA.
Articular cartilage degeneration is the main pathological change associated with OA (2). A variety of cytokines, growth factors and enzymes, such as interleukin (IL)-1β (3-5) and collagenase (6–8) are involved in articular cartilage degeneration. IL-1β is secreted from synovial cells and inflammatory cells in OA, and stimulates the production of proteolytic enzymes, such as stromelysin and collagenase, causing synovial inflammation and bone resorption (5). Collagenase is upregulated in OA-affected cartilage and animal models of OA have been successfully established by an intra-articular injection of collagenase (6–8). In addition, matrix metalloproteinases (MMPs) have been shown to play an important role in the pathogenesis of OA (9), and the activity of MMPs is regulated by tissue inhibitors of metalloproteinase (TIMP)-1 in OA (10). In our previous study, we demonstrated that inflammatory cytokines increase MMP-13 secretion, which in turn promotes the degradation of type II collagen in chondrocytes, resulting in the occurrence of OA (11).
The death of chondrocytes is a hallmark of cartilage degeneration in OA; however, the mechanisms responsible for chondrocyte death in OA-affected cartilage remain largely unknown. Autophagy plays a crucial role in maintaining cellular metabolism and homeostasis (12,13). However, excessive autophagy may lead to cell death (14,15). Autophagy is regulated by a series of autophagy-related genes (ATGs) (16), such as Beclin-1 (17) and light chain 3 (LC3) (18). The expression levels of these genes are commonly used to monitor autophagic activity and flux (19). LC3 is present in two forms (LC3-I and LC3-II) and several isoforms (LC3A, LC3B, LC3C). The conversion of LC3-I to LC3-II is an indicator of autophagosome formation (20). Accumulating evidence suggests that the deregulation of autophagy is closely related to the pathogenesis of OA (21–24). Some researchers have reported that autophagy is enhanced in OA-affected cartilage (25); however, others have reported that autophagy is significantly reduced in OA-affected cartilage, and the age-related loss of autophagy has been linked to cell death in OA-affected cartilage (26).
In order to elucidate the changes in autophagy occurring during the progression of OA and the specific role of autophagy in OA, in this study, we established a cellular model of OA by treating SW1353 chondrosarcoma cells with IL-1β. In addition, we created a rabbit model of OA by an intra-articular injection of collagenase, followed by treatment with the autophagy specific inhibitor, 3-methyladenine (3-MA). We then observed the degeneration of cells and cartilage and detected the levels of autophagy at different time points. The results demonstrated that autophagy was enhanced during the early stages of experimental OA and was weakened during the late stages; the inhibition of autophagy aggravated the degeneration of chondrosarcoma cells and cartilage. Our results determine the changes of autophagy during the different stages of OA and the role of impaired autophagy in the development of OA, suggesting that the regulation of autophagy may be a potential therapeutic strategy with which to attenuate OA.
Materials and methods
Cell culture and treatment
SW1353 human chondrosarcoma cells were purchased from the Institute of Life Science Cell Culture Center (Shanghai, China) and were cultured in α-minimal essential medium (α-MEM; HyClone, Logan, UT, USA) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, USA), 100 IU/ml penicillin and 100 μg/ml streptomycin (Gibco). The α-MEM contained RNA and DNA, 2 mM L-glutamine and 1 mM sodium pyruvate, but no vitamin C or glucose. The cells were incubated at 37°C in humidified air containing 5% CO2. Upon reaching 80% confluence, the cells were pre-treated with or without the autophagy inhibitor, 3-MA at 5 mmol/l (Sigma-Aldrich, St. Louis, MO, USA). One hour after pre-treatment, the cells were stimulated with 10 ng/ml IL-1β (PeproTech, Inc., Rocky Hill, NJ, USA) for 12, 24, 36 and 48 h, separately. The cells that were not exposed to 3-MA and IL-1β were used as controls.
Cell viability analysis
The viability of thecells was assessed by MTS assay. The SW1353 cells were seeded on 96-well plates (5,000 cells/well) and incubated at 37°C for 24 h, followed by pre-treatment with or without 3-MA (5 mmol/l) for 1 h, and then stimulation with IL-1β (10 ng/ml). The wells that were treated without 3-MA and IL-1β were used as controls. Each sample concentration was replicated 6 times. Following incubation for 0, 12, 24, 36, and 48 h, 10 μl of MTS (Sigma-Aldrich) were added to each well and the cells were incubated at 37°C for a further 2 h. The absorbance was measured using a multifunctional microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 490 nm. The relative cell viability (RCV) to the controls was calculated using the following equation: RCV = (ODtest/ODcontrol) x100%.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's instructions. First-strand cDNA was synthesized using the ReverTra Ace reverse transcriptase cDNA synthesis kit (Applied Biosystems Inc., Foster City, CA, USA). Quantitative PCR (qPCR) was performed using a 7500 real-time PCR system with SYBR-Green (both from Applied Biosystems Inc.). The forward and reverse primers used were as follows: MMP-13, 5′-CGACTTCTACCCATTTGA-3′ and 5′-TAGCCTTTGGAACTACTTGTC-3′; TIMP-1, 5′-AGATAGCCTGAATCCTGCC-3′ and 5′-CTGGGTGGTAACTCTTTATTTC-3′; β-actin, 5′-TCGACAACGGCTCCGGCAT-3′ and 5′-AAGGTGTGGTGCCAGATTTTC-3′, respectively. β-actin was used as an internal control. Each experiment was repeated 3 times in triplicate. The relative expression of target genes was calculated using the 2−ΔΔCq method.
Immunofluorescence
The cells were seeded at 1×105/ml on a glass coverslip into a 6-well plate. Following treatment, the cells were fixed with 4% cold paraformaldehyde (Solarbio, Beijing, China) for 30 min and permeabilized with 0.5% T riton X-100 (Sigma-Aldrich) in PBS for 5 min, followed by PBS washing 3 times. After blocking for 30 min with 2 ml of PBS-0.5% Triton-10% goat serum (Gibco), the cells were incubated with rabbit anti-LC3B antibody (1:400; Cat. no. 2775; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight. Following 3 washes with PBS, the cells were incubated with Alexa Fluor 555 goat anti-rabbit secondary antibody (1:1,000 dilution; Cat. no. ZB-2301; Zhongshan Golden Bridge Biotechnology, Beijing, China) at room temperature for 2 h followed by 3 separate PBS washes. Subsequently, DAPI staining (Cat. no. C1006; Beyotime Biotechnology, Shanghai, China) was used to stain the nuclei, and after rinsing with ddH2O, the slide was mounted with anti-fading agent and examined using a confocal microscope (Olympus Corp., Tokyo, Japan) to quantify the expression of LC3B protein.
Rabbit model of OA
A total of 54 healthy New Zealand white male rabbits (3 months old; weighing 2.30–2.56 kg) were obtained from the Animal Center of Capital Medical University (Beijing, China). All animal experiments were performed following the approval of the Ethics Committee of Capital Medical University. After 4 weeks, the rabbits were anesthetized with 10% chloral hydrate (2 ml/kg body weight) by an intraperitoneal injection. Subsequently, the first experimental group (collagenase-injected group) received an intra-articular injection in the right knee joint with 0.5 ml saline containing 2.0 mg/ml of collagenase (type II; Sigma-Aldrich), and the second experimental group (3-MA-treated group) received an intra-articular injection in the right knee joint with 0.5 ml saline containing 2.0 mg/ml of collagenase combined with 5 mmol/l 3-MA. The injection was administered on days 1 and 4 (injected twice) as previously described (6,27). The control group (rabbits injected twice with 0.5 ml normal saline) contained 6 rabbits, and the collagenase-injected experimental group and the 3-MA-treated experimental group each contained 24 rabbits. The rabbits from the control group were sacrificed at 8 weeks, while the rabbits from the collagenase-injected experimental group and 3-MA-treated experimental group were averagely sacrificed at 2, 4, 6 and 8 weeks.
Histological evaluation
Following sacrifice, the lateral femoral condyles of the rabbits were removed and fixed in 10% neutral buffered formalin (pH 7.4; VWR International, Radnor, PA, USA) for 48 h and decalcified with 20% EDTA (Gibco). This was followed by dehydration through a series of increasing concentrations of ethanol. The samples were paraffin-embedded, and 5-μm-thick microsections [created using a microtome (Leica RM2145; Leica, Wetzlar, Germany)] were stained with Safranin-O (Cat. no. G2540; Solarbio). The grading of Safranin-O staining was evaluated by blinded individuals according to the Mankin scoring system (28).
Transmission electron microscopy
The medial femoral condyles were first fixed with 2.5% glutaraldehyde in PBS (pH 7.0) for 48 h. This was followed by 3 PBS washing steps, and decalcification with 20% EDTA. The samples were further fixed with 1% OsO4 in PBS (pH 7.0) for 2 h and washed 3 times in PBS, and were then dehydrated with a series of ethanol concentrations (50, 70, 90, and 100%) for 15 min intervals. The samples were subsequently incubated in a mixture of alcohol and isoamyl acetate (v:v=1:1) for 30 min, followed by incubation with pure isoamyl acetate for 1 h. Finally, the samples were coated with gold-palladium, cut into ultrathin sections, and observed using a transmission electron microscope (TEM; Hitachi, Tokyo, Japan).
Western blot analysis
The cells were washed in ice-cold PBS and lysed in total protein lysis buffer (Sigma-Aldrich) supplemented with 1 mg/ml protease inhibitor cocktail (Roche, Indianapolis, IN, USA) at 4°C for 30 min. Cartilage from the tibial plateaus was cut into 1-mm-thin slices, and 200 mg of frozen cartilage was pulverized in liquid nitrogen. The cartilage dry weight was measured, and the same amount of cartilage was resuspended in total protein lysis buffer supplemented with 1 mg/ml protease inhibitor cocktail in order to attain homogenates using the T10 tissue homogenizer (IKA, Staufen, Germany). The homogenates were incubated at 4°C for 30 min. The samples were then centrifuged at 15,000 rpm for 30 min, and the supernatants were harvested to measure protein concentrations using the bicinchoninic acid reagent assay (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Equal amounts of protein were heated at 99°C for 10 min, and separated using Tris-glycine gels (Sigma-Aldrich). The proteins were then transferred onto nitrocellulose membranes (Millipore Corp., Billerica, MA, USA). After blocking with 5% dry milk in Tris-buffered saline Tween-20 (TBST; Merck Millipore) for 2 h, the membranes were incubated at 4°C (overnight) with rabbit polyclonal antibody specific for Beclin-1 (Cat. no. 3738) and LC3B (Cat. no. 2775) (Cell Signaling Technology), and mouse monoclonal antibody specific for β-actin (sc-69879; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Following three wash steps with TBST, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit (Cat. no. ZB-2301) or goat anti-mouse (Cat. no. ZB-5305) IgG (Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China) for 2 h. The membranes were washed 3 times with TBST, and subjected to signal development using enhanced chemiluminescence (ECL) substrate (Thermo Fisher Scientific, Inc.).
Statistical analysis
The data are expressed as the means ± standard deviation (SD). Statistical analysis was performed using one-way ANOVA and the Student's t-test using SPSS statistical software 19.0 (SPSS Inc., Chicago, IL, USA). P-values <0.05 were considered to indicate statistically significant differences.
Results
Cell viability
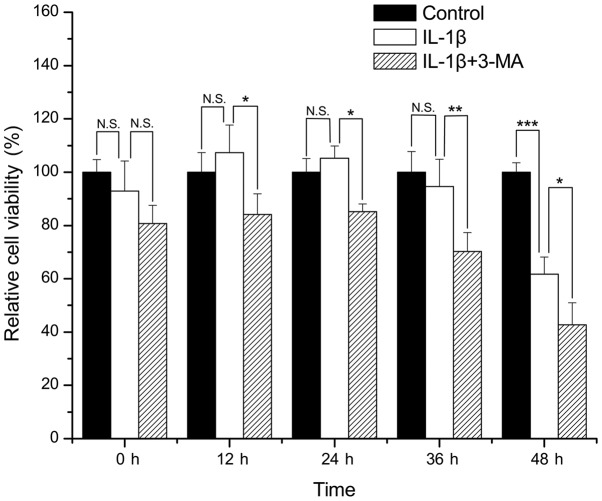
Compared with the control group, there was no apparent loss of viability in the SW1353 cells treated with IL-1β for 0, 12, 24 and 36 h (P=0.365, 0.402, 0.600 and 0.582, respectively). However, treatment of the SW1353 cells with IL-1β for 48 h resulted in a significant loss of cell viability when compared with the control group (P<0.001). The viability of the cells treated with IL-1β in combination with 3-MA was significantly decreased when compared with that of the cells treated only with IL-1β at 12, 24, 36 and 48 h (P=0.012, 0.024, 0.007 and 0.043 respectively) (Fig. 1).
Figure 1.
Comparison of the cell viability in interleukin (IL)-1β-stimulated SW1353 cells between the control and 3-methyladenine (3-MA)-treated cells. *P<0.05, **P<0.01, ***P<0.001. N.S., not significant.
Expression of MMP-13 and TIMP-1
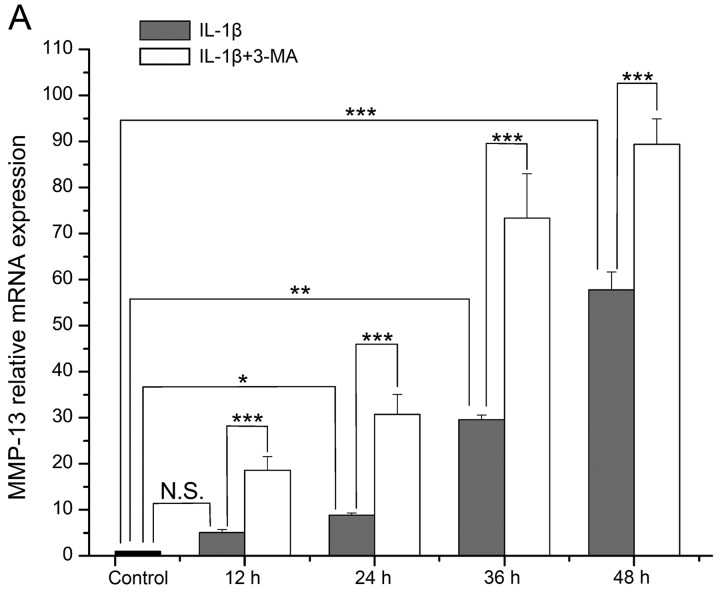
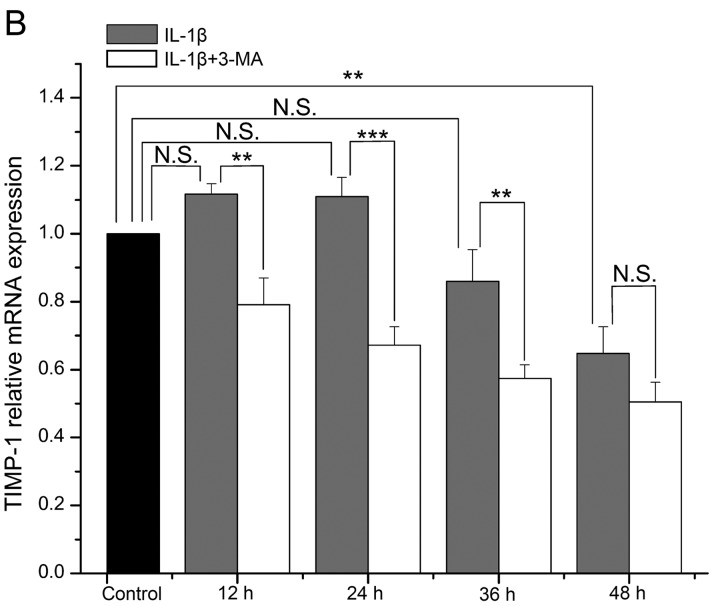
The mRNA level of MMP-13 in the IL-1β-stimulated SW1353 cells became increasingly higher with time, and it was increased at each time point when compared with the normal cells (P=0.078, 0.024, 0.002 and <0.001 respectively). The increase in MMP-13 expression was significantly enhanced by 3-MA in comparison with the cells treated with IL-1β only (all P<0.001) (Fig. 2A). The mRNA level of TIMP-1 in the IL-1β-stimulated SW1353 cells was highest at 12 h, and then it decreased with time. It was increased at 12 and 24 h (P=0.071 and 0.067), while it was decreased at 36 and 48 h when compared with the normal cells (P=0.071 and 0.001). In addition, the decrease in TIMP-1 expression was enhanced by 3-MA in comparison with cells treated with IL-1β only (P=0.001, <0.001, 0.003 and 0.057 respectively) (Fig. 2B).
Figure 2.
(A) Comparison of the mRNA expression level of matrix metalloproteinase (MMP)-13 in interleukin (IL)-1β-stimulated SW1353 cells between the control and 3-methyladenine (3-MA)-treated cells. (B) Comparison of the mRNA expression level of tissue inhibitor of metalloproteinase (TIMP)-1. *P<0.05, **P<0.01, ***P<0.001. N.S., not significant.
Expression of Beclin-1 and LC3B in IL-1β-stimulated SW1353 cells
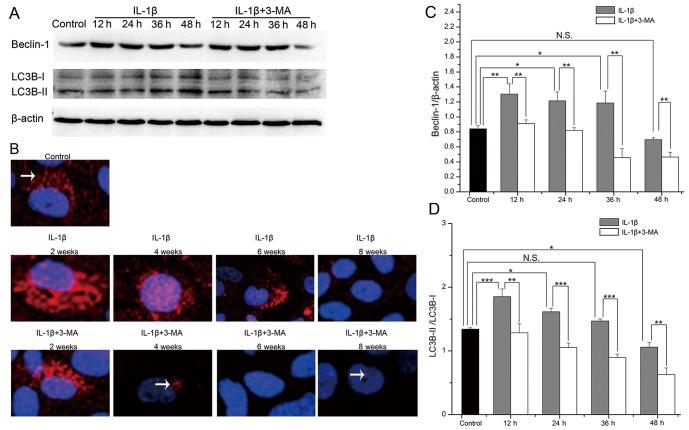
To detect the levels of autophagy in the IL-1β-stimulated SW1353 cells, we evaluated the protein expression levels of Beclin-1 and LC3B by western blot analysis (Fig. 3A). In the IL-1β-stimulated SW1353 cells, the expression levels of Beclin-1 and the LC3B-II/LC3B-I ratio were both highest at 12 h, and they then decreased with time. Both of these were increased at 12 (P=0.007 and <0.001), 24 (P=0.029 and 0.013) and 36 h (P=0.045 and 0.364), while they were decreased at 48 h (P=0.631 and 0.010) in the IL-1β-stimulated SW1353 cells when compared with the normal cells. At each time point, the expression levels of Beclin-1 (P=0.009, 0.005, 0.003 and 0.005 respectively) and the LC3B-II/LC3B-I ratio (P=0.006, <0.001, <0.001 and 0.004 respectively) were both decreased in the cells treated with 3-MA when compared with the cells treated only with IL-1β (Fig. 3C and D). We further evaluated the protein expression of LC3B by immunofluorescence and found that the results observed were the same as those of western blot analysis (Fig. 3B). These results demonstrated that autophagy was first enhanced and then weakened in the IL-1β-stimulated SW1353 cells, and the expression levels of Beclin-1 and the LC3B-II/LC3B-I ratio were inhibited by 3-MA treatment.
Figure 3.
(A) The protein expression levels of Beclin-1 and light chain 3 (LC3) B evaluated by western blot analysis in interleukin (IL)-1β-stimulated SW1353 cells, control and 3-methyladenine (3-MA)-treated cells. (B) The protein expression of LC3B (white arrows) evaluated by immunofluorescence. (C and D) Comparison of the protein expression level of Beclin-1 and the LC3B-II/LC3B-I ratio. *P<0.05, **P<0.01, ***P<0.001. N.S., not significant.
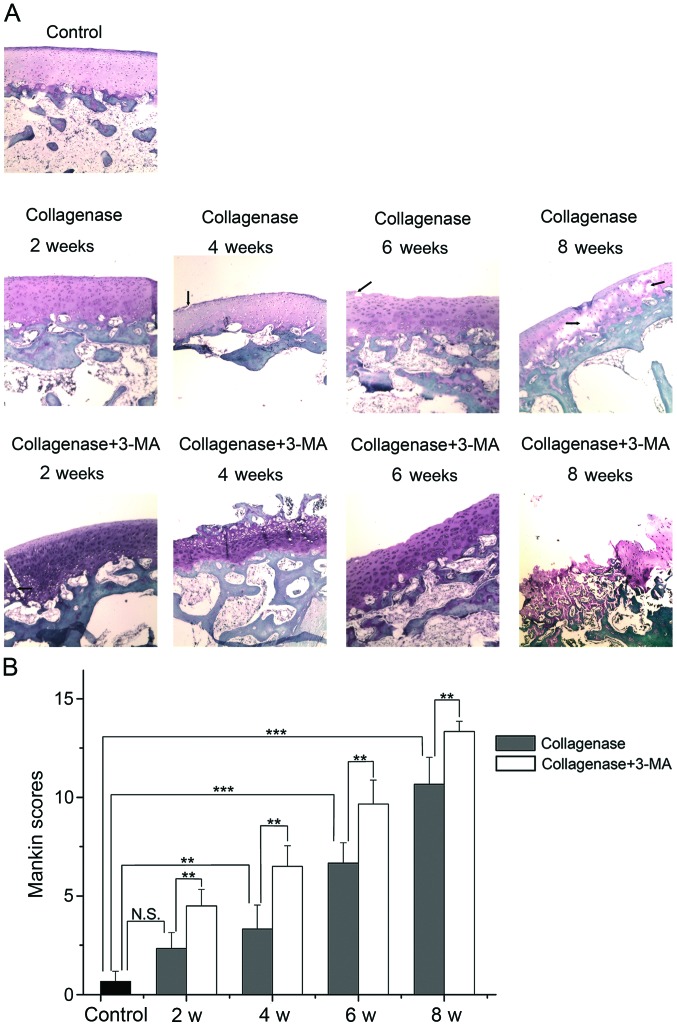
Histological evaluation
The cartilage of the lateral femoral condyles was stained with Safranin-O and examined under a light microscope (Fig. 4A), and the average scores of Safranin-O staining were evaluated by blinded observers (Fig. 4B). Photomicrographs of the cartilage revealed that the cartilage of the control group was not degenerated. In the collagenase-injected group, the cartilage surface was slightly irregular and the slight loss of Safranin-O staining was observed at 2 weeks after the injection. At 4 weeks, clefts were observed in the transitional zone, and cell proliferation and relatively reduced Safranin-O staining ability were observed in the transitional and radial zone. At 6 weeks, clefts and the disappearance of cells in the transitional zone, and reduced Safranin-O staining were evident. At 8 weeks, the loss of cartilage extended to the radial zone, and clefts and reduced staining of Safranin-O were apparent. The extent of OA was presented as Mankin scores (higher score, greater degeneration of cartilage). According to the summed score, the collagenase-injected group developed OA in a time-dependent manner and had a significantly higher score than the control group at 4, 6 and 8 weeks (P=0.004, <0.001 and <0.001 respectively). The score in the 3-MA-treated group was significantly higher than the collagenase-injected group at each time point (all P=0.001). These results indicated that the inhibition of autophagy by 3-MA significantly enhanced the collagenase-induced cartilage degeneration of OA.
Figure 4.
Histological evaluation. (A) Representative images of Safranin-O staining from control, collagenase-injected and 3-methyladenine (3-MA)-treated rabbits. (B) Graph indicating the Mankin scores. **P<0.01, ***P<0.001. N.S., not significant. Clefts in images are marked with black arrows (magnification, ×40).
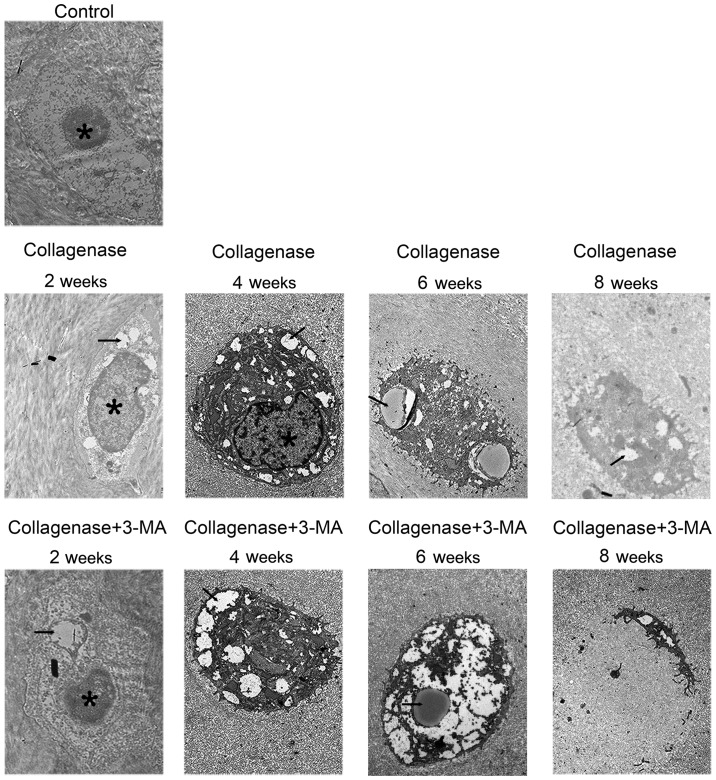
Transmission electron microscopy
The cartilage of the medial femoral condyles was examined by a TEM to observe autophagosomes and chondrocyte degeneration (Fig. 5). In the control group, chondrocytes containing round nuclei were observed in the lacunae. In the collagenase-injected group, much more autophagosomes in chondrocytes were observed at 2 and 4 weeks, and the autophagosomes became less at 6 and 8 weeks. Furthermore, chondrocyte degeneration increased with time. In addition, abundant rough endoplasmic reticulum and other organelles were observed in the cytoplasm. In the cartilage from the collagenase-injected group at 8 weeks, condensed chondrocytes with several autophagosomes were observed. In the 3-MA-treated group at all 4 time points, autophagy in chondrocytes was less and chondrocyte degeneration was greater than the collagenase-injected group. In the cartilage from the 3-MA-treated group at 8 weeks, apparent chondrocyte lysis and cell debris were observed in the lacunae of cellular materials.
Figure 5.
Representative images of transmission electron microscopy from control, collagenase-injected and 3-methyladenine (3-MA)-treated rabbits. Autophagosomes and chondrocyte degeneration were observed. Autophagosomes in images are marked with black arrows, the nuclei are marked with an asterisks (*). Scale bar, 1 μm.
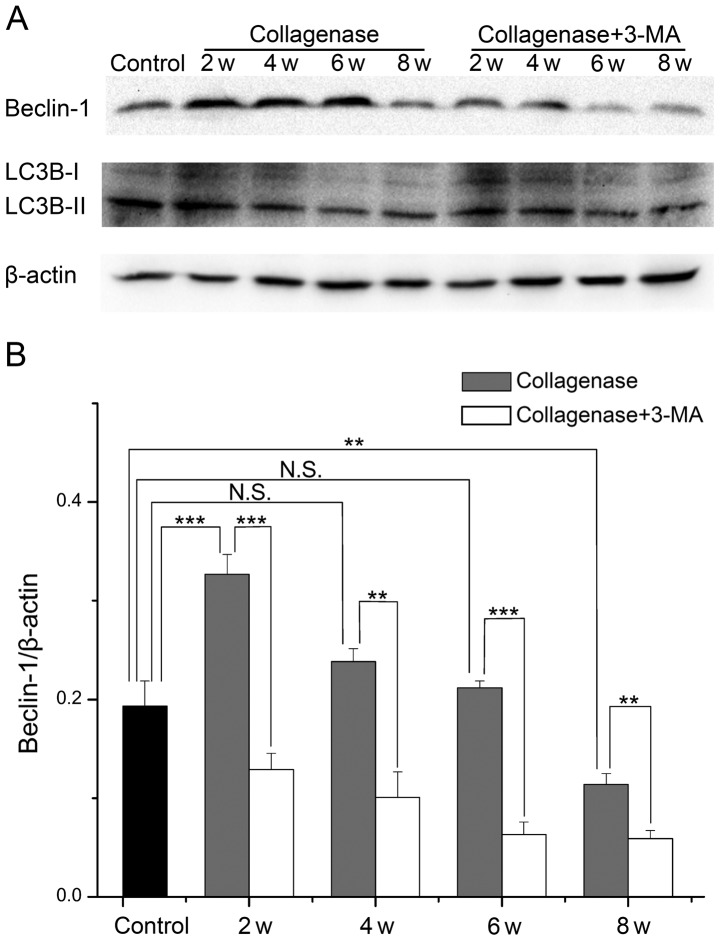
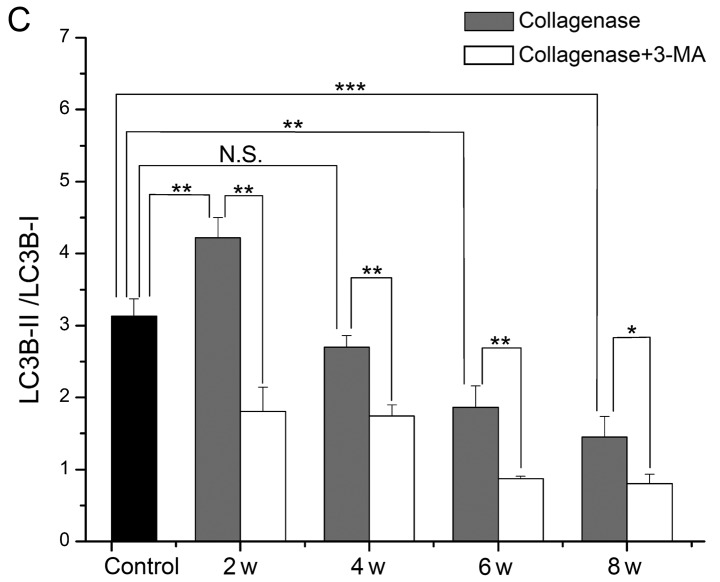
Expression of Beclin-1 and LC3B in cartilage from rabbit with OA
To further investigate the changes in autophagy and the role of impaired autophagy in the pathological process of OA, we evaluated the protein expression levels of Beclin-1 and LC3B in cartilage from rabbits with OA by western blot analysis (Fig. 6A). In cartilage from the collagenase-injected group, the expression levels of Beclin-1 and the LC3B-II/LC3B-I ratio were both highest at 2 weeks, and they then decreased with time. The expression level of Beclin-1 was increased at 2, 4 and 6 weeks (P<0.001, 0.094 and 0.768, respectively), and it was decreased at 8 weeks (P=0.003) compared with the cartilage from the control group. The LC3B-II/LC3B-I ratio was only increased at 2 weeks (P=0.007), and decreased at 4, 6 and 8 weeks (P=0.437, 0.002 and <0.001, respectively). At each time point, the expression levels of Beclin-1 and the LC3B-II/LC3B-I ratio were decreased in cartilage from the 3-MA-treated group compared to cartilage from the collagenase-injected group. These results demonstrated that autophagy was first enhanced and then weakened in cartilage from rabbits with collagenase-induced OA, and treatment with 3-MA enhanced the decrease in Beclin-1 expression and the LC3B-II/LC3B-I ratio.
Figure 6.
(A) The protein expression levels of Beclin-1 and light chain 3 (LC3) B evaluated by western blot analysis in cartilage from control, collagenase-injected and 3-methyladenine (3-MA)-treated rabbits. (B and C) Comparison of the protein expression level of Beclin-1 and the LC3B-II/LC3B-I ratio. *P<0.05, **P<0.01, ***P<0.001. N.S., not significant.
Discussion
OA is a complex joint disorder with multiple etiologies, including aging, obesity, mechanical and biochemical factors (29,30). The degeneration of cartilage is the main pathology of OA and is associated with many inflammatory cytokines, such as IL-1β (31,32) and collagenase (33,34). IL-1β stimulates the expression of collagenase in chondrocytes and is often applied to produce cellular OA models for in vitro studies (35), and a number of of studies have shown that SW1353 cells can take the place of human chondrocytes for research (36–38). In this study, IL-1β was applied to SW1353 cells to establish a cellular model of OA. We found that the expression of MMP-13 was significantly promoted, while the expression of TIMP-1 and cell viability were significantly suppressed at 48 h, further indicating that IL-1β induced the degeneration of chondrocytes.
Previous studies have successfully established models of OA using rabbits following the intra-articular injection of collagenase (39,40). Moreover, it has been reported that the injection of 1.0 mg of collagenase is sufficient to induce OA-like changes for 6 weeks (6). Accordingly, 1.0 mg of collagenase was injected into the right knees of rabbits in this study, and the results observed by light microscopy and TEM were roughly consistent with those of previous studies, and further demonstrated that collagenase may be involved in cartilage degeneration in OA. However, the time needed for OA-like changes to occur was not 6 weeks but 8 weeks in this study, which may be due to various reasons, such as individual differences between animals. Other methods such as the one described by Hulth et al (41) involve the opening of the joint, exposing the articular surface, resulting in swelling, edema and congestion, which may affect the experimental results. The intra-articular injection of drugs does not involve the exposure of the articular surface, and this thus avoids side-effects such as swelling, edema and congestion caused by surgery affecting the experimental results. Thus, this method is suitable for the investigation of the effects of drugs on OA.
Recent studies have demonstrated that autophagy is involved in certain bone and cartilage diseases, such as cervical disc degeneration (42), cartilage degeneration of the temporomandibular joint (23), degradation of Meckel's cartilage (43) and OA (44). However, the results regarding changes in autophagy and the specific role of autophagy in the progression of OA are sometimes contradictory (25,26). In this study, we found that the expression of Beclin-1 and LC3B was first increased and then decreased both in the IL-1β-stimulated SW1353 cells and in cartilage from rabbits with collagenase-induced OA. TEM observation revealed that much more autophagosomes in chondrocytes were observed at the early time points and less were observed at later time points in cartilage from rabbits with collagenase-induced OA. These results suggest that autophagy is enhanced during the early stages and is weakened during the late stages of experimental OA, which determines the changes in autophagy in different stages of OA and provides the possible cause of the contradictory reports.
During the early period, there was no apparent loss of cell viability in the IL-1β-stimulated SW1353 cells; the mRNA level of MMP-13 was increased at 12 h and that of TIMP-1 was increased at 12 and 24 h without statistical significance. Moreover, the OA score of cartilage from the collagenase-injected group at 2 weeks showed no significance compared to the control group. These results may be related to the enhancement of autophagy during the early period of experimental OA.
To further investigate the role of the loss of autophagy in the pathogenesis of OA, a chemical inhibitor of the autophagic process, 3-MA, was applied to treat IL-1β-stimulated SW1353 cells and was directly injected into the knee joints of rabbits (45). We found that 3-MA treatment significantly inhibited the expression of Beclin-1 and LC3B in cells and caitilage, and the autophagosomes in chondrocytes were reduced. In addition, the loss of cell viability, the upregulation of MMP-13 and the downregulation of TIMP-1 induced by IL-1β were significantly enhanced in the SW1353 cells treated with 3-MA. Importantly, the degeneration of cartilage and chondrocytes in rabbit joints with collagenase-induced OA was aggravated by treatment with 3-MA. These results demonstrated that 3-MA aggravated the severity of experimental OA by inhibiting autophagy.
In conclusion, our results demonstrated that autophagy was first enhanced and then weakened in IL-1β-stimulated SW1353 cells and in rabbits with collagenase-induced OA degenerative cartilage. 3-MA aggravated the severity of experimental OA via the inhibition of autophagy, suggesting that the regulation of autophagy may be a potential therapeutic strategy for the treatment of OA.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (no. 31171672). We are thankful to the Liver Research Center of Beijing Friendship Hospital of Capital Medical University for providing technical assistance.
References
- 1.Buckland J. Osteoarthritis: targeting cartilage erosion in OA. Nat Rev Rheumatol. 2010;6:64. doi: 10.1038/nrrheum.2009.266. [DOI] [PubMed] [Google Scholar]
- 2.Lahm A, Kasch R, Mrosek E, Spank H, Erggelet C, Esser J, Merk H. Semiquantitative analysis of ECM molecules in the different cartilage layers in early and advanced osteoarthritis of the knee joint. Histol Histopathol. 2012;27:609–615. doi: 10.14670/HH-27.609. [DOI] [PubMed] [Google Scholar]
- 3.Wang JH, Shih KS, Wu YW, Wang AW, Yang CR. Histone deacetylase inhibitors increase microRNA-146a expression and enhance negative regulation of interleukin-1β signaling in osteoarthritis fibroblast-like synoviocytes. Osteoarthritis Cartilage. 2013;21:1987–1996. doi: 10.1016/j.joca.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Hui W, Young DA, Rowan AD, Xu X, Cawston TE, Proctor CJ. Oxidative changes and signalling pathways are pivotal in initiating age-related changes in articular cartilage. Ann Rheum Dis. 2016;75:449–458. doi: 10.1136/annrheumdis-2014-206295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim HJ, So HS, Lee JH, Park C, Lee JB, Youn MJ, Kim SJ, Yang SH, Lee KM, Kwon KB, et al. Role of proinflammatory cytokines in cisplatin-induced vestibular hair cell damage. Head Neck. 2008;30:1445–1456. doi: 10.1002/hed.20892. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi T, Sakuta T, Yamaguchi T. Intra-articular injection of collagenase induces experimental osteoarthritis in mature rabbits. Osteoarthritis Cartilage. 1998;6:177–186. doi: 10.1053/joca.1998.0110. [DOI] [PubMed] [Google Scholar]
- 7.Adães S, Ferreira-Gomes J, Mendonça M, Almeida L, Castro-Lopes JM, Neto FL. Injury of primary afferent neurons may contribute to osteoarthritis induced pain: an experimental study using the collagenase model in rats. Osteoarthritis Cartilage. 2015;23:914–924. doi: 10.1016/j.joca.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Adães S, Mendonça M, Santos TN, Castro-Lopes JM, Ferreira-Gomes J, Neto FL. Intra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritis. Arthritis Res Ther. 2014;16:R10. doi: 10.1186/ar4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klatt AR, Klinger G, Paul-Klausch B, Kühn G, Renno JH, Wagener R, Paulsson M, Schmidt J, Malchau G, Wielckens K. Matrilin-3 activates the expression of osteoarthritis-associated genes in primary human chondrocytes. FEBS Lett. 2009;583:3611–3617. doi: 10.1016/j.febslet.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Naito K, Takahashi M, Kushida K, Suzuki M, Ohishi T, Miura M, Inoue T, Nagano A. Measurement of matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases-1 (TIMP-1) in patients with knee osteoarthritis: comparison with generalized osteoarthritis. Rheumatology (Oxford) 1999;38:510–515. doi: 10.1093/rheumatology/38.6.510. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Guo A, Gu JC. c-Jun N-terminal kinase and nuclear factor κB mediate nitric oxide-induced expression of matrix metalloproteinase-13. Int Orthop. 2011;35:1261–1266. doi: 10.1007/s00264-010-1056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryter SW, Cloonan SM, Choi AM. Autophagy: a critical regulator of cellular metabolism and homeostasis. Mol Cells. 2013;36:7–16. doi: 10.1007/s10059-013-0140-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang SY, Yu QJ, Zhang RD, Liu B. Core signaling pathways of survival/death in autophagy-related cancer networks. Int J Biochem Cell Biol. 2011;43:1263–1266. doi: 10.1016/j.biocel.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Chang J, Wang W, Zhang H, Hu Y, Wang M, Yin Z. The dual role of autophagy in chondrocyte responses in the pathogenesis of articular cartilage degeneration in osteoarthritis. Int J Mol Med. 2013;32:1311–1318. doi: 10.3892/ijmm.2013.1520. [DOI] [PubMed] [Google Scholar]
- 16.Klionsky DJ, Cregg JM, Dunn WA, Jr, Emr SD, Sakai Y, Sandoval IV, Sibirny A, Subramani S, Thumm M, Veenhuis M, et al. A unified nomenclature for yeast autophagy-related genes. Dev Cell. 2003;5:539–545. doi: 10.1016/S1534-5807(03)00296-X. [DOI] [PubMed] [Google Scholar]
- 17.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 18.Ravikumar B, Sarkar S, Davies JE, Futter M, Garcia-Arencibia M, Green-Thompson ZW, Jimenez-Sanchez M, Korolchuk VI, Lichtenberg M, Luo S, et al. Regulation of mammalian autophagy in physiology and pathophysiology. Physiol Rev. 2010;90:1383–1435. doi: 10.1152/physrev.00030.2009. [DOI] [PubMed] [Google Scholar]
- 19.Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS, Baba M, Baehrecke EH, Bahr BA, Ballabio A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175. doi: 10.4161/auto.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caramés B, Olmer M, Kiosses WB, Lotz MK. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis Rheumatol. 2015;67:1568–1576. doi: 10.1002/art.39073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barranco C. Osteoarthritis: activate autophagy to prevent cartilage degeneration? Nat Rev Rheumatol. 2015;11:127. doi: 10.1038/nrrheum.2015.12. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M, Zhang J, Lu L, Qiu ZY, Zhang X, Yu SB, Wu YP, Wang MQ. Enhancement of chondrocyte autophagy is an early response in the degenerative cartilage of the temporomandibular joint to biomechanical dental stimulation. Apoptosis. 2013;18:423–434. doi: 10.1007/s10495-013-0811-0. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Lang W, Ye H, Yu F, Li H, Chen J, Cai L, Chen W, Lin R, Huang Y, et al. Tougu Xiaotong capsule inhibits the tidemark replication and cartilage degradation of papain-induced osteoarthritis by the regulation of chondrocyte autophagy. Int J Mol Med. 2013;31:1349–1356. doi: 10.3892/ijmm.2013.1341. [DOI] [PubMed] [Google Scholar]
- 25.Almonte-Becerril M, Navarro-Garcia F, Gonzalez-Robles A, Vega-Lopez MA, Lavalle C, Kouri JB. Cell death of chondrocytes is a combination between apoptosis and autophagy during the pathogenesis of Osteoarthritis within an experimental model. Apoptosis. 2010;15:631–638. doi: 10.1007/s10495-010-0458-z. [DOI] [PubMed] [Google Scholar]
- 26.Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. doi: 10.1002/art.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mankin HJ, Johnson ME, Lippiello L. Biochemical and metabolic abnormalities in articular cartilage from osteoarthritic human hips. III. Distribution and metabolism of amino sugar-containing macromolecules. J Bone Joint Surg Am. 1981;63:131–139. doi: 10.2106/00004623-198163010-00017. [DOI] [PubMed] [Google Scholar]
- 29.van der Kraan PM. Understanding developmental mechanisms in the context of osteoarthritis. Curr Rheumatol Rep. 2013;15:333. doi: 10.1007/s11926-013-0333-3. [DOI] [PubMed] [Google Scholar]
- 30.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213:626–634. doi: 10.1002/jcp.21258. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Kondragunta V, Kornman KS, Wang HY, Duff GW, Renner JB, Jordan JM. IL-1 receptor antagonist gene as a predictive biomarker of progression of knee osteoarthritis in a population cohort. Osteoarthritis Cartilage. 2013;21:930–938. doi: 10.1016/j.joca.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santangelo KS, Nuovo GJ, Bertone AL. In vivo reduction or blockade of interleukin-1β in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthritis Cartilage. 2012;20:1610–1618. doi: 10.1016/j.joca.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bucknor MD, Nardo L, Joseph GB, Alizai H, Srikhum W, Nevitt MC, Lynch JA, McCulloch CE, Link TM. Association of cartilage degeneration with four year weight gain - 3T MRI data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2015;23:525–531. doi: 10.1016/j.joca.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Botter SM, van Osch GJ, Waarsing JH, van der Linden JC, Verhaar JA, Pols HA, van Leeuwen JP, Weinans H. Cartilage damage pattern in relation to subchondral plate thickness in a collagenase-induced model of osteoarthritis. Osteoarthritis Cartilage. 2008;16:506–514. doi: 10.1016/j.joca.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Roman-Blas JA, Contreras-Blasco MA, Largo R, Alvarez-Soria MA, Castañeda S, Herrero-Beaumont G. Differential effects of the antioxidant n-acetylcysteine on the production of catabolic mediators in IL-1beta-stimulated human osteoarthritic synoviocytes and chondrocytes. Eur J Pharmacol. 2009;623:125–131. doi: 10.1016/j.ejphar.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Schaefer JF, Millham ML, de Crombrugghe B, Buckbinder L. FGF signaling antagonizes cytokine-mediated repression of Sox9 in SW1353 chondrosarcoma cells. Osteoarthritis Cartilage. 2003;11:233–241. doi: 10.1016/S1063-4584(02)00354-0. [DOI] [PubMed] [Google Scholar]
- 37.Lu YC, Jayakumar T, Duann YF, Chou YC, Hsieh CY, Yu SY, Sheu JR, Hsiao G. Chondroprotective role of sesamol by inhibiting MMPs expression via retaining NF-κB signaling in activated SW1353 cells. J Agric Food Chem. 2011;59:4969–4978. doi: 10.1021/jf1046738. [DOI] [PubMed] [Google Scholar]
- 38.Tetsunaga T, Nishida K, Furumatsu T, Naruse K, Hirohata S, Yoshida A, Saito T, Ozaki T. Regulation of mechanical stress-induced MMP-13 and ADAMTS-5 expression by RUNX-2 transcriptional factor in SW1353 chondrocyte-like cells. Osteoarthritis Cartilage. 2011;19:222–232. doi: 10.1016/j.joca.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Kwon DR, Park GY, Lee SU. The effects of intra-articular platelet-rich plasma injection according to the severity of collagenase-induced knee osteoarthritis in a rabbit model. Ann Rehabil Med. 2012;36:458–465. doi: 10.5535/arm.2012.36.4.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SB, Kwon DR, Kwak H, Shin YB, Han HJ, Lee JH, Choi SH. Additive effects of intra-articular injection of growth hormone and hyaluronic acid in rabbit model of collagenase-induced osteoarthritis. J Korean Med Sci. 2010;25:776–780. doi: 10.3346/jkms.2010.25.5.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hulth A, Lindberg L, Telhag H. Experimental osteoarthritis in rabbits. Preliminary report. Acta Orthop Scand. 1970;41:522–530. doi: 10.3109/17453677008991540. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Xiong S, Wang H, Zhang M, Yu Y. The evidence and the possible significance of autophagy in degeneration model of human cervical end-plate cartilage. Exp Ther Med. 2014;7:537–542. doi: 10.3892/etm.2013.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang RT, Zhang C, Liu Y, Zhou HH, Li ZB. Autophagy prior to chondrocyte cell death during the degeneration of Meckel's cartilage. Anat Rec (Hoboken) 2012;295:734–741. doi: 10.1002/ar.22433. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Vasheghani F, Li YH, Blati M, Simeone K, Fahmi H, Lussier B, Roughley P, Lagares D, Pelletier JP, et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015;74:1432–1440. doi: 10.1136/annrheumdis-2013-204599. [DOI] [PubMed] [Google Scholar]
- 45.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]