Abstract
Background
The gene FOXO3, encoding the transcription factor forkhead box O-3 (FoxO3), is one of only two for which genetic polymorphisms have exhibited consistent associations with longevity in diverse human populations.
Objective
Here we review the multitude of actions of FoxO3 that are relevant to health, and thus healthy ageing and longevity.
Methods
Literature search for articles retrieved from PubMed using FoxO3 as keyword.
Results
We review the molecular genetics of FOXO3 in longevity, then current knowledge of FoxO3 function relevant to ageing and lifespan. We describe how FoxOs are involved in energy metabolism, oxidative stress, proteostasis, apoptosis, cell cycle regulation, metabolic processes, immunity, inflammation and stem cell maintenance. The single FoxO in Hydra confers immortality to this fresh water polyp, but as more complex organisms evolved this role has been usurped by the need for FoxO to control a broader range of specialized pathways across a wide spectrum of tissues assisted by the advent of as many as 4 FoxO subtypes in mammals. The major themes of FoxO3 are similar, but not identical, to other FoxOs and include regulation of cellular homeostasis, particularly of stem cells, and of inflammation, which is a common theme of age-related diseases. Other functions concern metabolism, cell cycle arrest, apoptosis, destruction of potentially damaging reactive oxygen species, and proteostasis.
Conclusions
The mechanism by which longevity-associated alleles of FOXO3 reduce age-related mortality is currently of great clinical interest. The prospect of optimizing FoxO3 activity in humans to increase lifespan and reduce age-related diseases represents an exciting avenue of clinical investigation. Research strategies directed at developing therapeutic agents that target FoxO3, its gene and proteins in the pathway(s) FoxO3 regulates should be encouraged and supported.
Keywords: Forkhead box O-3, Genetics, Human, Longevity, Aging
Introduction
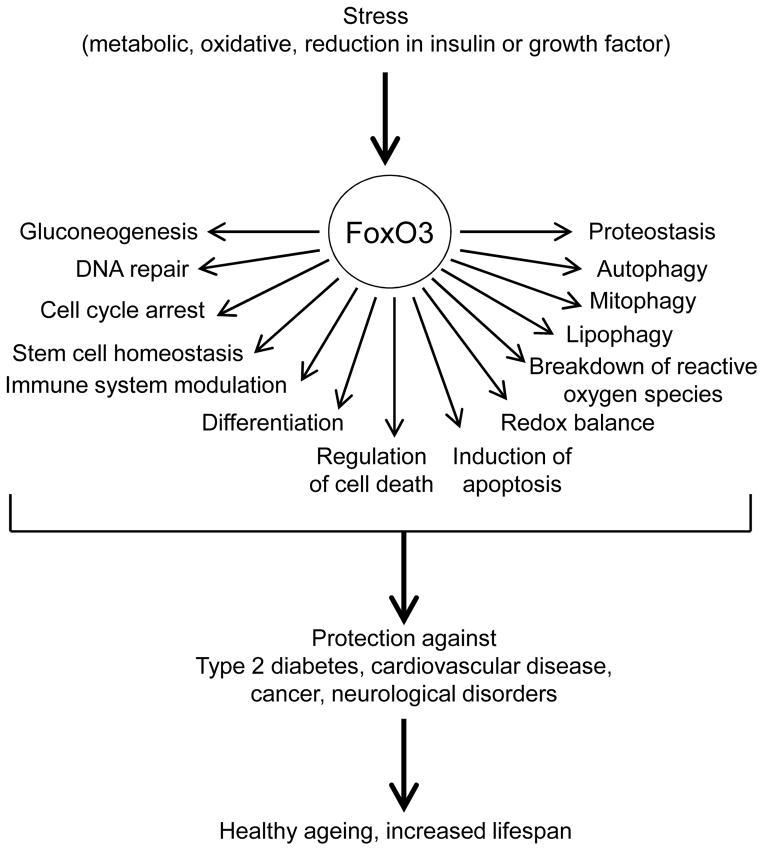
Forkhead/winged helix box gene, group O (FoxO) proteins are a set of evolutionarily-conserved transcription factors at a central integration hub for many important cellular stimuli (Fig. 1). Fig. 2 shows the specific factors that mediate these effects and Table 1 lists genes targetted.
Fig. 1.
Cellular processes regulated by FoxOs that serve as homeostatic regulators, particularly in response to stress, to potentially affect ageing and lifespan.
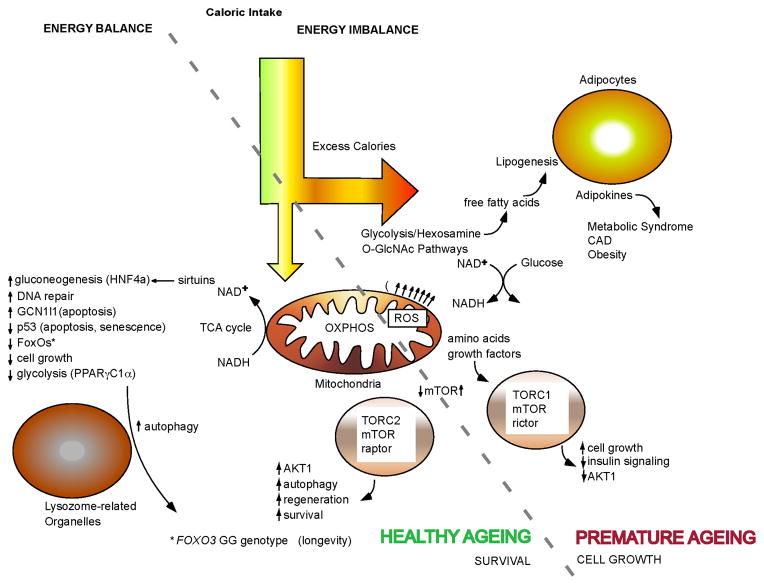
Fig. 2.
Mechanisms by which FoxOs influence healthy ageing. Shown are several of the key intracellular pathways targeted by FoxO transcription factors. FoxOs are master regulators that translate environmental stimuli arising from insulin, growth factors, nutrients and oxidative stress into specific gene expression programs. The role of FoxO3 in longevity may involve upregulation of target genes involved in stress resistance, metabolism, cell cycle arrest, and apoptosis. Effective control of FoxO3 in response to environmental stimuli is likely critical to prevent ageing and age related diseases including cardiovascular disease, type 2 diabetes, cancer and neurodegenerative diseases. The diagram shows how the well-known longevity-associated intervention of caloric restriction helps to maintain the redox state of the cell by cycling calories through the mitochondria so as to restore NAD+. Caloric restriction results in activation of sirtuins, leading to activation of FoxOs, improved autophagy, amino acid recycling via inhibition of mTOR activity, and other mechanisms leading to a healthy ageing phenotype. On the other hand, excess calories, particularly from carbohydrates, increase the NADH/NAD+ ratio and leads to lipogenesis, overproduction of ROS by mitochondria, poor autophagy and activation of mTOR as a result of an excess of protein intake.
Abbreviations: AKT1, a term derived from the “Ak” mouse strain that develops spontaneous thymic lymphomas (AKT1 is also known as protein kinase B); CAD, coronary artery disease; HNF4a, hepatocyte nuclear factor 4α; GCN1l1, general control of amino acid synthesis 1-like 1; mTOR, mechanistic target of rapamycin; O-GlcNAc, O-linked N-acetylglucosamine’ OXPHOS, oxidative phosphorylation; PPARγC1α, peroxisome proliferator activated receptor γ coactivator 1α; TCA, tricarboxylic acid; TORC1, mTOR complex 1; TORC2, mTOR complex 2.
Table 1.
Intracellular functions regulated by FoxO transcription factors, showing genes targetted
| Function | Target gene(s) |
|---|---|
| Metabolism | |
| Energy homeostasis | AGRP, GP17, NPY |
| Gluconeogenesis | APOC3, G6PC, PEPCK |
| ROS detoxification | CAT, PRDX3, SENP, SOD2 |
| Proteostasis | |
| Autophagy | ATG12, BCEN1, BNIP3, GABARAPL1 |
| Mitophagy | PINK1 |
| Degradation of proteasome | FBXO32, TRIM63 |
| Intracellular signaling | |
| PI3K pathway | IRS2, PI3K |
| EGF pathway | EGFR |
| mTORC suppression | RPTOR, SESN3 |
| mTORC2 activation | RICTOR |
| Apoptosis | BBC3, BCL2L11, BCL6, FASLG, TNF, TNFSF10 |
| Cell cycle arrest | CCND1, CCNG2, CDKN1A, CDKN1B, CDKN2A, CDKN2B, GADD45, RBL2 |
| Functions specific to cell type | |
| Maintenance of pluripotency | OCT4, SOX2 |
| Immune system | CCR7, FOXP3, IFNG, IL7R, IRF7, RAG1, RAG2, SELL |
| Vascular wall | CYR61 |
| LDL cholesterol | PCSK9 |
Abbreviations: AGRP, agouti-related protein; APOC3, apolipoprotein C3; BBC3, BCL-2-binding component 3; BCEN1, beclin 1; BCL6, B cell CLL lymphoma 6; CAT, catalase; CCND1, cyclin D1; CCNG2, cyclin G2; CCR7, C-C chemokine receptor 7; CYR61, cysteine-rich angiogenic inducer 61; EGFR, epidermal growth factor receptor; FASLG, Fas ligand; FBXO32, F-box protein 32; G6PC, glucose-6-phosphatase; GPR17, G protein-coupled receptor 17; IFNG, interferon gamma; IL-7R, interleukin 7 receptor; IRF7, interferon regulatory factor 7; IRS2, insulin receptor substrate 2; neuropeptide Y; PCSK9, proprotein convertase subtilisin kexin type 9; PEPCK, phosphoenolpyruvate carboxykinase; PINK1, PTEN induced putative kinase 1; PMAIP1, phorbol-12-myristate-13-acetate-induced protein 1; PRDX3, peroxiredoxin 3; RAG, recombination activating gene; RPTOR, regulatory associated protein of mTORC1; RBL2, retinoblastoma-like 2; RICTOR, rapamycin insensitive companion of mTOR; SELL, selectin L; SENP, sentrin specific peptidase 1; SESN3, sestrin 3; SOD2, superoxide dismutase 2; TNF, tumour necrosis factor; TNFSF10, tumour necrosis factor superfamily member 10.
In mammals there are four FoxOs – FoxO1, −3, −4 and 6. Although most are expressed ubiquitously, tissue-specific and temporal differences in expression are seen. FoxO3 may undergo a greater decline with ageing, at least in skeletal muscle [1]. Each FoxO can regulate different genes depending on cell type [2].
Here we review the nature of FoxOs and the essence of their control, then discuss findings from gene manipulation, human molecular genetics and the role of FoxOs in modulation of specific processes relevant to ageing and lifespan.
Intracellular signaling and posttranslational modification of FoxOs
FoxOs were originally implicated in insulin/insulin-like growth factor signalling (IIS). IIS results in decreased activity and/or expression of FoxOs, whereas oxidative stress, via JNK activity, leads to increased activity or expression of FoxOs. IIS activates phosphoinositide-3-kinase (PI3K)-AKT (also known as protein kinase B). AKT and serum and glucocorticoid-induced kinase (SGK) phosphorylate FoxOs directly at three conserved residues, leading to increased FoxO binding to the regulator protein 14-3-3 and the consequent exclusion of FoxOs from the nucleus. This results in a reduction in the ability of FoxOs to regulate transcription. JNK induces the release of FoxOs from 14-3-3, thereby overriding FoxO inhibition caused by insulin and growth factors. JNK also decreases insulin receptor substrate activity. Various kinases – PI3K, AKT, SGK, AMP kinase (AMPK), cyclin dependent kinases (CDK1 and CDK2), c-Jun N-terminal kinase (JNK), macrophage stimulating 1 (MST1), extracellular signal regulated kinase (ERK) and p38 mitogen activated protein kinase (MAPK) – phosphorylate FoxOs at a diversity of sites. Acetylation/deacetylation, ubiquitination and arginine/lysine methylation represent further modes of FoxO regulation [3]. Deacetylation of FoxOs by sirtuin family members 1, 2 and 3 results in FoxO activation [4]. The multitude of FoxO modifications may be crucial to binding of FoxOs to DNA recognition sequences and regulators [5]. Alterations in such modifications with ageing could potentially diminish FoxO activity in old age in various tissues, so contributing to effects on age-related disease and lifespan.
General cellular and organismal functions of FoxOs
FoxOs boost expression of genes encoding proteins involved in DNA repair and suppress members of the pro-growth mechanistic target of rapamycin (mTOR) kinase pathway [6] (Table 1). Both FoxO1 and FoxO3 suppress regulator associated protein of mTOR (Raptor) to lower the activity of growth-promoting mTOR complex 1 (TORC1) activity, but unlike FoxO1, FoxO3 is not able to upregulate the rapamycin insensitive companion of mTOR (Rictor) and sestrin 3, so has no effect on TORC2 activity [7].
IIS affects energy metabolism, oxidative stress, apoptosis, G0 to G1 and G1 to S phase progression in the cell cycle, and a diversity of other metabolic processes. The effects of FoxOs on gene expression are generally in the opposite direction as insulin and IGF-1.
The ability of FoxOs to protect against oxidative stress involves increase in expression of scavengers of reactive oxygen species (ROS), such as Mn superoxide dismutase (SOD2) in mitochondria, catalase and peroxiredoxin III (Table 1).
Evidence accumulated initially in model organisms has highlighted the ability of FoxOs to protect against environmental and biological stressors and to enhance lifespan [8], as will be discussed in more detail later.
Transcriptional and indirect effects of FoxOs
All FoxOs bind to the same consensus sequence (5′-TTGTTTAC-3′), so act redundantly. This short sequence is common in genomic DNA. Binding of FoxOs to promoters to activate transcription therefore requires interactions with other transcription factors [5]. FoxOs can activate transcription directly by binding to this enhancer sequence, whereas their ability to suppress transcription occurs through indirect mechanisms that do not involve DNA binding, as shown by FoxO3-specific transcriptome-wide RNA polymerase II profiling [9]. FoxOs also regulate various microRNAs and in turn these regulate FoxO expression [5].
FoxO confers longevity in model organisms
It has been suggested that, ‘over time the level of a rate-of-ageing regulator falls, crossing thresholds that trigger various aspects of ageing’ [10]. Various factors decline with age. In the case of FoxOs, decreases of 25% in protein levels of FoxO3 and 18% in FoxO4, but no change in FoxO1, have been seen in skeletal muscle of aged mice [1].
Experimental manipulation of FoxO expression in diverse model organisms can increase or decrease their lifespan [8]. Lifespan extension has, moreover, been seen when FoxO3 overexpression is confined to the fat body of Drosophila melanogaster [11] and adipose tissue of mice [12]. Mutation in the insulin receptor gene increases FoxO in Drosophila [13] and Caenorhabditis elegans [14].
In contrast to the strong molecular effects caused by manipulation of genes in model organisms, similar strong phenotypic effects in humans, arising from mutations in the same genes, are rare. In the absence of targetted gene manipulation, effect sizes tend to be small. Effects in model organisms can, moreover, differ between strains. Even calorie restriction, the most potent lifespan extending intervention, has little effect on the lifespan of wild mice. In calorie restricted rhesus monkeys contradictory findings, likely arising from the food used, means such studies need to be re-done taking into account more recent data showing the divergent effects on lifespan of diets differing in macronutrient composition [15].
The GenAge database list over 1,000 genes that have been associated with longevity or ageing in model organisms, including >1000 in C. elegans and >100 in mice, 51 of the latter being able to extend lifespan. There is, however, little evidence to date for any of these being involved in human longevity. This could, however, be a result of low power of human genetic studies to detect rare variants in such a complex polygenic condition as lifespan. Such limited power could be due to small numbers of long-lived cases, lack of proper controls (since birth cohort-matched controls are usually not available), population stratification artifacts, population specific effects, gender-specific effects and other issues. Furthermore, the cause of death in most laboratory mouse strains is cancer, so limiting the relevance of mouse studies to humans.
Studies in mammals of specific FoxO-related mechanisms that could increase lifespan will be discussed later in this review.
FoxO mediates the immortality of fresh water polyps
The fresh water polyp, Hydra, is regarded as effectively immortal. Its ability to escape death and senescence was initially thought to be due to its asexual mode of reproduction, which requires each individual polyp to maintain cells that are continuously proliferating [16]. It would now appear, however, that Hydra’s immortality is also contributed by its ability to continuously maintain the self-renewal capacity of its 3 stem cell lineages. The transcription factor shared between each stem cell lineage is its single FoxO protein [17]. In Hydra, constitutively expressed FoxO controls stem cells and the innate immune system [18]. The primary ability of FoxO to cause continuous stem cell proliferation and renewal, and ensure its immune system is continuously effective, is what confers immortality to this organism [17,18]. In more complex organisms FoxO function has become more diverse, so enabling adaptability, but at the cost of reduced regenerative capacity [19]. During evolution, the restricted role of FoxO in self-renewal and immunity that enabled FoxO to confer immortality to simple organisms like Hydra was largely abandoned in favour of a more diversified set of additional functions that in more complex organisms required regulation, so resulting in the prospect of eventual death.
FOXO3 is a major gene for human longevity
Because of its actions and strategic position in relation to intracellular pathways, FoxO3 has long been considered to play a pivotal role in the molecular basis of longevity [6]. This led researchers at Kuakini Medical Center in Honolulu to perform a genetic association study of single nucleotide polymorphisms (SNPs) spanning the human FoxO3 gene (FOXO3) and flanking DNA in a cohort of American men of Japanese ancestry well characterized for ageing phenotypes. Longevity ‘cases’ were men aged over 95 years and ‘controls’ were birth-cohort matched men of normal lifespan for this population (mean age 78.5 years). This revealed an association of three FOXO3 SNPs with living to extreme old age [20]. Eleven independent studies of populations of diverse ancestry in multiple different countries have now confirmed and extended this finding. A meta-analysis in 2014 of the various studies found that 5 of the FOXO3 SNPs tested retained statistically significant associations with longevity [21]. The strongest association was for the minor allele of the SNP reported originally to exhibit the most robust association, namely, the G allele of rs2802292 in men (odds ratio, 1.54; 95% confidence intervals (CI), 1.33–1.67). A subsequent meta-analysis by others of rs2802292 and longevity that included GWAS data from the CHARGE consortium led to an OR of 1.17 (P=1.85×10−10) [22]. In the CHARGE study rs10457180, which was in linkage disequilibrium with rs2802292, provided a stronger association with longevity, leading the authors to suggest that rs10457180 may be a better tagging SNP for the true causal variant. The coding region of FOXO3 has 99% sequence homology with that of its pseudogene, ZNF286B (previously termed FOXO3B). This would affect genotyping of polymorphisms in the FOXO3 coding region. However, since both rs2802292 and rs10457180 are intronic and intron sequences differ between FOXO3 and ZNF268B, genotyping of these SNPs should be accurate.
The various longevity-associated SNPs are located in or near intron 2 of the 125 kb FOXO3 gene [23–25]. After performing extensive sequence analyses of coding DNA the Kuakini team ruled out involvement of coding variants (amino acid differences) as an explanation for the genetic association [25]. To date, the causal SNP(s) and the reason underlying the protective effect of the longevity-associated allele(s) in human longevity remains to be delineated. The Leiden 85-plus study has, nevertheless, found an association of FOXO3 haplotypes with all-cause mortality, stroke and cardiovascular mortality [26]. The rs2802292 TT genotype is, moreover, associated with the rare hamartomatous polyposis syndromes [27]. Research is needed to compare FoxO3 levels in various tissues of long-lived and normal lifespan individuals with TT and GG genotypes. The findings might help inform experiments aimed at identifying factors that could be relevant to the genetic association findings in humans.
Genetic factors account for approximately one-third of the variability in human lifespan [23]. Based on research to date FOXO3 is one of only two genes (the other being APOE) that have been replicated consistently across diverse human populations for association with attainment of extreme old age [23,24]. In each gene, the longevity-associated minor alleles are common. While common variants in other genes may also contribute to longevity, there is likely to be a significant contribution of rare variants in multiple genes, just as is well-known for other common complex polygenic conditions.
The pathways whereby FoxO3 exerts its lifespan-extending effects should, by revealing biological targets for future therapies, be of considerable importance in devising ways of helping humans not just to live longer, but grow old with a reduced burden of ill-health. This has enormous implications for society, social insurance programs and health care
We will now review the specific actions of FoxO3 that may affect health, ageing and lifespan.
Insulin signalling and glucose homeostasis
Organs involved in control of glucose homeostasis (liver, pancreas, adipose tissue and skeletal muscle) exhibit higher FoxO1 than FoxO3 activity, with FoxO1 being activated under low glucose conditions in order to help maintain glucose homeostasis [5,28]. FoxO4 levels are higher in skeletal muscle, while FoxO3 exhibits greater abundance in brain, heart, kidney and spleen, and FoxO6 is primarily detected in the brain, both during development and in adulthood [3,28]. A study of 196 monozygotic Danish twins found that the longevity-associated FOXO3 rs2800292 G allele was associated with more favorable peripheral and hepatic insulin sensitivity and increased FOXO3 mRNA expression in skeletal muscle, suggesting that the beneficial effects of FoxO3 on glucose metabolism may be mediated through enhanced FOXO3 transcription [29]. However, in the population-based Inter99 cohort of 5,768 individuals, the association became non-significant after adjustment for body mass index [29]. Consistent with a role for FoxO3 in suppression of IIS and thus growth so as to extend lifespan, the longevity-associated G allele of the FOXO3 rs28002292 variant is associated with shorter stature [30].
Apoptosis
FoxOs promote cell growth inhibitory and/or apoptosis signalling pathways by inducing multiple pro-apoptotic members of the Bcl2 family of mitochondrial targetting proteins, stimulating expression of death receptor ligands such as Fas ligand and tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), or enhancing expression of various CDK inhibitors [28].
Immunity and inflammation
FoxO3 contributes to regulation of the immune system [31]. In old age the immune system deteriorates, so increasing risk of infection. As a result an infection is a common cause of death in the elderly. Following cytokine or growth factor withdrawal FoxO3 upregulates Puma and Bim, leading to apoptosis of T cells [32]. Foxo3 induces the synthesis of antimicrobial peptides in human kidney, lung and gut [33]. These fight microbial infections in diverse species and serve as effector molecules of innate immunity.
Evidence from knock-down experiments in mice show that FoxO3 controls cytokine production, opposes NF-κB activation, suppresses T cell activation and proliferation, reduces the proliferation of lymphatic cells and lowers inflammation [34].
Its anti-inflammatory actions involve inhibition of production of inflammatory cytokines such as interleukin-2 [35] and interleukin-6 [36]. In response to infection the pleiotropic cytokine interleukin-6 is activated [36] and this regulates the immune system in a variety of ways, such as production of antibodies, the function of T cells, haematopoiesis and inflammation [37]. In the elderly, elevated interleukin-6 is associated with increase in risk of all-cause mortality and deaths from CHD and cancer [37].
The minor allele of FOXO3 SNP rs12212067 is associated with a milder course of the inflammatory conditions Crohn’s disease and rheumatoid arthritis, consistent with this variant having an anti-inflammatory role [38]. This role for FoxO3 is not shared with other FoxO proteins. The SNP rs12212067 is in close proximity to, although not in strong linkage disequilibrium with, the longevity-associated SNP rs2802292, and similar to rs2802292, rs12212067 is non-coding. In monocytes of subjects with the minor allele the inflammatory responses was less severe, and appeared to be a result of a reduction in pro-inflammatory cytokines, including TNFα, mediated by an effect of TGF-β1. As well there was an increase in production of anti-inflammatory cytokines, including interleukin-10.
As they age, centenarians appear to be relatively free of age-related diseases that feature inflammation, i.e., have lower “inflammageing” [39]. This, despite elevated interleukin-6, fibrinogen and coagulation factors. It may be that these pro-inflammatory factors are countered by increased expression of anti-inflammatory cytokines such as TGF-β1 [39]. Alternatively, it might be the case that age-related diseases that feature inflammation are delayed in centenarians. The longevity-associated alleles of FOXO3 are associated with stronger inhibition of cytokines [38].
Proteostasis and autophagy
FoxOs regulate the protein homeostasis network, which is involved in cellular quality control [3]. This may underlie the role of FoxO proteins in longevity. The ubiquitin-proteosome system and autophagy help eliminate damaged and aggregated proteins from the cell. Autophagy decreases with age, and a reduction in autophagy is associated with premature ageing and disease [3]. In response to starvation, a variety of autophagy genes are activated in skeletal muscle, liver, neurons and other cells to induce autophagy, mitophagy (by which mitochondria are also targetted for autophagy via the lysosomal pathway), and, in liver, lipophagy [40]. Inhibition of the major regulator of protein homeostasis, mTORC1, is another way FoxOs control autophagy. The ability of FoxO-induced proteostasis to promote survival in neurons, but degeneration in skeletal muscle, might be a result of cell-type specific expression, FoxO3 and FoxO6 being highly enriched in brain, whereas muscle is enriched in FoxO4 [3]. FoxO3 could therefore counter toxic protein aggregation seen in neurodegenerative diseases that shorten lifespan.
Cardiovascular disease
FoxO3 protects the heart and blood vessels. Overexpression of FoxO3 in vivo increases p27 (kip1) in vascular smooth muscle, so inhibiting vascular smooth muscle cell proliferation and neointimal hyperplasia [41]. Such effects also involve binding to a FoxO3 motif in the promoter of the cyteine-rich angiogenic inducer 61 gene (CYR61) [42], an immediate early gene expressed in these cells in response to stimulation by growth factors. CYR61 expression is associated with postangioplasty restenosis. Ageing-associated diseases and ageing of the vasculature all involve increased inflammation. Loss of microvascular density and plasticity is a major feature of human ageing. This is accompanied by dysfunction of adult stems in vascular niches. Inflammatory cytokines appear to drive this process. FoxO3 inhibits these cytokines. Angiogenesis can be induced by infection of vascular endothelial cells with cytomegalovirus, a process that is mediated by upregulation of microRNA-217 to lower sirtuin 1 and FoxO3 [43]. The ageing of the vasculature thus appears to involve the processes of loss of microvascular density and plasticity, dysfunction of adult stem cells, and inflammation. Such effects would likely impede the health of various organ systems in the body.
Deletion of FoxO1, FoxO3 and FoxO4 from myeloid cells of atherosclerosis-prone LDL receptor knockout mice results in plaques that are not only larger, but contain more macrophages having higher proliferative and reduced apoptotic ability [44]. These myeloid cells exhibited increased generation of ROS, which are known to accelerate atherogenesis. FoxO3 lowers LDL cholesterol by recruiting sirtuin 6 to the proximal promoter of the gene for proprotein convertase subtilisin/kexin type 9, which has a crucial role in LDL receptor degradation [45].
FoxO3 acts in cardiomyocytes to regulate autophagy, proliferation, cell size and cell survival [46]. It has been suggested that FoxO3 serves both a positive and negative role in autophagy-related cardiomyopathy such as ischaemic and cardiac hypertrophy. Overexpression of FoxO3 in vivo reduces cardiomyocyte size by increasing autophagosome formation, expression of atrogin-1 and the autophagy-related genes encoding microtubule-associated protein 1A/1B-light chain 3, GABA receptor-associated protein-like 1, and autophagy related 12 protein [47]. In cardiac fibroblasts FoxO3 reduces oxidative stress [48].
Cancer
Foxo3 suppresses tumour growth [2]. Tumour suppression can occur in concert with p53 [28]. The p53 protein positively regulates FoxO expression and FoxOs steer the induction of p53-dependent apoptosis [5]. In leukaemia [49] and breast cancer [50] cells anticancer drugs upregulate FoxO3 leading to increased Bim expression and consequent inhibition of tumour growth. FoxO3 downregulates Myc, a stimulator of tumour cell proliferation and survival [51]. Alternatively, FoxO factors appear to behave as oncogenes in a subset of acute myeloid leukaemia patients [52]. The antioxidant actions of FoxO3 can, moreover, enhance survival of drug-resistant tumour cells [53].
Stem cells
In humans FoxO3 is crucial for regulation of adult stem cell homeostasis, including that in neural, leukaemic and haemopoietic [54] stem cells, whereas FoxO1 maintains embryonic stem cell renewal and pluripotency [55]. In neural stem/progenitor cells FoxO3 is the predominant isoform. It protects these cells by increasing expression of genes encoding SOD2, catalase, ataxia telangiectasia mutated (ATM) and CDK inhibitor 2A (CDKN2A) that target ROS for destruction. Erythroid cells are particularly prone to oxidative damage during maturation and depend on FoxO3 for protection [56].
A feature of many age-related disorders is the loss of adult stem cells. This is seen in coronary artery disease, insulin resistance and type 2 diabetes. FoxO3 is the primary driver maintaining the stem cell pool in mammals. It does this by ensuring the operation of a protective autophagy program, as well as other processes such as notch-1 and notch-2 receptor mediated quiescence in muscle satellite cells [57], adult mesenchymal, neural [58], Sca-1-positive cardiac [59], and haemopoietic stem cells (HSC).
Maintenance of the HSC pool by FoxO3 has been demonstrated in mouse strains in which FoxO3 has been knocked out or knocked down [54,60]. The ability of sirtuin 1 to control HSC homeostasis is via FoxO3 [61]. FoxO3 is not, however, important for the differentiation potential HSCs [54]. A critical reduction in telomere length in extreme old age in humans results in the disappearance of many HSCs from the HSC pool [62].
The ability of FoxO3 to restrain Achaete-scute homolog 1 (ASCL1)-dependent neurogenesis may help preserve the neural stem cell pool [63]. Stem cells are moreover involved in the regeneration of damaged cells after myocardial infarction [64]. FoxO3 agonists could potentially be used to protect adult stem cells and so prevent their loss with ageing.
Interventions that increase FoxO3 level or activity may extend lifespan
It would appear then that FoxO3 should be considered as a potential target for therapeutic interventions to promote healthy ageing and extend lifespan. Agents that boost FoxO3 may be especially beneficial in high-risk patient populations, such as those with type 2 diabetes and obesity. Finding the functional variant responsible for differences in FOXO3 expression might provide a valuable target for therapy. The longevity-associated variant rs2802292 is associated with an increased expression of FOXO3 in skeletal muscle [29]. The region containing the 125 kb FOXO3 gene is, however, not only large, but displays a high degree of linkage disequilibrium. This presents a challenge to finding the variant that confers increased FOXO3 expression.
Phytochemicals, including polyphenols, appear to have promise for upregulation of the function of FoxOs. For example, treatment of rats from 5 weeks of age with 25 mg.kg−1 per day of the green tea phytochemical epigallocatenin gallate (EGCG) in drinking water increased sirtuin 1, FoxO3 (by 67–100%), SOD, glutathione peroxidase and lifespan (by 14%), reduced NF-κB, IL-6, TNF-α, ROS, inflammation, oxidative stress in serum, liver and kidney, and protected against organ damage [65]. In a human population it was shown recently that associations between tea drinking and risk for cognitive disability among the oldest old were dependent upon FoxO genotype, with carriers of FOXO1 SNP rs17630266 and FOXO3 SNPs rs2253319 and rs2802292 displaying significantly reduced risk for cognitive disability at advanced ages [66]. The polyphenol curcumin reduces FoxO3 phosphorylation, causing nuclear translocation and a 2-fold increase in FoxO3-mediated gene expression[67]. In vivo, curcumin lowers lipid levels in peritoneal macrophages of obese mice. Curcumin, by increasing FoxO3 activity, may protect against oxidant- and lipid-induced damage in the inflammatory cells of the vascular system, so reducing the risk for age associated cardiovascular disease [67].
In cultured human dermal fibroblasts, the phytoallexin resveratrol inhibited expression of the senescence mediator INK4a [68]. Resveratrol and sirtuin-activating compounds can, in certain cases, delay ageing, age-related diseases and increase lifespan [69]. In response to resveratrol transcriptome-wide microarray experiments identified the differential expression of 47 genes, including ones involved in transcription, growth, cell division, cell signalling and apoptosis, such as Ras-GRF1, RAC3 and UBE2D3 in the Ras and ubiquitin pathways [68]. Inhibition of Ras signalling by resveratrol would counter the Ras-PI3K mediated activation of protein kinase B, so leading to an increase in FoxO3 activity. The fact that SIRT1 controls HSC homeostasis via FoxO3 [61] further highlights the value of SIRT1 activation therapeutically.
Conclusions
The present review has shown how FoxO3, and other FoxOs, are not only pivotal to a wide array of processes associated with the healthy ageing of cells and thus the organism, but protective allele(s) of FOXO3 are the second most-replicated genetic factor(s) found so far to be associated with extended human lifespan. FoxOs share the same target sequence in promoters, so what is it that makes FOXO3 a longevity gene, but not the other three family members? Could it be tissue-specific differences in expression of each, subtle differences in posttranslational modification, or something else?
Natural compounds such as resveratrol, curcumin, astaxanthin, amongst others, by stimulating the effect of FoxOs on numerous genes important for cellular health and amelioration of diseases of ageing, could theoretically help improve organismal health and boost lifespan. More details of the delicately balanced FoxO3 signalling pathways are crucial to development of pharmacological interventions for healthy ageing and longevity [5].
Acknowledgments
This article was supported by: Kuakini Medical Center and US National Institutes of Health Grants 5R01AG027060 (Kuakini Hawaii Lifespan Study) and 5R01AG038707 (Kuakini Hawaii Healthspan Study).
Footnotes
The authors have no financial conflicts of interest.
Contributor Information
Brian J. Morris, Email: brian.morris@sydney.edu.au.
D. Craig Willcox, Email: dcwillcox@hotmail.com.
Timothy A. Donlon, Email: donlon@hawaii.edu.
Bradley J. Willcox, Email: willcox@hawaii.edu.
References
- 1.Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech. 2002;59:331–334. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- 2.Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb AE, Brunet A. FOXO transcription factors: key regulators of cellular quality control. Trends Biochem Sci. 2014;39:159–169. doi: 10.1016/j.tibs.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris BJ. Seven sirtuins for seven deadly diseases of aging. Free Radic Biol Med. 2013;56:133–71. doi: 10.1016/j.freeradbiomed.2012.10.525. [DOI] [PubMed] [Google Scholar]
- 5.Eijkelenboom A, Burgering BM. FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol. 2013;14:83–97. doi: 10.1038/nrm3507. [DOI] [PubMed] [Google Scholar]
- 6.Morris BJ. A forkhead in the road to longevity: the molecular basis of lifespan becomes clearer. (Review) J Hypertens. 2005;23:1285–1309. doi: 10.1097/01.hjh.0000173509.45363.dd. [DOI] [PubMed] [Google Scholar]
- 7.Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, Park Y, Hay N. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592–604. doi: 10.1016/j.devcel.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 9.Eijkelenboom A, Mokry M, de Wit E, Smits LM, Polderman PE, van Triest MH, van Boxtel R, Schulze A, de Laat W, Cuppen E, Burgering BM. Genome-wide analysis of FOXO3 mediated transcription regulation through RNA polymerase II profiling. Mol Syst Biol. 2013;9(article 638):1–15. doi: 10.1038/msb.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenyon C. A pathway that links reproductive status to lifespan in Caenorhabditis elegans. Ann N Y Acad Sci. 2010;1204:156–162. doi: 10.1111/j.1749-6632.2010.05640.x. [DOI] [PubMed] [Google Scholar]
- 11.Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004;305:361. doi: 10.1126/science.1098219. [DOI] [PubMed] [Google Scholar]
- 12.Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 13.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 14.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 15.Solon-Biet SM, McMahon AC, Ballard JW, Ruohonen K, Wu LE, Cogger VC, Warren A, Huang X, Pichaud N, Melvin RG, Gokarn R, Khalil M, Turner N, Cooney GJ, Sinclair DA, Raubenheimer D, Le Couteur DG, Simpson SJ. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bosch TCG, Anton-Erxleben F, Hemmrich G, Khalturin K. The Hydra polyp: nothing but an active stem cell community. Dev Growth Differ. 2010;52:15–25. doi: 10.1111/j.1440-169X.2009.01143.x. [DOI] [PubMed] [Google Scholar]
- 17.Boehm A-M, Rosenstiel P, Bosch TC. Stem cells and aging from a quasi-immortal point of view. Bioessays. 2013;35:994–1003. doi: 10.1002/bies.201300075. [DOI] [PubMed] [Google Scholar]
- 18.Boehm AM, Khalturin K, Anton-Erxleben F, Hemmrich G, Klostermeier UC, Lopez-Quintero JA, Oberg HH, Puchert M, Rosenstiel P, Wittlieb J, Bosch TC. FoxO is a critical regulator of stem cell maintenance in immortal Hydra. Proc Natl Acad Sci USA. 2012;109:19697–19702. doi: 10.1073/pnas.1209714109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaible R, Sussman M. FOXO in aging: did evolutionary diversification of FOXO function distract it from prolonging life? BioEssays. 2013;35:1101–1110. doi: 10.1002/bies.201300078. [DOI] [PubMed] [Google Scholar]
- 20.Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao JM, Song XL, Hong YQ, Zhu HL, Li C, Zhang T, Chen W, Zhao SC, Chen Q. Association between FOXO3A gene polymorphisms and human longevity: a meta-analysis. Asian Journal of Andrology. 2014;16(3):446–452. doi: 10.4103/1008-682X.123673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broer L, Buchman AS, Deelen J, Evans DS, Faul JD, Lunetta KL, Sebastiani P, Smith JA, Smith AV, Tanaka T, Yu L, Arnold AM, Aspelund T, Benjamin EJ, De Jager PL, Eirkisdottir G, Evans DA, Garcia ME, Hofman A, Kaplan RC, Kardia SL, Kiel DP, Oostra BA, Orwoll ES, Parimi N, Psaty BM, Rivadeneira F, Rotter JI, Seshadri S, Singleton A, Tiemeier H, Uitterlinden AG, Zhao W, Bandinelli S, Bennett DA, Ferrucci L, Gudnason V, Harris TB, Karasik D, Launer LJ, Perls TT, Slagboom PE, Tranah GJ, Weir DR, Newman AB, van Duijn CM, Murabito JM. GWAS of longevity in CHARGE consortium confirms APOE and FOXO3 candidacy. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks-Wilson AR. Genetics of healthy aging and longevity. Hum Genet. 2013;132:1323–1338. doi: 10.1007/s00439-013-1342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murabito JM, Yuan R, Lunetta KL. The search for longevity and healthy aging genes: insights from epidemiological studies and samples of long-lived individuals. J Gerontol A Biol Sci Med Sci. 2012;67:470–479. doi: 10.1093/gerona/gls089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donlon TA, Curb JD, He Q, Grove JS, Masaki KH, Rodriguez B, Elliott A, Willcox DC, Willcox BJ. FOXO3 gene variants and human aging: Coding variants may not be key players. J Gerontol A Biol Sci Med Sci. 2012;67:1132–1139. doi: 10.1093/gerona/gls067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuningas M, Mägi R, Westendorp RG, Slagboom PE, Remm M, van Heemst D. Haplotypes in the human Foxo1a and Foxo3a genes; impact on disease and mortality at old age. Eur J Hum Genet. 2007;15:294–301. doi: 10.1038/sj.ejhg.5201766. [DOI] [PubMed] [Google Scholar]
- 27.Forte G, Grossi V, Celestini V, Lucisano G, Scardapane M, Varvara D, Patruno M, Bagnulo R, Loconte D, Giunti L, Petracca A, Giglio S, Genuardi M, Pellegrini F, Resta N, Simone C. Characterization of the rs2802292 SNP identifies FOXO3A as a modifier locus predicting cancer risk in patients with PJS and PHTS hamartomatous polyposis syndromes. BMC Cancer. 2014;14:661. doi: 10.1186/1471-2407-14-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011;1813:1978–1986. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Banasik K, Ribel-Madsen R, Gjesing AP, Wegner L, Andersson A, Poulsen P, Borglykke A, Witte DR, Pedersen O, Hansen T, Vaag A. The FOXO3A rs2802292 G-allele associates with improved peripheral and hepatic insulin sensitivity and increased skeletal muscle-FOXO3A mRNA expression in twins. J Clin Endocrinol Metab. 2011;96:E119–E124. doi: 10.1210/jc.2010-0881. [DOI] [PubMed] [Google Scholar]
- 30.He Q, Morris BJ, Grove JS, Petrovitch H, Ross W, Masaki KH, Rodriguez B, Chen R, Donlon TA, Willcox DC, Willcox BJ. Shorter men live longer: association of height with longevity and FOXO3 genotype in American men of Japanese ancestry. PLoS One. 2014;9(5):e94385. doi: 10.1371/journal.pone.0094385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng SL. Immune regulation by Foxo transcription factors. Autoimmunity. 2007;40:462–469. doi: 10.1080/08916930701464913. [DOI] [PubMed] [Google Scholar]
- 32.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, Carrera P, Inhester T, Schultze JL, Hoch M. FOXO-dependent regulation of innate immune homeostasis. Nature. 2010;463:369–373. doi: 10.1038/nature08698. [DOI] [PubMed] [Google Scholar]
- 34.Lin L, Hron JD, Peng SL. Regulation of NF-κB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Oh HM, Yu CR, Golestaneh N, Amadi-Obi A, Lee YS, Eseonu A, Mahdi RM, Egwuagu CE. STAT3 protein promotes T-cell survival and inhibits interleukin-2 production through up-regulation of Class O Forkhead transcription factors. J Biol Chem. 2011;286:30888–30897. doi: 10.1074/jbc.M111.253500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dejean AS, Beisner DR, Ch’en IL, Kerdiles YM, Babour A, Arden KC, Castrillon DH, DePinho RA, Hedrick SM. Transcription factor Foxo3 controls the magnitude of T cell immune responses by modulating the function of dendritic cells. Nat Immunol. 2009;10:504–513. doi: 10.1038/ni.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JK, Bettencourt R, Brenner D, Le TA, Barrett-Connor E, Loomba R. Association between serum interleukin-6 concentrations and mortality in older adults: the Rancho Bernardo study. PLoS One. 2012;7:article e34218. doi: 10.1371/journal.pone.0034218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JC, Espéli M, Anderson CA, Linterman MA, Pocock JM, Williams NJ, Roberts R, Viatte S, Fu B, Peshu N, Hien TT, Phu NH, Wesley E, Edwards C, Ahmad T, Mansfield JC, Gearry R, Dunstan S, Williams TN, Barton A, Vinuesa CG, Parkes M, Lyons PA, Smith KG. Human SNP links differential outcomes in inflammatory and infectious disease to a FOXO3-regulated pathway. Cell. 2013;155:57–69. doi: 10.1016/j.cell.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 40.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abid MR, Yano K, Guo S, Patel VI, Shrikhande G, Spokes KC, Ferran C, Aird WC. Forkhead transcription factors inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. J Biol Chem. 2005;280:29864–29873. doi: 10.1074/jbc.M502149200. [DOI] [PubMed] [Google Scholar]
- 42.Lee HY, Chung JW, Youn SW, Kim JY, Park K, Koo BK, Oh BH, Park YB, Chaqour B, Walsh K, Kim HS. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2007;100:372–380. doi: 10.1161/01.RES.0000257945.97958.77. [DOI] [PubMed] [Google Scholar]
- 43.Zhang S, Liu L, Wang R, Tuo H, Guo Y, Yi L, Wang D, Wang J. MicroRNA-217 promotes angiogenesis of human cytomegalovirus-infected endothelial cells through downregulation of SIRT1 and FOXO3A. PLoS One. 2013;8(article e83620):1–8. doi: 10.1371/journal.pone.0083620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsuchiya K, Tanaka J, Shuiqing Y, Welch CL, DePinho RA, Tabas I, Tall AR, Goldberg IJ, Accili D. FoxOs integrate pleiotropic actions of insulin in vascular endothelium to protect mice from atherosclerosis. Cell Metab. 2012;15:372–381. doi: 10.1016/j.cmet.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tao R, Xiong X, DePinho RA, Deng CX, Dong XC. FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein (LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9 (Pcsk9) gene expression. J Biol Chem. 2013;288:29252–29259. doi: 10.1074/jbc.M113.481473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skurk C, Izumiya Y, Maatz H, Razeghi P, Shiojima I, Sandri M, Sato K, Zeng L, Schiekofer S, Pimentel D, Lecker S, Taegtmeyer H, Goldberg AL, Walsh K. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 2005;280:20814–20823. doi: 10.1074/jbc.M500528200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chiribau CB, Cheng L, Cucoranu IC, Yu YS, Clempus RE, Sorescu D. FOXO3A regulates peroxiredoxin III expression in human cardiac fibroblasts. J Biol Chem. 2008;283:8211–8217. doi: 10.1074/jbc.M710610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Essafi A, Fernández de Mattos S, Hassen YA, Soeiro I, Mufti GJ, Thomas NS, Medema RH, Lam EW. Direct transcriptional regulation of Bim by FoxO3a mediates STI571-induced apoptosis in Bcr-Abl-expressing cells. Oncogene. 2005;24:2317–2329. doi: 10.1038/sj.onc.1208421. [DOI] [PubMed] [Google Scholar]
- 50.Sunters A, Fernández de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 51.Delpuech O, Griffiths B, East P, Essafi A, Lam EW, Burgering B, Downward J, Schulze A. Induction of Mxi1-SR alpha by FOXO3a contributes to repression of Myc-dependent gene expression. Mol Cell Biol. 2007;27:4917–4930. doi: 10.1128/MCB.01789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sykes SM, Lane SW, Bullinger L, Kalaitzidis D, Yusuf R, Saez B, Ferraro F, Mercier F, Singh H, Brumme KM, Acharya SS, Scholl C, Tothova Z, Attar EC, Fröhling S, DePinho RA, Armstrong SA, Gilliland DG, Scadden DT. AKT/FOXO signaling enforces reversible differentiation blockade in myeloid leukemias. Cell. 2011;146:697–708. doi: 10.1016/j.cell.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hui RC, Gomes AR, Constantinidou D, Costa JR, Karadedou CT, Fernandez de Mattos S, Wymann MP, Brosens JJ, Schulze A, Lam EW. The forkhead transcription factor FOXO3a increases phosphoinositide-3 kinase/Akt activity in drug-resistant leukemic cells through induction of PIK3CA expression. Mol Cell Biol. 2008;28:5886–5898. doi: 10.1128/MCB.01265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell. 2007;1:101–112. doi: 10.1016/j.stem.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Zhang X, Yalcin S, Lee DF, Yeh TY, Lee SM, Su J, Mungamuri SK, Rimmelé P, Kennedy M, Sellers R, Landthaler M, Tuschl T, Chi NW, Lemischka I, Keller G, Ghaffari S. FOXO1 is an essential regulator of pluripotency in human embryonic stem cells. Nat Cell Biol. 2011;13:1092–1099. doi: 10.1038/ncb2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, Ghaffari S. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. J Clin Invest. 2007;117:2133–2144. doi: 10.1172/JCI31807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gopinath SD, Webb AE, Brunet A, Rando TA. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Reports. 2014;2:414–426. doi: 10.1016/j.stemcr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yeo H, Lyssiotis CA, Zhang Y, Ying H, Asara JM, Cantley LC, Paik JH. FoxO3 coordinates metabolic pathways to maintain redox balance in neural stem cells. EMBO J. 2013;32:2589–2602. doi: 10.1038/emboj.2013.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Uchida S, De Gaspari P, Kostin S, Jenniches K, Kilic A, Izumiya Y, Shiojima I, Grosse Kreymborg K, Renz H, Walsh K, Braun T. Sca1-derived cells are a source of myocardial renewal in the murine adult heart. Stem Cell Reports. 2013;1:397–410. doi: 10.1016/j.stemcr.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegué E, DePinho RA, Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128:325–339. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Rimmelé P, Bigarella CL, Liang RBI, Dieguez-Gonzalez R, Barbet G, Donovan M, Brugnara C, Blander JM, Sinclair DA, Ghaffari S. Aging-like phenotype and defective lineage specification in SIRT1-deleted hematopoietic stem and progenitor cells. Stem Cell Reports. 2014;3:44–59. doi: 10.1016/j.stemcr.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holstege H, Pfeiffer W, Sie D, Hulsman M, Nicholas TJ, Lee CC, Ross T, Lin J, Miller MA, Ylstra B, Meijers-Heijboer H, Brugman MH, Staal FJ, Holstege G, Reinders MJ, Harkins TT, Levy S, Sistermans EA. Somatic mutations found in the healthy blood compartment of a 115-yr-old woman demonstrate oligoclonal hematopoiesis. Genome Res. 2014;24:733–742. doi: 10.1101/gr.162131.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Webb AE, Pollina EA, Vierbuchen T, Urbán N, Ucar D, Leeman DS, Martynoga B, Sewak M, Rando TA, Guillemot F, Wernig M, Brunet A. FOXO3 shares common targets with ASCL1 genome-wide and inhibits ASCL1-dependent neurogenesis. Cell Rep. 2013;4:477–491. doi: 10.1016/j.celrep.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niu Y, Na L, Feng R, Gong L, Zhao Y, Li Q, Li Y, Sun C. The phytochemical, EGCG, extends lifespan by reducing liver and kidney function damage and improving age-associated inflammation and oxidative stress in healthy rats. Aging Cell. 2013;12:1041–1049. doi: 10.1111/acel.12133. [DOI] [PubMed] [Google Scholar]
- 66.Zeng Y, Chen H, Ni T, Ruan R, Feng L, Nie C, Cheng L, Li Y, Tao W, Gu J, Land KC, Yashin A, Tan Q, Yang Z, Bolund L, Yang H, Hauser E, Willcox DC, Willcox BJ, Tian XL, Vaupel JW. GxE Interactions between FOXO genotypes and tea drinking are significantly associated with cognitive disability at advanced ages in China. J Gerontol A Biol Sci Med Sci. 2014;xx doi: 10.1093/gerona/glu060. (Epub ahead of print 3 Jun 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zingg JM, Hasan ST, Cowan D, Ricciarelli R, Azzi A, Meydani M. Regulatory effects of curcumin on lipid accumulation in monocytes/macrophages. J Cell Biochem. 2012;113(3):833–40. doi: 10.1002/jcb.23411. [DOI] [PubMed] [Google Scholar]
- 68.Stefani M, Markus MA, Lin RC, Pinese M, Dawes IW, Morris BJ. The effect of resveratrol on a cell model of human aging. Ann N Y Acad Sci. 2007;1114:407–418. doi: 10.1196/annals.1396.001. [DOI] [PubMed] [Google Scholar]
- 69.Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends Pharmacol Sci. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]