Abstract
The traditional diet in Okinawa is anchored by root vegetables (principally sweet potatoes), green and yellow vegetables, soybean-based foods, and medicinal plants. Marine foods, lean meats, fruit, medicinal garnishes and spices, tea, alcohol are also moderately consumed. Many characteristics of the traditional Okinawan diet are shared with other healthy dietary patterns, including the traditional Mediterranean diet, DASH diet, and Portfolio diet. All these dietary patterns are associated with reduced risk for cardiovascular disease, among other age-associated diseases. Overall, the important shared features of these healthy dietary patterns include: high intake of unrefined carbohydrates, moderate protein intake with emphasis on vegetables/legumes, fish, and lean meats as sources, and a healthy fat profile (higher in mono/polyunsaturated fats, lower in saturated fat; rich in omega-3). The healthy fat intake is likely one mechanism for reducing inflammation, optimizing cholesterol, and other risk factors. Additionally, the lower caloric density of plant-rich diets results in lower caloric intake with concomitant high intake of phytonutrients and antioxidants. Other shared features include low glycemic load, less inflammation and oxidative stress, and potential modulation of aging-related biological pathways. This may reduce risk for chronic age-associated diseases and promote healthy aging and longevity.
Keywords: healthy aging, Okinawa, diet, Portfolio, DASH, longevity
Introduction
Most deaths across nations (including low and middle income countries) are now due to chronic disease and the proportion of worldwide mortality from chronic age-associated disease is projected to escalate further, reaching 66 per cent in 2030 (World Health Organization, 2005).
This global increase in disease burden from cardiovascular disease, cancer, diabetes and other chronic age-associated diseases reflects social and economic changes, including lifestyle and diet, as well as population aging. Although the world-wide increase in life expectancy (at birth) is among the world`s greatest achievements, the potential socio-economic costs of a higher chronic disease burden rise sharply with an aging society. The good news is that mounting evidence suggests effective public health policies and programs can do much to mitigate this risk and help people remain healthy as they age.
Reflecting this untapped potential for preventive public health efforts, the World Health Organization (WHO) and the U.S. Centers for Disease Control and Prevention (CDC) have estimated that 80 percent of coronary heart disease (CHD) and type-2 diabetes mellitus (T2DM) as well as 40 percent of cancers, could be prevented by improving three health behaviors: eating habits, physical activity, and tobacco use (World Health Organization, 2005; Centers for Disease Control and Prevention, 2009). Although difficult to quantify, of these three risk factors, dietary habits may have become the most important modifiable risk factor in many nations. Backing up this contention is a recent study that assessed 17 major risk factors and found that composition of the diet constituted the largest cluster of risk factors responsible for death (26%) and the highest percentage of disability-adjusted life years lost (14%) in the US (US Burden of Disease Collaborators et al. 2013).
Because nutritional issues play such a key role in a wide range of age-associated diseases and contribute so much to morbidity, disability and mortality as we age, the potential for better nutritional habits to improve health outcomes in older populations is a largely untapped (yet urgently needed) measure. Although some dietary patterns are well known to be associated with the prevention of chronic age-associated diseases, such as the traditional Mediterranean diet, the focus of this manuscript will be to explore other, less well known, dietary patterns that have also been linked to decreased risk for chronic age-associated diseases, such as the Okinawan Diet. Okinawan elders, many of whom still eat a very healthy diet, represent one of the healthiest populations of seniors on the planet.
Achieving Healthy Aging: The Art of the Possible
What can we realistically achieve in terms of healthy human aging? There is ongoing debate that seems to swing between two poles. Some scientists optimistically argue that technological breakthroughs may soon extend human lifespan to a thousand or more years (de Grey et al. 2002). Others argue that we may have already “hit the wall” in terms of the potential for growth in human life expectancy and we might even witness declines in the 21st century due to obesity and the re-emergence of infectious disease threats (Olshansky et al. 2005).
Caloric restriction is among the most robust interventions in model organisms of aging for extending lifespan (Masoro, 2005). With the plethora of recent studies of primates, including humans, some argue that dietary interventions such as caloric restriction have the potential to significantly extend human lifespan--as they have in invertebrate and animal models (Anderson & Weindruch 2012; Mercken et al. 2012). Although the evidence for dietary restriction effects in primates (including humans) is promising, and there are individuals who follow such a regimen, it is not practical as a public health policy. Nor are mechanistic studies of model organisms always applicable to humans thus caution must be used when extrapolating such findings to human populations.
On a more practical level, substantial population health gains may be possible in the future if we can delay the onset of common age-related diseases by currently available risk factor modification (Willcox B et al, 2006; de la Torre, 2012; Yaffe et al., 2012; Willcox et al, 2013). In order to further quantify the potentially achievable population-wide benefits of such an approach, public health scientists Olshansky and colleagues (2007) estimated that delaying typical age-related morbidity in Americans by just seven years would decrease the age-specific risk of disability and death by 50%, allowing a substantial improvement in both lifespan and more importantly, in healthspan. The authors label this the “longevity dividend”.
Combining what we already know about modifying risk factors for chronic disease with a better understanding of the genetics of healthy aging may help optimize future targets for intervention. For example, a review by Cluett and Melzer (2009) of over 50 GWAS studies of four major aging-related phenotypes found that cell cycle, regrowth and tissue repair were the most common biological pathways across these aging-related phenotypes, and may represent good targets for intervention. Nevertheless, whether one uses pharmacologically active foods or food extracts to target specific aging-related biological pathways or nutritional interventions that target multiple pathways related to aging, it will be important to identify individual genetic susceptibility to particular risk factor interventions, such as optimizing blood sugar or reducing sodium or cholesterol (de Magalhães et al., 2011; Mercken et al., 2012; Morris et al., 2005; Rattan, 2012).
The scientific discipline of epidemiology, which includes genetic epidemiology and nutritional epidemiology, may provide clues as to where to begin and which path to follow. From an epidemiology of aging perspective, wide variability exists in the global prevalence of age-related diseases and past studies have suggested that while genes are important, the majority of the variation in overall human lifespan (Gögele et al., 2011) and perhaps more importantly, in healthspan (Rattan, 2012), has been shown to be environmental. That is, dietary habits, physical activity, smoking and other risk behaviors, access to health care, immunization and other public health practices, and other social determinants of health, account for the majority of variation in risk for age-related morbidity and mortality.
Backing up this contention is recent epidemiological research that has focused upon risk factors for healthy aging which has shown that avoiding nine common risk factors in mid-life may increase odds of healthy aging into octogenarian and nonagenarian years by over four-fold (Willcox et al. 2006). Moreover, nutritional epidemiological research on risk factor modification as well as dietary intervention studies have shown that the benefits on particular lipid and inflammatory-related risk factors (e.g. LDL cholesterol, C-reactive protein) from shifting to a healthier dietary pattern can be substantial and even rival that of pharmacotherapy (Jenkins et al. 2005). Therefore, it stands to reason that a population-wide shift to a healthier dietary pattern may facilitate significant delays in age-related morbidity and decrease the age-specific risk of disability and death---allowing for substantial improvement in both lifespan and healthspan. This would bring us closer to realizing the “longevity dividend”.
Some large scale population-wide public health interventions that have used nutritional approaches (usually as part of a multi-intervention strategy) for age-associated diseases, such as cardiovascular disease reduction in the North Karelia Project, have been very successful, while others have been less so (Papadakis and Moroz, 2008; Puska, 2010). In North Karelia, Finland, a comprehensive lifestyle intervention that has lasted over three decades has been associated with an 80% risk reduction for cardiovascular disease (CVD)—three quarters of that risk was explained by reduction in common risk factors (e.g. cholesterol, blood pressure and smoking) (Vartiainen et al, 2010). While pharmacotherapy and other medical therapies also became more common during the intervention period, dietary change was a major factor in the CVD risk reduction. For example, statins for cholesterol reduction became popular during the public health intervention period but changes in dietary fat quality and cholesterol intake explained 65 % of the cholesterol decrease in men and 60 % of the decrease in women, with reductions in dietary saturated fat as the main explanatory factor (47 % in men and 41 % in women) (Valsta et al, 2010) The impact of lipid-lowering medication on observed cholesterol levels was found to be less important, at 16 % among men, and 7 % among women. Such comprehensive, long-term population studies are rare and there is a need for more such studies to support epidemiological findings and prior short-term, risk factor intervention trials.
Illustrating the potential of this approach for age-related diseases, some long-lived populations, such as the Japanese, already appear to be delaying typical age-related morbidity and have achieved a significantly longer life expectancy and much lower rates of disability than many western nations such as the US. (US Burden of Disease Collaborators et al., 2013; Ikeda et al 2011; Willcox et al, 2013). Such populations (or sub-populations) tend also to have higher numbers of oldest-old or long-lived individuals, such as nonagenarians or centenarians. The most remarkable of these populations have been referred to as “Blue Zones”, a concept that refers to a demographic and/or geographic area with high population longevity and originating from the blue color on demographic maps (Poulain et al. 2004; Appel, 2008). There is even some preliminary evidence that the “Blue Zones” share some common healthy eating patterns (Davinelli et al. 2012; Appel 2008). This is encouraging. The fact that the Japanese went from longevity laggards (low average life expectancy at birth) in the first half of the 20th century to longevity leaders in the second half (world’s longest lifespan and healthspan) is due, in large part, to focused investment in public health infrastructure and programs and a better educated population (Ikeda et al. 2011; Mori et al. 2012; Sugiura et al. 2010; US Burden of Disease Collaborators et al. 2013; Willcox et al, 2013). This buttresses belief in the “art of the possible.” That is, through broad public health efforts that engage major stakeholders, including industry and government, we can educate, promote and implement healthy lifestyle habits in places that have major community health impacts, such schools and workplaces. By doing so we might substantially expand the “Blue Zones”.
Reducing Risk Factors, Improving Biomarkers: The Potential of Dietary Therapy
Dietary knowledge has evolved substantially over the past few decades from calories, macronutrients and micronutrients to exploring the pharmaceutical or “nutraceutical” effects of particular foods and food components. A whole research and commercial industry has sprung up around the concept of “functional foods”, some of which might be as efficacious as commonly prescribed medications for ameliorating some medical conditions. For example, various foods, such as soy protein, fiber (ie.oat bran) and plant sterols can have an additive LDL-cholesterol-lowering effect and substantially attenuate risk for CHD (Jenkins et al. 2005). Direct comparison of a dietary portfolio of cholesterol-lowering foods versus pharmacotherapy in hypercholesterolemic patients has revealed that this dietary approach can be as effective as first generation statins in achieving current lipid goals for primary prevention of CHD (Jenkins et al. 2005).
Dietary intervention studies are now being undertaken using combinations of risk lowering foods as an overall eating pattern rather than encouraging single foods, in order to achieve more effective results and increase dietary compliance. This trend has been driven in large part by the response to new dietary recommendations for the management of common risk factors for CHD, in order to hit stricter targets (such as cholesterol), that in turn helps reduce the risk of other chronic age-associated diseases. There is also added impetus from the drive by industry to obtain health claims for particular “functional foods” or food components. This dietary pattern (or portfolio) approach has increased the potential relevance of dietary therapy and may yield nutrition strategies that help bridge the gap between a healthy diet and pharmacotherapy (see Table 1 below).
Table 1.
Dietary Patterns of Selected Healthy Diets
| Major Nutrient | Mediterraneanb | Traditional Okinawac | Okinawan Elder’s Dietd | Modern Okinawad | DASHe | Portfoliof |
|---|---|---|---|---|---|---|
| Carbohydrate (% kcal) a | 43% | 85% | 58% | 58% | 55% | 49% |
| Protein (% kcal)* | 13% | 9% | 16% | 15% | 18% | 20% |
| Fat (% kcal)* | 42% | 6% | 26% | 28% | 27% | 30% |
| Sat. Fat (% kcal) | 9% | 2% | 7% | 7% | 6% | 6% |
| Cholesterol (mg/1000 kcal) | 75 mg | -- | 156 mg | 164 mg | 72 mg | 49 mg |
| Sodium (mg/d) | 2157 mgg | 1113 mg | 3100 mg | 3256 mg | 1150 mg | 1921 mg |
| Potassium (mg/d) | 3637 mgg | 5199 mg | 1999 mg | 1901 mg | 4700 mg | 3382 mg |
Macronutrient data may not equal exactly 100% due to unaccounted alcohol calories and/or rounding error.
Is There an Ideal Dietary Pattern for Healthy Aging?
In a discouraging trend, modern societies seem to be converging on a common dietary pattern—one that is not ideal for healthy aging. This diet is high in saturated fat, sugar, and refined carbohydrates but low in fiber and phytonutrients, and is often labeled the "Western diet." In fact, recent research on the “nutrition transition” has concluded that the majority of nations in Asia, Latin America, Northern Africa, the urban areas of sub-Saharan Africa, and the Middle East share remarkably similar dietary and disease pattern shifts (Popkin 2001). Common to this shift is the consumption of more animal products, more fat and more sugar. Conversely, there have been substantial decreases in cereal products and fiber. Different foods drive this dietary “Westernization” in different regions (Popkin 2001). In Asia, as one example, more edible oil consumption is a large aspect of an overall increase in dietary fat in that region. Regardless of the heterogeneity in particular details of this dietary shift, the overall dietary pattern is from low to high caloric (energy) density with a concomitant high to low shift in nutrient density. This dietary shift correlates well with an increase in chronic, degenerative diseases and with reduced healthspan.
The Western Diet
The relation between a nutrient-poor energy dense diet (e.g. Western dietary pattern), sedentary lifestyle and increased chronic disease risk, has been well established (Cordain et al. 2005; Hu 2008; Popkin 1999; World Health Organization, 2003). This dietary pattern is characterized by high caloric density, a high intake of meat (especially red and processed meats) and accompanying saturated fat, an unfavorable n-6:n-3 polyunsaturated fatty acid (PUFA) ratio, a high intake of refined carbohydrates, and a low intake of fruits, vegetables, fiber and phytonutrients. This is a common dietary pattern in the United States and many other so-called “Western” nations. However, it is also a dietary pattern that, as previously mentioned, modernizing societies are adopting.
The USDA reports that, in general, Americans (this could be extrapolated to all who follow the Western dietary pattern) consume too many calories and too much saturated and trans fats, cholesterol, sugar, and salt. They typically lack sufficient dietary fiber, calcium, magnesium, potassium, and the anti-oxidant vitamins A (as carotenoids), C and E. This kind of dietary pattern not only leads to nutritional deficiencies but also promotes a cluster of metabolic problems including obesity, reduced insulin sensitivity, glucose intolerance, dyslipidemia, as well as systematic inflammation-all risk factors for the most common age associated diseases that include cardiovascular diseases, particular cancers, type 2 diabetes, among others.
As indicated above, dietary pattern analysis may represent a useful addition to the repertoire of researchers who study the relationship between diet and chronic disease. However, the reality, until very recently, has been that researchers have focused mainly upon the effects of individual nutrients and sometimes foods, but rarely on dietary patterns on disease risk factors, biomarkers, or morbidity. Prospective, nutrition-related cohort studies with all-cause mortality or other aging-related outcomes are not common since a large cohort must be followed for adequate statistical power and/or the duration of follow-up needs to be long for enough events to occur—a component than most studies can ill afford (Willcox et al, 2013).
The DASH Diet
High blood pressure affects approximately 1 in 3 Americans (Chobanian et al, 2003). The Dietary Approaches to Stop Hypertension (DASH) diet is, arguably, the most common physician prescribed diet to fight high blood pressure and was, in fact, originally developed by the National Heart, Lung, and Blood Institute to do just that, therefore, the acronym (Champagne 2006; Savika et al, 2010). The DASH dietary pattern is rich in fruits and vegetables, whole grains, low-fat dairy products, fish, poultry, beans, nuts and seeds. It also contains less sodium; sugar; fats; and red meat than the usual western diet as described above. Designed with cardiovascular health in mind, the DASH diet is also lower in saturated and trans fatty acids and cholesterol and rich in nutrients such as potassium, magnesium, calcium, protein, and fiber that are helpful for lowering blood pressure. Research on the DASH dietary pattern has shown that it not only can lower blood pressure but also improve other risk factors for cardiovascular disease such as HDL cholesterol levels, triglycerides or blood sugar. Long-term studies of the DASH dietary pattern have been associated with lower risk for hypertension and other cardiovascular diseases, diabetes, and several types of cancer, among other chronic age associated diseases (Fung et al. 2010; Shirani et al. 2013; Salehi-Abargouei et al. 2013)
The Portfolio Diet
As briefly discussed earlier, in an attempt to increase the effectiveness of diet in reducing serum cholesterol, the National Cholesterol Education Program (NCEP) and the American Heart Association recently recommended the use of functional foods, or foods high in components that reduce cholesterol, as options in dietary strategies. With these recommendations in mind the Portfolio Diet was designed by University of Toronto researchers to test the effectiveness of this dietary approach against standard drug therapy (statins) in hypercholesterolemic participants (Jenkins et al, 2003, 2005). Plant foods are emphasized in this vegetarian dietary pattern rich in vegetables such as broccoli, eggplant, tomatoes, onions, okra. Whole grains are plentiful, including oats and barley, and other prominent foods include vegetable-based margarine, almonds, and soy protein.
The study found that when these phytonutrient-rich foods were mixed into an already healthy diet (by NCEP standards), LDL cholesterol was reduced by a further 30% (Jenkins et al. 2003). This finding suggests that a combination of LDL-lowering dietary strategies (e.g., vicous fiber, plant stanols, soy protein, almonds) has additive effects when added to a healthy diet and can produce clinically significant reductions in CHD risk (Kendall and Jenkins 2004). A number of other benefits such as lower inflammation lower CRP (c-reactive protein) blood levels and weight loss were also witnessed (Jenkins et al. 2003). Viscous fibers facilitate bile acid loss from the gut (which binds cholesterol), plant sterols reduce cholesterol absorption from the gut, soy proteins reduce hepatic cholesterol synthesis and may increase hepatic LDL receptor uptake of cholesterol. Almonds, which contain monounsaturated fat, plant sterols, plant protein and fiber, and other phytochemicals, operate through a variety of mechanisms to reduce LDL (Jenkins et al 2005). There was a clear advantage for the Portfolio diet for cholesterol reduction versus a comparison vegetarian, low saturated fat diet without these four “functional foods.” (Jenkins et al. 2011).
Is variety the spice of life (and health)?
Is consuming a variety of different foods important for health? The Japanese certainly believe this is true. A common lesson for children, illustrated in a popular children’s’ book, is to bring a daily lunch bento (box) with “something from the mountains and something from the sea”. This popular expression illustrates the importance placed on vegetables and sea foods--and variety. The Japan Ministry of Health, Labour and Welfare recommends that one consume 30 different foods daily in order to get a wide variety of nutrients (Willcox et al, 2004).
When one examines healthy eating patterns around the world (such as the Seven Countries Study) one is surprised at the range of foods, cuisines, cooking styles, tastes, and use of spices and herbs. These are obvious places of difference that seem to fascinate scientists and lay people alike. However, of enduring interest (and intense debate), there is also significant variation in macronutrient intake, in healthy eating patterns. One only need compare the traditional diets of Okinawa, which is high in carbohydrate but low in fat, to that of the Mediterranean, which is high in fat, but low in carbohydrate (see Table 1) to see this fact. Both diets, however, are relatively low in calories but nutrient dense.
The key, upon further analysis, is that these nutrient-dense diets are anchored by high quality foods—despite a range in macronutrients, both diets are dominated by low glycemic carbohydrates, lean proteins (much of it from plant sources), and healthy fats monounsaturated, omega-3), which is associated with reduced risk for chronic age associated diseases. A diet portfolio rich in fruits and vegetables, legumes and whole grains, but reduced in animal products and accompanying saturated fat, salt, sweets, and refined carbohydrates may be the most “prudent” to recommend for healthy aging. Adding in a DASH of Okinawan herbs and spices may also help keep sodium levels in check while boosting ones antioxidant and anti-inflammatory network capacity, which as we shall see below may help keep one on the right track to a healthy old age.
The Okinawan Diet
Much of the longevity advantage in Okinawa is thought to be related a healthy lifestyle; this includes the traditional diet (Willcox et al, 2004; Willcox et al. 2007), which is low in calories, yet nutritionally dense, particularly with regard to vitamins, minerals, and phytonutrients, several of which have neutraceutical potential.
“Haute cuisine” Okinawan style
Many wonder how the Okinawan traditional dietary pattern differs from the Japanese. Are they the same? Some jokingly state the Okinawan diet is Japanese with salsa, due to the influence of other Asian cuisines with a tendency toward spicier dishes. Although many similarities exist, including the high intake of vegetables and soy products, the low fat content, the taste for miso, plentiful fish and sea vegetables, and the lack of dairy products, the traditional Okinawan diet differs dramatically in some key areas (Willcox et al, 2004;2007). For example, the staple of the Okinawan diet was the ubiquitous sweet potato---not rice or other grains. Over half of daily caloric intake was from these colorful sweet tasting tuberous roots from the morning glory family. Other key areas of difference include the Okinawan taste for flavorings, such dashi or bonito, and the routine use of herbs and spices in place of salt in Okinawan “haute cuisine”.
Traditional Okinawan cuisine centers on the staple sweet potato, green-leafy or yellow-root vegetables, and soy (e.g. miso soup, tofu or other incarnations of this legume) which accompanied almost every meal. Smaller servings of fish, noodles, or lean meats flavored with herbs, spices, and cooking oil often accompanied these staples (Willcox et al, 2004). See Figure 1
Figure 1.
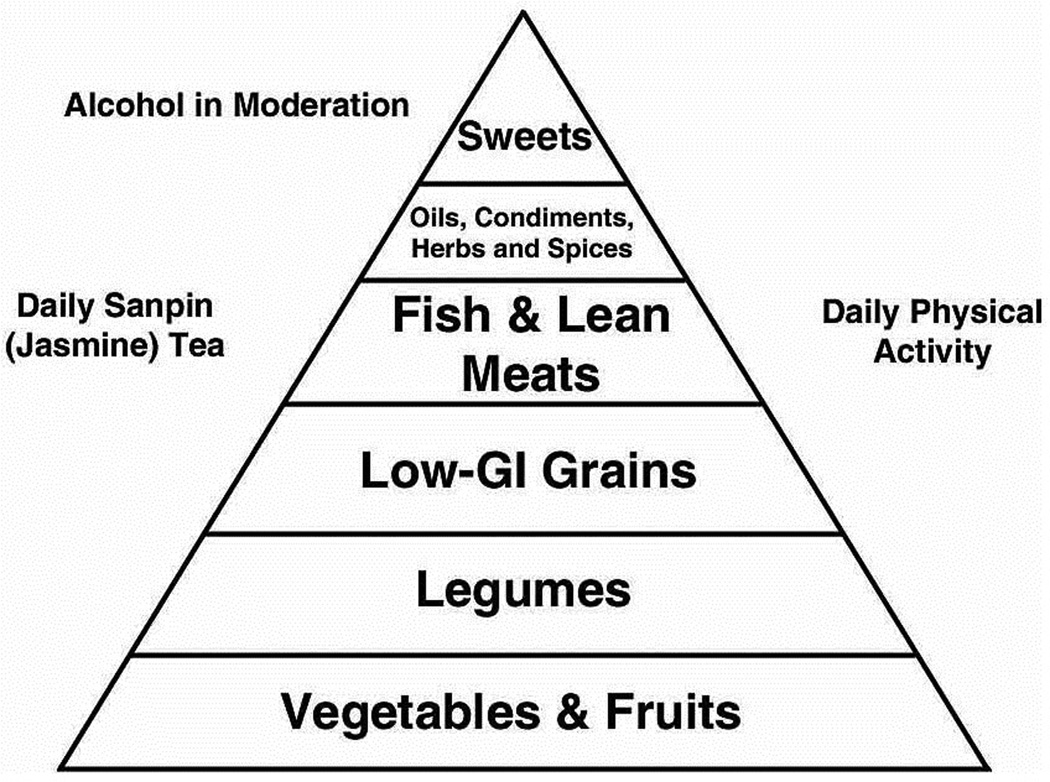
Traditional Okinawan diet food pyramid
A meal would typically begin with Okinawan-style miso soup. Unlike the Japanese version, Okinawans preferred to garnish their miso soup with small amounts of tofu, fish, pork, or vegetables. There are three main cooking styles: champuru, nbushi and irichi. The main dish was typically a champuru (stir-fried) vegetable dish, which dominated by vegetables such as bitter melon, cabbage, bamboo shoots or others accompanied by a side dish, such as konbu seaweed. This is typically simmered with a small amount of oil or pork fat, bonito broth (for flavor), and small amounts of fish or boiled pork. Nbushi style uses water rich vegetables such as daikon (a type of large white radish), Chinese okra, carrots, or pumpkin; seasons them with miso; and simmers them in their own juices. Irichi style focuses on less water-rich vegetables, and therefore uses a combination of simmering and stir-frying. Burdock, seaweed, dried daikon, or green papayas are favorites. The meal would typically be served with freshly brewed sanpin (jasmine) tea, on occasion followed with locally brewed awamori (millet brandy) (Willcox et al, 2004).
Almost vegetarians—by circumstance
As can be seen from the above descriptions of a typical meal, the Okinawans of old were not vegetarians but they were close to this eating pattern, but not by any particular conscious choice. Like most Asian populations in the first half of the 20th century, the average family simply could not afford meat, nor processed foods such as sugar, salt, cooking oil, or in the case of Okinawa, imported polished white rice. Staple foods were what could grow locally. Since Okinawa consists of a string of subtropical islands with seasonal, quite severe tropical storms, they had two growing seasons, which favored fresh plant foods. Some vegetables grew very well but rice did not. In fact, rice was supplanted in the 1600s by the sweet potato as the main staple, when it was first imported from China (Willcox et al, 2004: Todoriki et al, 2004: Robine et al, 2012). The sweet potato is hardy and can survive severe climates and thus became the main calorie source. Most other plant foods were also grown and consumed locally.
All families raised pigs, and chickens and sometimes other farm animals, such as goats. The majority of the population was engaged in farming or fishing or combined farming with local cottage industries such as carpentry or weaving (Willcox et al, 2004; 2006; 2007; Todoriki et al, 2004).
Living on an island meant ready access to fish, other sea creatures, and marine vegetables (particularly in the coastal areas), which were readily consumed. Of land animal meats, pork was the most commonly consumed meat and when pigs were slaughtered nothing was wasted. Typically, a pig was slaughtered early in the year at festival time and what was not eaten at that time was stored and consumed over the remainder of the year. A common expression is that “everything is eaten but the voice.” The entrails and the ears are still commonly consumed today (Willcox et al, 2004).
Although animal fat (from pigs) was often used for cooking due to infrequent access to other edible oils, the livestock rearing practices were far different from that of modern meat processing plants. The animals were “free range” and fed mostly throw-away vegetables, such as blemished sweet potatoes (and their stems and leaves) that were not to the tastes of their keepers. The result was a relatively higher level of beneficial n-3 fatty acids and less pro-atherogenic fats, such as saturated fat and n-6 PUFAs in the lean meat and fat stores of these animals (Willcox et al, 2004; Todoriki et al, 2004).
Moreover, the total amount of animal products in the diet was minuscule, with total fatty acid intake under 10% of energy intake. Even with the sharp increase in fat intake in the latter half of the 20th century (up to 27%) saturated fat intake in Okinawa still remains low and totals only about 7% of calories, which is well under most recommended allowances (the USDA recommends a total fat intake of less than 30% of energy intake with 10% or less from saturated fats) (See Table 1).
Ten Characteristics of the traditional Okinawa diet
As can be deduced from these descriptions of a typical meal, the traditional dietary pattern in Okinawa has the following characteristics:
Low caloric intake,
High consumption of vegetables (particularly root and green-yellow vegetables),
High consumption of legumes (mostly soybean in origin),
Moderate consumption of fish products (more in coastal areas),
Low consumption of meat products (mostly lean pork),
Low consumption of dairy products,
Low fat intake (high mono and polyunsaturated-to-saturated-fat ratio; low omega 6:3 ratio),
Emphasis on low-GI carbohydrates,
High fiber intake,
Moderate alcohol consumption.
The downside of East Asian diets in general (and the Japanese diet in particular) has been the high sodium content, mainly a result of the high intake of soy sauce, miso, salted fish, and pickled vegetables. Studies of the Japanese support a relation between higher intakes of sodium and higher rates of hypertension, cardiovascular diseases, in particular, cerebrovascular disease (Kawano et al. 2007; Miura et al. 2010; Nagata et al. 2004; Umesawa et al. 2008) as well as stomach cancer (Shikata et al. 2006; Tsugane et al. 2007). However, sodium intake has always been much lower in Okinawa when compared to other Japanese prefectures (Willcox et al, 2007).
As discussed above, local Okinawan cuisine has strong southern Chinese, South Asian and Southeast Asian influences (bitter greens, spices, peppers, turmeric), that results from active participation in the spice trade. Okinawa was an independent seafaring trading nation known as the Kingdom of the Ryukyus (from the 14th to the late 19th century) before it became a Japanese prefecture. Hypertensive effects of sodium consumption in the diet were also attenuated by the high consumption of vegetables rich in anti-hypertensive minerals (potassium, magnesium, and calcium) as well as the sodium wasting from their hot and humid subtropical climate (Willcox et al, 2004). See Table 2
Table 2.
Food Group Intake in Traditional Diets of Okinawans and Other Japanese
| Okinawa, 1949a | Japan, 1950 | |
|---|---|---|
| Food Group | Weight in grams (% total calories) | |
| Grains | 192 (33) | 481 (75) |
| Nuts, Seeds | <1 (<1) | <1 (<1) |
| Sugars | 3 (<1) | 8 (1) |
| Oils | 3 (2) | 3 (1) |
| Legumes (e.g., soy and other beans) | 71 (5) | 55 (4) |
| Fish | 15 (1) | 62 (5) |
| Meat (incl. poultry)b | 3 (<1) | 11 (<1) |
| Eggs | 1 (<1) | 7 (<1) |
| Dairy | <1 (<1) | 8 (<1) |
| Vegetables | 965 (58) | 301 (8) |
| Fruit b,c | <1 (<1) | 44 (1) |
| Pickled Vegetables | 0 (0) | 35 (1) |
Data derived from analysis of U.S. National Archives, archived food records, 1949 and Japan National Nutrition Survey, 1950.
Data derived from analysis of U.S. National Archives, archived food records, 1949 and Japan National Nutrition Survey, 1950.
Raw papaya classified as vegetable
Differences between the Traditional Okinawan and Japanese Diets
The dietary differences between Okinawans and other Japanese were once stark but have markedly narrowed in post-World War II birth cohorts, and in particular, since reversion of Okinawa from U.S. to Japanese administrations in 1972 (Todoriki et al, 2004; Willcox et al, 2008; 2012). This phenomenon has also been observed in the INTERMAP Study (Dennis et al, 2003; Zhou et al, 2003), where differences in traditional diets that were observed in older population cohort studies, such as the Seven Countries Study in the 1960s (Keys et al, 1966), had markedly narrowed by the 1990s. Therefore, in order to understand potential dietary influence on aging-related disease and longevity in older cohorts of Okinawans and other Japanese, where health and longevity advantages are the starkest, it is helpful to assess the food choices that may have influenced these aging-related phenotypes for most of their adult lives.
Table 2 illustrates several important points: One, differences in the intake of grains. 75% of the caloric intake of the Japanese diet originated from grains, principally refined (polished) white rice. In contrast, only 33% of the calories in the traditional Okinawan diet originated from grains, which was less dominated by white rice and more heavily dominated by millet and other lower glycemic load grains (Willcox et al, 2007; 2009). Two, vegetable/fruit intake was quite different. While both the traditional Japanese and Okinawan diets were not heavy in fruit and had some small differences in type of fruit (Okinawans had more tropical fruit)—both diets derived 1% or less of their caloric intake from fruit. Fruit tended to be a condiment or eaten as an after meal sweet. However, vegetable intake was markedly different between the two diets. While the traditional Japanese diet provided about 8% of caloric intake as vegetables the intake in Okinawans was seven times greater, in terms of caloric intake, at 58% of the diet. The majority of this vegetable intake originated from sweet potatoes, which were the staple food in the traditional Okinawan diet (Willcox et al, 2006; 2007; 2009).
The Healthiest of All Vegetables: The Staple Sweet potato
The sweet potato (Ipomoea batatas) is a dicotyledonous plant from the Convolvulaceae family, and although it is a perennial root vegetable similar in shape to the white “Irish potato” (Solanum tuberosum), it is only a distant cousin of the Irish tuber, which actually belongs to the Nightshade family. The edible tuberous root of the sweet potato is long and tapered, with a smooth and colorful skin that in Okinawa comes mainly in yellow, purple, or violet, or orange, shades. Some varieties are even close to red in appearance. The flesh of the most common Okinawan sweet potato (Satsuma Imo) is orange-yellow or dark purple (Beni Imo), however violet, beige, or white varieties can also be seen. The leaves and shoots (known as kandaba in Okinawa) are often consumed as greens and added to miso soup (Willcox et al, 2004; 2009).
It was only roughly a half century ago that the sweet potato was unceremoniously known as a food staple of the masses, mostly poor farmers or fisher-folk. Those in higher socioeconomic classes consumed more polished white rice, which was associated with an upper class lifestyle, and imported from mainland Japan where growing conditions are more hospitable to rice. By the 1990s, the health qualities of the lowly sweet potato, the staple food of the common men and women of Okinawan, were becoming increasingly apparent. The Center for Science in the Public Interest (CSPI) even ranked their “lowly” sweet potato as the healthiest of all vegetables, mainly for its high content of dietary fiber, naturally occurring sugars, slow digesting low GI carbohydrates, protein content, anti-oxidant vitamins A and C, potassium, iron, calcium, and low levels of fat (saturated fat in particular), sodium and cholesterol (see Table 3 below). The American Cancer Society, the American Heart Association and other organizations that recognize the value of a healthy diet for reducing risk for chronic disease have also heartily endorsed the sweet potato for its nutritional properties that may aid in decreasing risk for chronic age associated diseases such as cancer or cardiovascular disease (Willcox et al, 2004; 2009).
Table 3.
Nutrient Content of One Medium Okinawan Sweet Potatoa
| Macronutrients | %DV b | |
|---|---|---|
| Calories | 251 kcal | 13 |
| Total Carbohydrate | 61 g | 20 |
| Dietary Fiber | 6 g | 24 |
| Total Fat | 0 g | |
| Protein | 1 g | 2 |
| Micronutrients and other Constituents c | ||
| Vitamin A | 1643 RE | 100 c |
| Vitamin C | 34 mg | |
| Thiamin | 0.2 mg | 13 |
| Riboflavin | 0.07 mg | 12 |
| Niacin | 0.97 mg | 5 |
| Vitamin B6 | 0.5 mg | 25 |
| Calcium | 58 mg | 6 |
| Iron | 1 mg | 6 |
| Magnesium | 29 mg | 7 |
| Phosphorus | 55 mg | 6 |
| Potassium | 812 mg | 23 |
| Other | ||
| GI d | 34 ± 2.3 | |
| GL e | 11 |
Nutrient data source other than GI & GL: University of Hawaii’s http://hawaiifoods.hawaii.edu/index.asp. Percent Daily Values (%DV) are for adults or children aged 4 or older, and are based on a 2,000 calorie reference diet.
DV = Daily Value
5000 IU calculated into 1650 RE with methods provided by UMIN Nutrition Coordinating Center
Allen, J et al. The Open Nutrition Journal 2012:6:1–11.
GL calculated as GI/100 × grams available carbohydrate (total carb – fiber) per serving.
Moreover, as an excellent source of the antioxidant vitamin A (mainly in the form of beta-carotene) and a good source of antioxidant vitamins C and E, and other anti-inflammatory phytochemicals, sweet potatoes are potent food sources of free radical quenchers. Some varieties of sweet potatoes contain many times the daily recommended value of vitamin A. For example, a large baked orange sweet potato commonly available in North America (often mistakenly called the ‘‘yam’’) contains 789% of the USDA daily value of vitamin A. This comes in the form lacking most in the American diet (carotenoids) (Willcox et al. 2009). Moreover, vitamin E, is also relatively high in sweet potatoes. As a fat-soluble vitamin, it is found mainly in high-fat foods, such as oils or nuts; however, the sweet potato is rare because it delivers vitamin E in a low fat dietary vehicle. Since these nutrients are also anti-inflammatory, they may be helpful in reducing age-associated body inflammation, which is linked to chronic age associated diseases, such as atherosclerosis, cancer and Type 2 diabetes (Willcox et al, 2004;2009).
In light of the strong interplay between inflammation, age-associated diseases and longevity (Baylis et al 2013; Chung et al. 2009; Demartinis et al 2006; Franceschi 2007; Vasto et al. 2007) or inflammaging, as aptly coined by Franceschi and colleagues (Franceschi et al. 2000) it is of particular interest that the sweet potato (including the leaves) has been shown to have significant anti-inflammatory properties (Chao et al. 2013; Hwang et al. 2001; Shan et al 2009; Wang et al. 2010; Zhang et al 2009) as well as strong anti-oxidant effects (Dini et al. 2006; Hou et al. 2001; Hwang et al. 2001; Johnson & Pace 2010; Kano et al. 2005;; Zhang et al. 2009). - Although human interventional studies and clinical trials are necessary to confirm the promising preliminary work in vivo and in vitro, it should also be noted that sweet potatoes are also good sources of B vitamins, including folate, thiamine, riboflavin, and vitamin B6. Interestingly, folate and vitamin B6 help converts homocysteine into cysteine. Since high homocysteine levels have been shown to be associated with an increased risk of cardiovascular disease and dementia, it is noteworthy that serum homocysteine levels are particularly low in Okinawa (Alfthan et al. 1997) and cardiovascular mortality and dementia also follow this pattern (Ogura et al, 1995; Willcox B et al, 2007). See Table 3.
Traditional medical uses for sweet potato for a modern age
In addition to being the main food staple in Okinawa and an important starch throughout the southern Japanese prefectures, sweet potatoes and their extracts have also been consumed throughout Japan as folk remedy. Indications have included anemia, hypertension, and diabetes.
Building upon this folk knowledge base, Japanese scientists have extracted pharmacologically-active compounds from sweet potatoes for a variety of medicinal purposes. For example, Caiapo extract (from white skinned sweet potato) is sold commercially in Japan without medical prescription as a neutraceutical for the Type 2 diabetes mellitus. Although more work is needed in this area, preliminary studies of peel-based extracts from white-skinned sweet potatoes have revealed the ability to lower blood glucose by increasing insulin sensitivity---without affecting insulin secretion (Ludvik et al. 2003). Beneficial effects have also been shown on short term (fasting glucose) and long-term (glycosylated hemoglobin) blood sugar control in diabetic patients and these findings were accompanied by increased levels of adiponectin and a decrease in fibrinogen (Ludvik et al. 2002).
Research has also confirmed the beneficial effects of sweet potato on cholesterol levels (total cholesterol and LDL) in patients with type 2 diabetes (Ludvik et al. 2002). Preliminary research favors many traditional Japanese medical folk uses of the sweet potato, revealing it to be a natural insulin sensitizer with antiatherogenic and anti-inflammatory properties. Ultimately, more randomized and placebo-controlled clinical trials will be needed to support health claims. See Table 4.
Table 4.
Micronutrient Content of the Traditional Okinawan and Japanese Diets
| Amount | % RDAJ a | Amount | % RDAJ a | |
|---|---|---|---|---|
| Okinawa, 1949 b | Japan, 1950c | |||
| Calcium (mg) | 505.3 | 75 | 325.5 | 48 |
| Magnesium (mg) | 396.1 | 127 | 327.4 | 105 |
| Potassium (mg) | 5199.6 | 180 | 2712.3 | 94 |
| Sodium (mg) d,e | 1133 | 35 | 2451 | 76 |
| Vitamin A (RE) | 1252 | 165 | 337 | 44 |
| Vitamin E (mg) | 16.6 | 246 | 6.3 | 93 |
| Vitamin K (mcg) | 87.6 | 126 | 65.8 | 95 |
| Vitamin B1: thiamin (mg) | 1.4 | 118 | 1.1 | 92 |
| Vitamin B2: riboflavin (mg) | 0.5 | 38 | 0.5 | 38 |
| Niacin (mg) | 13.2 | 105 | 18.1 | 144 |
| Vitamin B6: pyridoxine (mg) | 3 | 240 | 1.6 | 128 |
| Folate (mcg) | 557.4 | 232 | 267.2 | 111 |
| Vitamin B12: cobalamin (mcg) | 0.6 | 25 | 4.0 | 167 |
| Vitamin C (mg) | 273.4 | 273 | 94.9 | 95 |
Calculated from U.S. National Archive, archived food records, 1949.
Calculated from the Japan National Nutrition Survey and the Statistics Record of the Ministry of Agriculture, Forestry and Fisheries, Government of Japan, 1950.
This is the upper LIMIT RDAJ recommends. 8.25 grams of salt (mean of men 9 g, women 7.5 g) is 3242.25 mg of sodium.
Sodium intake is calculated from food sources only and therefore underestimated. The main sources of sodium intake in Japan are added to foods in the form of soy based sauces (including miso flavorings), salting of fish, pickling of vegetables, and adding while cooking.
The free radical scavenging spud
Recent research has also revealed impressive free radical–scavenging abilities. Sweet potatoes contain root storage proteins, such as trypsin inhibitors, that have significant antioxidant capacities that rival even those of glutathione, one of the body’s more potent endogenous antioxidants (Hou et al. 2001). Other studies have shown that sweet potatoes are rich in particular polyphenols (such as 4,5-di-O-caffeoyldaucic acid) that show greater antioxidant activity than such antioxidant standards as l-ascorbic acid, tert-butyl-4-hydroxy toluene, and gallic acid (Dini et al. 2006). Interestingly, anthocyanins from an extract of the tuber of purple sweet potato (Ayamurasaki) have shown stronger radical-scavenging activity than anthocyanins from grape skin, red cabbage, elderberry, or purple corn, and ascorbic acid (Kano et al. 2005). Polyphenols from the leaves of sweet potatoes have also been shown to suppress the growth of human cancer cells (Kurata et al. 2007).
Low glycemic load
Finally, despite their sweet taste, the Glycemic Index of the sweet potato is not high. It ranges from low to medium, depending upon the specific variety of sweet potato, as well as the method of preparation (Willcox et al, 2004:2009). The most commonly consumed varieties of sweet potato in Okinawa rate low to medium on the Glycemic Index, ranging from 34 (see Table 3) for the purple sweet potato (referred to as the “Okinawan potato” in Hawaii) to 55 for the Satsuma Imo (Willcox et al. 2009), Thus, consuming sweet potatoes as a staple, as the Okinawans did when they followed a more traditional diet, would result in a meal with a low glycemic load (see Table 3).
Food is Medicine: The Okinawan Apothecary of Hormetic Phytochemicals
In Okinawa there is a saying Nuchi Gusui which means Food is Medicine. Reflected in this thinking is the blurring of the distinction between food and medicine since commonly consumed foods, herbs or spices are also used as a source of medicines. These foods include sweet potatoes (and their leaves), bitter melon, turmeric, seaweeds, among others (Willcox et al, 2004; 2009).
Although many of these plants or plant extracts have long histories of use in traditional Okinawan or Chinese medicine, it has only been in recent years that researchers have begun concerted efforts to assess, in an evidence-based manner, the potentially beneficial effects of plant-derived extracts to prevent or treat age associated diseases. It is now well known that plants have the potential to synthesize phytochemicals to protect their stems and leaves from pathogens, insects, bacteria, viruses, or other environmental stress stimuli. Carotenoids and flavonoids are often synthesized to help scavenge and quench free radicals formed due to UV light exposure. Since the sun in Okinawa is particularly strong, many locally grown plants contain powerful antioxidants, with high amounts of carotene, flavonoids or other antioxidant properties. Murakami et al (2005) reported that compared to typical mainland Japanese food items, those in Okinawa tend to have stronger free radical scavenging properties. Of 138 food items they tested for anti-inflammatory action, many were promising and wild turmeric and zedoary from Okinawa showed particularly promising anti-oxidative and anti-nitrosative properties.
These phytochemicals (such as polyphenols, flavonoids, terpenoids, sesquiterpenoids and others with strong anti-oxidant properties) can induce a cellular stress response and subsequent adaptive stress resistance involving several molecular adaptations collectively referred to as “hormesis”. The role of hormesis in aging, in particular its relation to the lifespan extending effects of caloric restriction, has been explored in depth by Rattan et al (2008). Davinelli, Willcox and Scapagnini (2012) propose that the anti-aging responses induced by phytochemicals are caused by phytohormetic stress resistance involving the activation of Nrf2 signaling, a central regulator of the adaptive response to oxidative stress. Since oxidative stress is thought to be one of the main mechanisms of aging, the enhancement of anti-oxidative mechanisms and the inhibition of ROS production are potentially powerful pathways to protect against damaging free radicals and therefore decrease risk for age associated disease and, perhaps, modulate the rate of aging itself.
Hormetic phytochemicals, including polyphenols such as resveratrol, have received great attention for their potential pro-longevity effects and ability to act as sirtuin activators. They may also be activators of FOXO3, a key transcription factor and part of the IGF-1 pathway. FOXO3 is essential for caloric restriction to exert its beneficial effects. Willcox et al (2008) first showed that allelic variation in the FOXO3 gene is strongly associated with human longevity. This finding has since been replicated in over 10 independent population samples (Anselmi et al. 2009; Flachsbart et al. 2009; Li et al. 2009; Pawlikowska et al. 2009) and now is one of only two consistently replicated genes associated with human aging and longevity (Donlon et al, 2012).
Space limitations preclude an in-depth analysis, but a brief review of four popular food items (bitter melon, Okinawan tofu, turmeric and seaweeds) in the traditional Okinawan diet, each of which has been receiving increasing attention from researchers for their anti-aging properties, appears below.
Bitter melon
Bitter melon is a vegetable that is shaped like a cucumber but with a rough, pockmarked skin. It is perhaps the vegetable that persons from mainland Japan most strongly associate with Okinawan cuisine. It is usually consumed in stir fry dishes but also in salads, tempura, as juice and tea, and even in bitter melon burgers in fast food establishments. Likely bitter melon came from China during one of the many trade exchanges between the Ryukyu Kingdom and the Ming and Manchu dynasties. Bitter melon is low in caloric density, high in fiber, and vitamin C, and it has been used as a medicinal herb in China, India, Africa, South America, among other places (Willcox et al, 2004;2009).
Traditional medical uses include tonics, emetics, laxatives and teas for colds, fevers, dyspepsia, rheumatic pains and metabolic disorders. From a pharmacological or nutraceutical perspective, bitter melon has primarily been used to lower blood glucose levels in patients with diabetes mellitus (Willcox et al, 2004;2009). Anti-diabetic compounds include charantin, vicine, and polypeptide-p (Krawinkel & Keding 2006), as well as other bioactive components (Sathishsekar & Subramanian 2005). Metabolic and hypoglycemic effects of bitter melon extracts have been demonstrated in cell cultures and animal and human studies; however, the mechanism of action is unclear, and whether bitter melon acts principally via regulation of insulin release or through altered glucose metabolism, is still under investigation (Krawinkel & Keding 2006). In vitro studies have demonstrated anticarcinogenic and antiviral activities (Lee-Huang et al. 1995).
Bitter melon as a functional food and/or nutraceutical supplement is becoming more commonplace as research is gradually unlocking its mechanism of action, however, randomized, placebo-controlled trials are needed to properly assess safety and efficacy before bitter melon can be routinely recommended (Basch et al. 2003).
Okinawan tofu
The high legume content in the traditional Okinawan diet mainly originates from soybean-based products. In the traditional diet, soy was the main source of protein, and older Okinawans have arguably consumed more soy (e.g. tofu, miso) than any other population (Willcox et al, 2004;2009). Soy is rich in flavonoids, which have antioxidant-like effects and exhibit hormetic properties which can activate cell signaling pathways such as the Sirtuin-FOXO pathway. For example flavonoids, such as genestein, are potent activators of gene expression in FOXO3, a gene that is strongly associated with healthy aging and longevity, among other health-promoting properties (Speciale et al. 2011). Isoflavones, the type of flavonoids most common in soy, also regulate the Akt/FOXO3a/GSK-3beta/AR signaling network in prostate cancer cells. Specifically, they inhibit cell proliferation and foster apoptosis (cell death) suggesting that isoflavones might prove useful for the prevention and/or treatment of prostate cancer (Li et al. 2008). More evidence is required from clinical studies of human populations to better assess organ or disease-specific effects, as well as overall health effects of flavonoids in humans.
The tofu in Okinawa is lower in water content than typical mainland Japan versions and higher in healthy fat and protein. This makes tofu more palatable and may be a factor in the exceptionally high consumption in Okinawa (Willcox et al, 2004). The high consumption of soy in Okinawa may be connected to the low rates of breast and prostate cancer observed in older Okinawans (Douglas et al. 2013; Willcox et al. 2009; Wu et al. 1996; Yan & Spitznagel 2005).
Soy phytochemicals such as isoflavones, saponins, or trypsin inhibitors have also been shown to have strong anti-inflammatory effects (Dia et al. 2008; Kang et al. 2005; Hooshmand et al. 2007). Some isoflavones are potent dual PPARα/γ agonists and/or aryl hydrocarbon receptor (AhR) agonists and induce cell cycle arrest and modulate xenobiotic metabolism (Medjakovic et al. 2010). Moreover, soy protein hydrolysates can decrease expression of inflammatory genes in vitro (Martinez-Villaluenga et al. 2009) and, more importantly have potential clinical applications, in vivo (Nagarajan et al. 2008). Further therapeutic potential is present in soy-derived di-and tripeptides which have shown recent promise in alleviating colon and ileum inflammation, in vivo (Young et al. 2012). Genistein, a soy derived isoflavone, also can prevent azoxymethane-induced up-regulation of WNT/β-catenin signalling and reduce colon pre-neoplasia in vivo (Zhang et al. 2013). More work is needed in human populations since most of this work has been in vitro.
Clinical studies have shown that ingestion of soy proteins can modulate risk factors for cardiovascular disease. This property originally led to the approval of the food-labeling health claim for soy proteins for prevention of coronary heart disease by the U.S. FDA (FDA, 1999). More recent meta-analyses have shown that the average LDL lowering effect of soy protein is only about 3%, which is lower than the previously reported 8% reduction that led to the original health claim, and additional analyses suggested no contribution to this effect from isoflavones (Sacks et al, 2006). A subsequent meta-analysis of randomized controlled trials suggested that soy isoflavones indeed contributed, in part, to reduction of serum total and LDL cholesterol in humans (Taku et al. 2007). The American Heart Association still advocates substitution of high animal fat foods with soy since it has other cardiovascular benefits in addition to LDL-lowering effects (Sacks et al, 2006).
However, evidence for other health benefits for soy isoflavones, such as the ability to lessen vasomotor symptoms of menopause, to slow postmenopausal bone loss, and to help prevent or treat various cancers, is less convincing, and more complicated than it initially appeared a couple of decades ago . The basis for the hypothesis originates manly from Japan, where observational studies show that soy consumption is high and women experience fewer menopausal symptoms and fewer hip fractures, and there has been far less hormone-associated cancer incidence and mortality (e.g. breast, endometrium, prostate, colon) versus Western nations (Willcox et al. 2004; 2009). Nevertheless, despite the encouraging ecological evidence and the generally positive results from observational and epidemiological studies that indicate soy reduces breast cancer risk (Qin et al. 2006), beneficial as well as adverse effects in relation to cell proliferation and cancer risk is still under study (Rietjens et al. 2013).
Brain health is an additional area of interest. For example, enzymes from fermented soy (natto) may help prevent the buildup of certain plaques in the brain linked to Alzheimer’s disease (Hsu et al. 2009). Finally, soy rates very low on the GI, and helps regulate blood sugar and insulin fluctuations (Willcox et al, 2009).
While we await more evidence regarding soy isoflavones for multiple health conditions, there does seem to be strong consensus that soy foods are of potential benefit to cardiovascular health due to multiple other factors as well---high content of fiber, polyunsaturated fats, vitamins, and minerals, and low content of saturated fat (Sacks et al. 2006). Definitive conclusions regarding other health-related outcomes as well as pharmacokinetic issues that critically influence the biological activity of isoflavones (Vitale et al. 2013) will need to await further evidence.
Marine-based Carotenoids: Fucoxanthin, Astaxanthin, and Fucoidan
Marine-based carotenoids, such seaweed, algae, kelp are very low in caloric density, nutrient-dense, high in protein, folate, carotenoids, magnesium, iron, calcium, iodine, and have significant antioxidant properties. They represent relatively untapped potential for plant-based therapeutic products, including new and useful nutraceuticals.
Fucoxanthin is a xanthophyll that is found as a pigment in the chloroplasts of brown algae and most other heterokonts (ranging in size from very large multicellular kelp to unicellular diatoms of plankton), which have a brown or olive-green color. These foods are commonly consumed in the Okinawan diet (Willcox et al, 2004). Some interesting studies in animal models show that this carotenoid has multiple beneficial effects on metabolism, including reducing blood glucose and insulin levels, increasing the level of hepatic docosahexanoic acid, and attenuating weight gain, thereby holding promise as a potential dietary intervention for obesity, metabolic syndrome and Type 2 diabetes mellitus, among other related metabolic disorders (Maeda et al. 2008; Kim and Pangestuti, 2011; Miyashita et al, 2011). Fucoxanthin may also promote thermogenesis within fat cells in white adipose tissue (Maeda et al. 2008; Miyashita et al, 2011). One double-blind placebo-controlled human trial in obese women with showed that a seaweed extract containing fucoxanthin and pomegranate seed oil lost an average 4.9 kg weight loss over a 16-week period (Abidove et al, 2009). Studies of fucoxanthin show diverse potential health benefits, principally though biological activities including antioxidant, anticarcinogenic, anti-inflammatory, antiobesity, and neuroprotection (Kim and Pangesttuti, 2011: Miyashita et al, 2011).
Astaxanthin, a xanthophyll carotenoid, is a powerful, broad-ranging antioxidant from microalgae that also occurs naturally in a wide variety of living organisms such as fungi, complex plants, and sea life such as crustaceans and reddish colored fish (Guedes et al, 2011). As such, is makes its way into the Okinawa diet through widespread means (Willcox et al, 2004). Results from multiple studies have revealed significant antioxidant and anti-inflammatory properties for astaxanthin compounds and suggest that there is promise as a nutraceutical and cosmaceutical (Anunciato and da Rocha Filho , 2012). Data support this carotenoid as a novel potential candidate for prevention and treatment of cardiovascular oxidative stress and inflammation, with thus far no evidence of the potentially fatal complications of NSAIDs (e.g. GI bleeding) or steroids, such as prednisone (bone less, GI bleeding, adrenal suppression) (Pashkow et al. 2008; Fasset and Coombs, 2011). Recent evidence suggests that that astaxanthin has promise for modulating aging through activation of the insulin signaling pathway and FOXO3 gene in particular (Yazaki, 2011). A recent review highlights clinical trials in model organisms and humans for astaxanthin in aging and age-related diseases (Kidd, 2011).
Fucoidan is another carotenoid with potential promise consumed in popular Okinawan marine foods, coming from sulfated polysaccharide found mainly in various species of brown seaweed such as kombu, wakame, mozuku, and hijiki (Senni et al, 2011). Research on fucoidan has focused primarily on two distinct forms: F-fucoidan, which is mainly composed of sulfated esters of fucose, and U-fucoidan, which is has a relatively abundant level of glucuronic acid, although there is variation in both depending upon the source and the season (Morya et al, 2011; Ale et al, 2011). Both U-fucoidan and F-fucoidan are popular neutraceuticals in Japan and other nations due to their potent free radical–quenching capabilities (Wang et al 2008) and other health-enhancing potential (Fitton, 2011; Morya, 2011). For example, fucoidan may have anti-carcinogenic properties (Fitton, 2011). F-fucoidan can induce apoptosis in human lymphoma cell lines (Aisa et al. 2005), and other studies have shown it can inhibit hyperplasia in animal models (Deux et al. 2002). The algal and invertebrate polysaccharides are also potent anticoagulant agents of mammalian blood and may represent a potential source of compounds for antithrombotic therapies (Pomin & Mourao 2008; Morya, 2011). See Figure 2.
Figure 2.
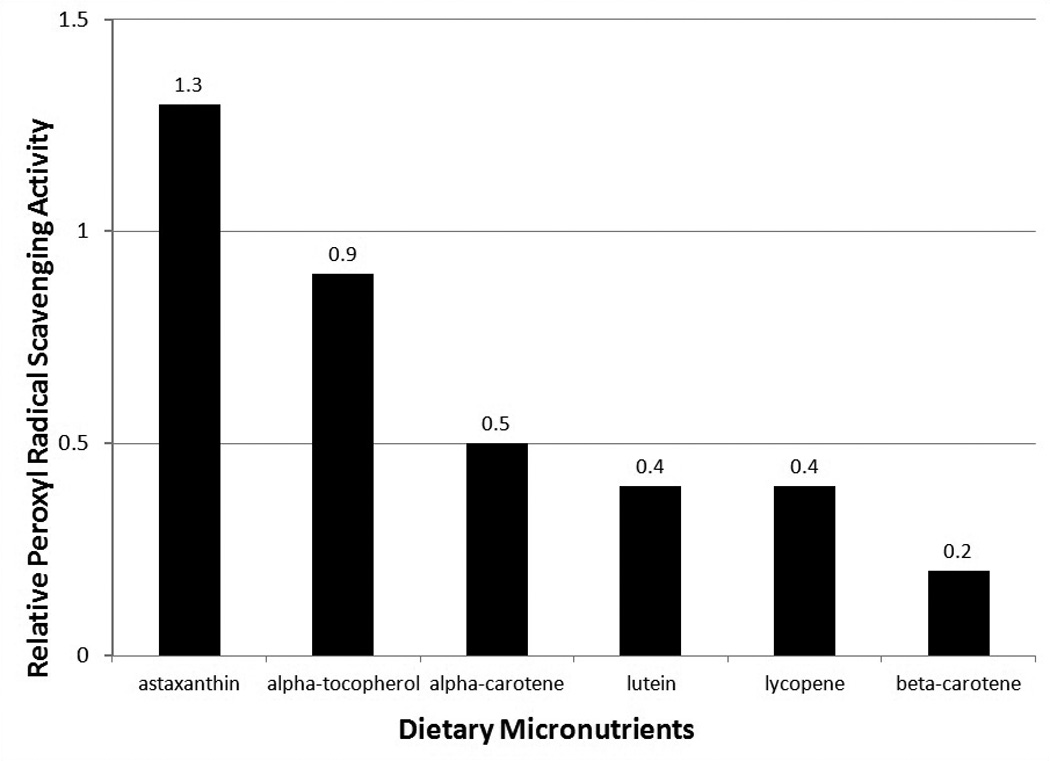
Antioxidant activity of Carotenoids and other Dietary Micronutrient
Turmeric
Turmeric is a very popular spice in Okinawa which is used for cooking in soups or curries, or drank as a tea (Willcox et al. 2004). Recently it has become popular to consume in tablet or nutritional drink form as a liver “detoxifier” (especially when alcohol is consumed) or overall energy enhancer. Originally from India, turmeric is from the rhizome of Curcuma longa, and belongs to the ginger family. Tumeric was likely brought to the Ryukyu Kingdom (now Okinawa prefecture) through the spice trade, in which the Ryukyu Kingdom was an avid participant (Willcox et al, 2004). Traditional Indian medicine (Ayurvedic medicine), and other traditional medical systems in Asia, use turmeric or turmeric components, such as curcumin, for a wide variety of diseases and conditions, including those of the integumentary (skin), pulmonary, and gastrointestinal systems, and for pain, wounds, and liver disorders, among other conditions (Gupta et al, 2013).
Curcumin is a phenolic compound concentrated in the roots of Curcuma longa and has been extensively studied for its numerous biological activities including anti-inflammatory, antioxidant and anticancer properties (Ahser and Spelman, 2013). The anti-inflammatory capacity of curcumin correlates with a reduction of the activity of nuclear transcription factors in the NFkβ signaling pathway (Singh & Aggarwal 1995), which regulate the transcription of several proinflammatory genes.
In C. elegans, curcumin extended lifespan and reduced intracellular ROS and lipofuscin during aging. It also affected body size and the pharyngeal pumping rate (a measure of healthspan) but not reproduction of wild-type C. elegans. The lifespan extension found by use of curcumin in C. elegans was attributed to its antioxidative properties. Specific genes implicated were osr-1, sek-1, mek-1, skn-1, unc-43, sir-2.1, and age-1 (Liao et al, 2011).
One of the mechanisms for curcumin’s anti-inflammatory properties is the inhibition of release of proinflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), IL-1β,and IL-6 (Jin et al. 2007). In one study, curcumin abolished the proliferative effects of IL-6 through blocking phosphorylation of the signal transducer and activator of transcription 3 (STAT3) (Bharti et al. 2003). In a similar manner, curcumin downregulates the transcription factor activator protein 1 (AP1) through direct interaction with its DNA binding motif (Bierhaus et al. 1997) and inducing the inhibition of IL-1α and TNF-α (Xu et al. 1997). Likely, the inhibition of AP1 and NF-kβ occurs through the chromatin remodeling activity of curcumin, where it may modulate histone deacetylase (HDAC) activity (Rahman et al. 2004). Moreover, curcumin attenuates inflammatory responses through the inhibition of lipoxygenase and cyclooxygenase-2 (COX-2) enzymes, which are responsible for the synthesis of proinflammatory prostaglandins and leukotrienes (Bengmark 2006).
Curcumin also acts as a strong anti-oxidant, having the potential to inhibit lipid peroxidation and to effectively intercept and neutralize ROS (Priyadarsini 1998) and NO-based free radicals (Sreejayan & Rao 1997). In this regard, curcumin demonstrates greater potency than vitamin E (Zhao et al. 1989).
The free radical chemistry of curcumin is based on the redox peculiarities of its phenol ring, and the possible involvement of the beta-diketone moiety, both of which may influence the antioxidant action of curcumin (Masuda et al. 1999). Beyond its ROS quencher activity, curcumin effects have been mostly associated with its ability to interfere at a molecular level with numerous cellular antioxidant pathways. Curcumin has been demonstrated to activate the nuclear factor erythroid 2-related factor 2 (Nrf2), leading to induction of the antioxidant responsive element (ARE) activated reporter genes (Balogun et al. 2003). Nrf2 belongs to the CnC (Cap’n’Collar) family leucine zipper transcription factors and is a conserved master regulator of cellular antioxidant responses. In this pathway (Nrf2/ARE), curcumin strongly induces expression of some cellular stress response genes (phase II detoxification enzymes, such as glutathione synthetase (GSS), and heme oxygenase-1), resulting in enhanced cell protection and better cell survival (Scapagnini et al. 2011).
Curcumin also appears as a potential blocker of cancer cell growth both in vitro and in vivo. The activity of curcumin reported against numerous diverse cancers (e.g. the hematologic cancers leukemia and lymphoma, gastrointestinal cancers, genitourinary cancers, breast cancer, ovarian cancer, head and neck squamous cell carcinoma, lung cancer, skin cancers including melanoma, neurological cancers, and cancers of muscle tissue such as sarcoma) reflects its ability to affect multiple, diverse targets (Sung et al. 2012).
However, cancer is not the only chronic disease for which turmeric holds promise. Epidemiological studies suggest that curcumin, as one of the most prevalent nutritional and medicinal compounds used by the population of India, may be partly responsible for the significantly reduced (4.4-fold) prevalence of Alzheimer’s disease (AD) in India compared to United States (Chandra et al. 2001). Further studies on this issue are warranted, particularly since the prevalence of dementia among elderly population appears to be lower in the curcumin-consuming Okinawans when compared to the US or Japan populations (Ogura et al. 1995). Numerous pieces of evidence suggest that curcumin may be a promising therapy for AD because it has different neuroprotective activities, including antioxidant, anti-inflammatory and antiamyloidogenic properties. In a transgenic mouse model of Alzheimer’s disease, dietary supplementation with curcumin (160–5000 ppm) decreased the accumulation of amyloid beta-peptide, and markers of oxidative stress and inflammation in the cerebral cortex (Lim et al. 2001). Curcumin can directly protect cultured neurons against death induced by oxidative insults by the activation of nrf2 pathway (Scapagnini G et al. 2006). Of note, curcumin exhibits protective effects on neuronal cells by inhibiting the aggregation of Aβ into oligomers and has a clearance effect on the existing Aβ (Cole et al. 2007). A very interesting in vivo approach with multiphoton microscopy showed the ability of curcumin to cross the blood-brain barrier (BBB) and disrupt amyloid plaques (Garcia-Alloza et al. 2007).
Interestingly, curcumin possesses both MAO-A- and MAO-B-inhibiting properties and has been shown to modulate the levels of noradrenaline, dopamine and serotonin in the brain, demonstrating antidepressant effects in animal models of depression (Scapagnini et al. 2012) and in patients with major depressive disorder (Sanmukhani et al. 2013).
Much of the research conducted to date on curcumin has been focused on exploring its protective and therapeutic effects against age-related degeneration. Recently the possibility that curcumin and its metabolites can modulate pathways directly involved in the determination of lifespan and extension of longevity, has been also highlighted (Shen et al. 2013). Tetrahydrocurcumin (THC), an active metabolite of curcumin, produced after its ingestion, has been shown to extend lifespan of drosophila under normal conditions, by attenuating oxidative stress via FOXO and Sir2 modulation (Xiang et al. 2011). Curcuminoids may also affect mammalian longevity, as shown in mice fed diets containing THC starting at the age of 13 months, which showed significantly increased mean lifespan (Shen et al. 2013).
Summary and Conclusions
The traditional diet in Okinawa is based on green and yellow vegetables, root vegetables (principally sweet potatoes), soybean-based foods, and other plants, many with medicinal properties. This is supplemented by regular seafood consumption and consumption of smaller amounts of lean meats, fruit, and medicinal garnishes and spices. Sanpin (jasmine) tea is the principal beverage, consumed with meals and awamori (Okinawan sake) is the social drink of choice.
The dietary composition over the past half-century has changed from a low calorie diet dominated by low glycemic index carbohydrates, low in protein and fat, to one more moderate in all three macronutrients. While the caloric content has increased due to higher consumption of calorically dense foods, the diet remains very healthy by most expert criteria including the National Cholesterol Education Program (NCEP), the U.S. Dietary Guidelines for Americans Advisory Committee, and the Unified Dietary Guidelines. Many of the characteristics of the traditional Okinawan diet are shared with other healthy dietary patterns, such as the traditional Mediterranean diet, the modern DASH diet, and the modern Portfolio diet. All these dietary patterns have been found to be associated with reduced risk for cardiovascular disease (Appel, 2008; Fung et al. 2001; Jenkins et al. 2007b; Sacks et al. 2001; Willcox et al. 2009). Healthy fat intake is very likely one mechanism for reducing CVD risk factors, however, other mechanisms, such as the high amounts of phytochemicals, high antioxidant intake, low glycemic load and resultant lowered oxidative stress are also likely playing a role in reducing risk for cardiovascular disease and other age-associated diseases.
A comparison of the nutrient profiles of these dietary patterns in Table 1 showed that the traditional Okinawan diet is the lowest in fat, particularly in terms of saturated fat, and highest in carbohydrate. This is due to the very high intake of phytonutrient-rich yet calorie-poor orange-yellow-purple root vegetables, such as sweet potatoes, and green leafy vegetables (Willcox et al. 2004; 2009). However, the traditional Okinawan diet has undergone extensive post-war change, most notably in terms of an increase in fat intake and a decrease in carbohydrate quality. The sweet potato has largely been replaced by white rice, bread, and noodles, as the main sources of carbohydrate. Despite the large increase in fat consumption in Okinawa since the 1950’s, fat intake for elders in Okinawa is still comparable to that of the DASH diet (at approximately 27% of total daily energy intake) and lower than that of the traditional Mediterranean diet (42%) (Kromhout et al. 1989; Sacks et al. 2001). Saturated fat remains less than 10% of total energy intake (around 7% versus 6% in DASH and 9% in Mediterranean), consistent with NCEP and Unified Dietary recommendations.
Despite a reduction of dietary carbohydrate, this macronutrient remains the highest in Okinawa versus other healthy diets (58% versus a low of 42% for Mediterranean) and protein intake, at 16%, falls between the lower Mediterranean (13%) intake and the higher Portfolio (20%) intake.
Overall, the important shared features of the aforementioned healthy dietary patterns include the following:
-
▪
Relatively high consumption of unrefined, low GI carbohydrates: principally vegetables, legumes, and fruits;
-
▪
Moderate fish and marine food consumption
-
▪
Lower intake of meat with emphasis on lean meats
-
▪
Liberal use of medicinal plants, herbs, spices or oils
-
▪
Regular tea consumption and moderate alcohol consumption.
These dietary patterns result in:
-
▪
Healthy fat profile (higher in mono and polyunsaturated fats and lower in saturated fat; relatively high in omega-3 fat);
-
▪
Higher phytonutrient intake;
-
▪
Lower caloric density and intake;
-
▪
Less inflammation;
-
▪
Potential modulation of biological pathways linked to aging.
These shared features have contributed to the lower rates of cardiovascular disease (CHD, stroke), some cancers, diabetes and several other age-associated chronic diseases witnessed in the long-living Okinawan elders (Suzuki et al. 2001; Willcox et al. 2007; 2009; Sho 2001). Indeed, interventional studies of the Okinawan diet have shown improvements in several risk factors that reflect odds for healthy aging, particular risk factors for cardiovascular disease.. For example, the Okinawan diet has been shown to be able to increase potassium excretion in normotensive healthy young women (Tuekpe et al. 2006) as well as raise levels of circulating endothelial progenitor cells (Mano et al. 2007). Circulating endothelial progenitor cells (EPCs) are playing an increasingly important role as biomarkers of cardiovascular disease and may improve risk stratification, as well as offer novel tools for monitoring disease progression and response to therapy (Grisar et al. 2011).
While the Okinawan elders have maintained a relatively healthy version of the Okinawan diet, dietary change in the post-war period has been mostly negative among younger Okinawans. Less healthy food choices in post-war generations has resulted in an increase in calories and a less nutritious diet; when combined with less physical activity, there has been a worsening risk factor profile in post-war generations (men in particular), who are at higher risk of obesity and possess higher prevalence of several other chronic disease risk factors (Todoriki et al. 2004; Willcox et al. 2012) versus previous generations and other Japanese. The contrast is particularly stark when viewed from a generational perspective. In two generations Okinawans have gone from the lowest BMI to the highest BMI among the Japanese population (Willcox et al, 2007).
As a consequence, there has been a resurgence of interest from public health professionals in the health enhancing effects of the traditional Okinawan diet and a movement to re-educate younger persons in eating a more traditional dietary pattern. Other similar movements exist in Japan, such as the slow food movement, and in America, such as the Oldways movement (www.oldways.org). All share in common a mission to educate the public about the health, family, and societal benefits of traditional diets.
In conclusion, the Okinawan diet, particularly the traditional diet represents a real-world dietary pattern that is among the healthiest in the world of traditional diets. While the food choices are more common to Asian diets, it shares many of the nutritional characteristics of other healthy traditional (Mediterranean) and modern diets (DASH, Portfolio) and is good choice for those who have a taste for healthy Asian cuisine and wish to embark on a path toward healthier aging.
Highlights.
Nutrition can alter risk for aging-related diseases rivaling pharmacotherapy in efficacy
Such eating patterns include the Okinawan, Mediterranean, DASH and Portfolio diets
These diets have abundant vegetable, legume, and marine functional foods
The Okinawan diet is especially rich in pharmacologically active phyto- and marine compounds
Several potential anti-aging compounds modulate insulin signaling and inflammatory pathways
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abidov M, Ramazanov Z, Seifulla R, Grachev S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes, Obesity and Metabolism. 2010;12:72:1463–1326. doi: 10.1111/j.1463-1326.2009.01132.x. [DOI] [PubMed] [Google Scholar]
- Aisa Y, Miyakawa Y, Nakazato T, et al. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of RK pathways. Am. J. Hematol. 2005;78:7–14. doi: 10.1002/ajh.20182. [DOI] [PubMed] [Google Scholar]
- Ale MT, Mikkelsen JD, Meyer AS. Important determinants for fucoidan bioactivity: a critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar Drugs. 2011;9(10):2106–2130. doi: 10.3390/md9102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfthan G, Aro A, Gey KF. Plasma homocysteine and cardiovascular disease mortality. Lancet. 1997;1997:349–397. doi: 10.1016/S0140-6736(97)80014-1. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Healthy Entertaining for Any Occasion. ACS Publishing. 2001:1–272. [Google Scholar]
- [Accessed 12/15/2013];American Heart Association: https://www.heart.org/HEARTORG/GettingHealthy/NutritionCenter/HeartSmartShopping/Heart-Check-Mark-Nutrition-Requirements. [Google Scholar]
- Anderson RM, Weindruch R. The caloric restriction paradigm: implications for healthy human aging. Am. JM. Hum. Biol. 2012;24:101–106. doi: 10.1002/ajhb.22243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi CV, Malovini A, Roncarati R, et al. Association of the FOXO3A locus with extreme longevity in a southern Italian centenarian study. Rejuvenation Res. 2009;12:95–104. doi: 10.1089/rej.2008.0827. [DOI] [PubMed] [Google Scholar]
- Anunciato TP, da Rocha Filho PA. Carotenoids and polyphenols in nutricosmetics, nutraceuticals, and cosmeceuticals. J Cosmet Dermatol. 2012;11(1):51–54. doi: 10.1111/j.1473-2165.2011.00600.x. [DOI] [PubMed] [Google Scholar]
- Appel L. Dietary patterns and longevity. Expanding the Blue Zones. Circulation. 2008;118:213–215. doi: 10.1161/CIRCULATIONAHA.108.788497. [DOI] [PubMed] [Google Scholar]
- Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19(2):20–22. [PubMed] [Google Scholar]
- Balogun E, Hoque M, Gong P, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem. J. 2003;371:887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basch E, Gabardi S, Ulbricht C. Bitter melon (Momordica charantia): a review of efficacy and safety. Am. J. Health Syst. Pharm. 2003;60:356–359. doi: 10.1093/ajhp/60.4.356. [DOI] [PubMed] [Google Scholar]
- Baylis D, Bartlett DA, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longevity & Healthspan. 2013;2:8. doi: 10.1186/2046-2395-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengmark S. Curcumin, an atoxic antioxidant and natural NFκB, cyclooxygenase-2, lipooxygenase, and inducible nitric oxide synthase inhibitor: a shield against acute and chronic diseases JPEN. J. Parenter. Enteral. Nutr. 2006;30:45–51. doi: 10.1177/014860710603000145. [DOI] [PubMed] [Google Scholar]
- Bharti AC, Donato N, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive and IL-6-inducible STAT3 phosphorylation in human multiple myeloma cells. J Immunol. 2003;171:3863–3871. doi: 10.4049/jimmunol.171.7.3863. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Zhang Y, Quehenberger P, et al. The dietary pigment curcumin reduces endothelial tissue factor gene expression by inhibiting binding of AP-1 to the DNA and activation of NF-kappa B. Thromb Haemost. 1997;77(4):772–782. [PubMed] [Google Scholar]
- Butler RN, Miller RA, Perry D, et al. New model of health promotion and disease prevention for the 21st century. BMJ. 2008;337:a399. doi: 10.1136/bmj.a399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. The Power of Prevention. Chronic disease the public health challenge of the 21st century. National Center for Chronic Disease Prevention and Health Promotion. Department of Health and Human Services; 2009. [Google Scholar]
- Chandra V, Pandav R, Dodge HH, et al. Incidence of Alzheimer’s disease in a rural community in India: the Indo-US study. Neurology. 2001;57:985–989. doi: 10.1212/wnl.57.6.985. [DOI] [PubMed] [Google Scholar]
- Chao PY, Huang YP, Hsieh WB. Inhibitive effect of purple sweet potato leaf extract and its components on cell adhesion and inflammatory response in human aortic endothelial cells. Cell Adh Migr. 2013 Mar-Apr;7(2):237–245. doi: 10.4161/cam.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003 May 21;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. Epub 2003 May 14. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GM, Teter B, Frautchy SA. Neuroprotective effects of curcumin. Adv. Exp. Med. Biol. 2007;595:197–212. doi: 10.1007/978-0-387-46401-5_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne CM. Dietary interventions on blood pressure: the Dietary Approaches to Stop Hypertension (DASH) trials. Nutr Rev. 2006;64(2 Pt 2):S53–S56. doi: 10.1111/j.1753-4887.2006.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Cluett C, Melzer D. Human genetic variations: Beacons on the pathways to successful ageing. Mechanisms of Ageing and Development. 2009;130(9):553–563. doi: 10.1016/j.mad.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Cordain L, Eaton S, Sebastian A, et al. Origins and evolution of the western diet: health implications for the twenty-first century. Am.J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- Davinelli S, Willcox DC, Scapagnini G. Extending healthy ageing: nutrient sensitive pathway and centenarian population. Immun. Ageing. 2012;9:9. doi: 10.1186/1742-4933-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Grey AD, Ames BN, Andersen JK, et al. Time to talk SENS: critiquing the immutability of human aging. Ann. N.Y. Acad. Sci. 2002;959:452–462. doi: 10.1111/j.1749-6632.2002.tb02115.x. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. A turning point for Alzheimer's disease? Biofactors. 2012;38:78–83. doi: 10.1002/biof.200. [DOI] [PubMed] [Google Scholar]
- de Magalhães JP, Wuttke D, Wood SH, Plank M, Vora C. Genome-environment interactions that modulate aging: powerful targets for drug discovery. Pharmacol. Rev. 2012;64:88–101. doi: 10.1124/pr.110.004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflamm-ageing and lifelong antigenic load as major determinants of ageing rate and longevity. FEBS Lett. 2005;579:2035–2039. doi: 10.1016/j.febslet.2005.02.055. [DOI] [PubMed] [Google Scholar]
- Dennis B, Stamler J, Buzzard M, Conway R, Elliott P, Moag-Stahlberg A, Okayama A, Okuda N, Robertson C, Robinson F, Schakel S, Stevens M, Van Heel N, Zhao L, Zhou BF INTERMAP Research Group, 2003. INTERMAP: the dietary data--process and quality control. J Hum Hypertens. 2003 Sep;17(9):609–622. doi: 10.1038/sj.jhh.1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deux J-F, Meddahi-Pelle A, Le Blanche AF, et al. Low molecular weight fucoidan prevents neointimal hyperplasia in rabbit iliac artery in-stent restenosis model. Arterioscler. Thromb. Vasc. Biol. 2002;22:1604–1609. doi: 10.1161/01.atv.0000032034.91020.0a. [DOI] [PubMed] [Google Scholar]
- Dia VP, Berhow MA, Gonzalez, De Mejia E. Bowman-Birk inhibitor and genistein among soy compounds that synergistically inhibit nitric oxide and prostaglandin E2 pathways in lipopolysaccharide-induced macrophages. J Agric Food Chem. 2008;56:11707–11717. doi: 10.1021/jf802475z. [DOI] [PubMed] [Google Scholar]
- Dini I, Tenore GC, Dini A. New polyphenol derivative in Ipomoea batatas tubers and its antioxidant activity. J. Agric. Food. Chem. 2006;54:8733–8737. doi: 10.1021/jf061687v. [DOI] [PubMed] [Google Scholar]
- Donlon TA, Curb JD, He Q, Grove JS, Masaki KH, Rodriguez B, Elliott A, Willcox DC, Willcox BJ. FOXO3 gene variants and human aging: coding variants may not be key players. J Gerontol A Biol Sci Med Sci. 2012;67(11):1132–1139. doi: 10.1093/gerona/gls067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas CC, Johnson SA, Arjmandi BH. Soy and Its Isoflavones: The Truth Behind the Science in Breast Cancer. Anticancer Agents Med Chem. 2013 Aug 6; doi: 10.2174/18715206113139990320. 2013. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Fassett RG, Coombes JS. Astaxanthin in cardiovascular health and disease. Molecules. 2012;20;17(2):2030–2048. doi: 10.3390/molecules17022030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton JH. Therapies from fucoidan; multifunctional marine polymers. Mar Drugs. 2011;9(10):1731–1760. doi: 10.3390/md9101731. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbart F, Caliebe S, Kleindorp R, et al. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc. Natl. Acad. Sci. USA. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA Food Labeling: Health Claims; Soy Protein and Coronary Heart Disease In: Federal Register: (Volume 64, Number 206)]; 1999:57699–57733. [PubMed] [Google Scholar]
- Franceschi C. Inflammaging as a major characteristic of old people: can it be prevented or cured? Nutr Rev. 2007;65:S173–S176. doi: 10.1111/j.1753-4887.2007.tb00358.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- Fung TT, Willett WC, Stampfer MJ, et al. Dietary patterns and the risk of coronary heart disease in women. Arch. Intern. Med. 2001;161:1857–1862. doi: 10.1001/archinte.161.15.1857. [DOI] [PubMed] [Google Scholar]
- Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr. 2010;92:1429–1435. doi: 10.3945/ajcn.2010.29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Alloza M, Borrelli LA, Rozkalne A, Hyman BT, Bacskai BJ. Curcumin labels amyloid pathology in vivo disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007;102:1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39(1):2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- Guedes AC, Amaro HM, Malcata FX. Microalgae as sources of Carotenoids. Drugs. 2011;11;9(4):625–644. doi: 10.3390/md9040625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gögele M, Pattaro C, Fuchsberger C, Minelli C, Pramstaller PP, Wjst M. Heritability analysis of life span in a semi-isolated population followed across four centuries reveals the presence of pleiotropy between life span and reproduction. J. Gerontol A. Biol. Sci. Med. Sci. 2011;66:26–37. doi: 10.1093/gerona/glq163. [DOI] [PubMed] [Google Scholar]
- Grisar JC, Haddad F, Gomari FA, Wu JC. Endothelial progenitor cells in cardiovascular disease and chronic inflammation: from biomarker to therapeutic agent. Biomark Med. 2011;5:731–744. doi: 10.2217/bmm.11.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooshmand S, Soung, do Y, Lucas EA, Madihally SV, Levenson CW, Arjmandi BH. Genistein reduces the production of proinflammatory molecules in human chondrocytes. J Nutr Biochem. 2007;18:609–614. doi: 10.1016/j.jnutbio.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Hou WC, Chen YC, Chen HJ, Lin YH, Yang LL, Lee MH. Antioxidant activities of trypsin inhibitor, a 33 KDa root storage protein of sweet potato (Ipomoea batatas (L.) Lam cv. Tainong 57) J. Agric. Food. Chem. 2001;49:2978–2981. doi: 10.1021/jf0100705. [DOI] [PubMed] [Google Scholar]
- Hsu RL, Lee KT, Wang JH, Lee LY, Chen RP. Amyloid degrading ability of nattokinase from B acillus subtilis natto. J. Agric. Food Chem. 2009;57:503–508. doi: 10.1021/jf803072r. [DOI] [PubMed] [Google Scholar]
- Hu B. Globalization of food patterns and cardiovascular disease risk. Circulation. 2008;118:1913–1914. doi: 10.1161/CIRCULATIONAHA.108.808493. [DOI] [PubMed] [Google Scholar]
- Hwang YP, Choi JH, Yun HJ, et al. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2001;49:93–99. doi: 10.1016/j.fct.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Ikeda N, Saito E, Kondo N, et al. What has made the population of Japan healthy? Lancet. 2011;378:1094–1105. doi: 10.1016/S0140-6736(11)61055-6. [DOI] [PubMed] [Google Scholar]
- Japan Ministry of Health, Labour and Welfare. 2010 Recommended Dietary Allowances for the Japanese (RDAJ) 2010 http://www.mhlw.go.jp/shingi/2009/05/s0529-4.html.
- Jenkins DJ, Kendall CW, Marchie A, et al. Effects of a dietary portfolio of cholesterol-lowering foods vs lovastatin on serum lipids and C-reactive protein. JAMA. 2003;290:502–510. doi: 10.1001/jama.290.4.502. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Kendall CW, Marchie A, et al. Direct comparison of a dietary portfolio of cholesterol-lowering foods with a statin in hypercholesterolemic participants. Am. J. Clin. Nutr. 2005;81:380–387. doi: 10.1093/ajcn.81.2.380. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Kendall CWC, Faulkner DA, et al. Long-term effects of a plant-based dietary portfolio of cholesterol-lowering foods on blood pressure. Eur. J. Clin. Nutr. 2007a:1–8. doi: 10.1038/sj.ejcn.1602768. 2007. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Josse AR, Wong JM, Nguyen TH, Kendall CW. The portfolio diet for cardiovascular risk reduction. Curr. Atheroscler. Rep. 2007b;9:501–507. doi: 10.1007/s11883-007-0067-7. [DOI] [PubMed] [Google Scholar]
- Jenkins DJ, Jones PJH, Lamarche B, et al. Effect of a dietary portfolio of cholesterol-lowering foods given at 2 levels of intensity of dietary advice on serum lipids in Hyperlipidemia. JAMA. 2011;306:831–839. doi: 10.1001/jama.2011.1202. [DOI] [PubMed] [Google Scholar]
- Jin CY, Lee JD, Park C, Choi YH, Kim GY. Curcumin attenuates the release of pro-inflammatory cytokines in lipopolysaccharide-stimulated BV2 microglia. Acta. Pharmacol. Sin. 2007;28:1645–1651. doi: 10.1111/j.1745-7254.2007.00651.x. [DOI] [PubMed] [Google Scholar]
- Johnson M, Pace RD. Sweet potato leaves: properties and synergistic interactions that promote health and prevent disease. Nutr. Rev. 2010;68:604–615. doi: 10.1111/j.1753-4887.2010.00320.x. [DOI] [PubMed] [Google Scholar]
- Kang JH, Sung MK, Kawada T, Yoo H, Kim YK, Kim JS, Yu R. Soybean saponins suppress the release of proinflammatory mediators by LPS stimulated peritoneal macrophages. Cancer Lett. 2005;230:219–227. doi: 10.1016/j.canlet.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Kano M, Takayanagi T, Harada K, Makino K, Ishikawa F. Antioxidative activity of anthocyanins from purple sweet potato, Ipomoea batatas cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005;69:979–988. doi: 10.1271/bbb.69.979. [DOI] [PubMed] [Google Scholar]
- Kawano Y, Ando K, Matsuura H, et al. Report of the Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension:(1) Rationale for salt restriction and salt-restriction target level for the management of hypertension. Hypertens Res. 30:879–886. doi: 10.1291/hypres.30.879. [DOI] [PubMed] [Google Scholar]
- Kendall CW, Jenkins DJ. Curr Atheroscler Rep. 2004;6:492–498. doi: 10.1007/s11883-004-0091-9. [DOI] [PubMed] [Google Scholar]
- Keys A, Aravanis C, Blackburn HW, Van Buchem FS, Buzina R, Djordjević BD, Dontas AS, Fidanza F, Karvonen MJ, Kimura N, Lekos D, Monti M, Puddu V, Taylor HL. Epidemiological studies related to coronary heart disease: characteristics of men aged 40-59 in seven countries. Acta Med Scand Suppl. 1966;460:1–392. [PubMed] [Google Scholar]
- Kidd P. Astaxanthin, cell membrane nutrient with diverse clinical benefits and anti-aging potential. Altern Med Rev. 2011;16(4):355–364. [PubMed] [Google Scholar]
- Kim SK, Pangestuti R. Biological activities and potential health benefits of fucoxanthin derived from marine brown algae. Adv Food Nutr Res. 2011;64:111–128. doi: 10.1016/B978-0-12-387669-0.00009-0. [DOI] [PubMed] [Google Scholar]
- Krawinkel MB, Keding GB. Bitter gourd (Momordica Charantia): a dietary approach to hyperglycemia. Nutr. Rev. 2006;64:331–337. doi: 10.1301/nr.2006.jul.331-337. [DOI] [PubMed] [Google Scholar]
- Kromhout D, Keys A, Aravanis C, et al. Food consumption patterns in the 1960s in seven countries. J. Clin. Nutr. 1989;49:889–894. doi: 10.1093/ajcn/49.5.889. [DOI] [PubMed] [Google Scholar]
- Kurata R, Adachi M, Yamakawa O, Yoshimoto M. Growth suppression of human cancer cells by polymorphenolics from sweet potato (Ipomoea batatas L.) leaves. J. Agric. Food. Chem. 2007;55:185–190. doi: 10.1021/jf0620259. [DOI] [PubMed] [Google Scholar]
- Lee-Huang S, Huang PL, Chen HC, et al. Anti-HIV and anti-tumor activities of recombinant MAP30 from bitter melon. Gene. 1995;161:151–156. doi: 10.1016/0378-1119(95)00186-a. [DOI] [PubMed] [Google Scholar]
- Liao V, Yu CW, Chu YJ, Li WH, Hsieh YC, Wang TT. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mechanisms of Ageing and Development. 2011;132(10):480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Li Y, Wang WJ, Cao H, et al. Genetic association of FOXO1A and FOXO3A with longevity trait in Han Chinese populations. Hum. Mol. Genet. 2009;18:4897–4904. doi: 10.1093/hmg/ddp459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang Z, Kong D, Li R, Sarkar SH, Sarkar FH. Regulation of Akt/FOXO3a/GSK-3beta/AR signaling network by isoflavone in prostate cancer cells. J Biol Chem. 2008;283:27707–27716. doi: 10.1074/jbc.M802759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvik BH, Mahdjoobian K, Waldhaeusl W, et al. The effect of Ipomoea batatas (Caiapo) on glucose metabolism and serum cholesterol in patients with type 2 diabetes: a randomized study. Diabetes Care. 2002;25:239–240. doi: 10.2337/diacare.25.1.239. [DOI] [PubMed] [Google Scholar]
- Ludvik B, Waldhäusl W, Prager R, Kautky-Willer A, Pacini G. Mode of action of ipomoea batatas (Caiapo) in type 2 diabetic patients. Metabolism. 2003;52:875–880. doi: 10.1016/s0026-0495(03)00073-8. [DOI] [PubMed] [Google Scholar]
- Maeda H, Tsukui T, Sashima T, Hosokawa M, Miyashita K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac. J. Clin. Nutr. 2008;(suppl 1):196–199. [PubMed] [Google Scholar]
- Mano R, Ishida A, Ohya Y, Todoriki H, Takishita S. Dietary intervention with Okinawan vegetables increased circulating endothelial progenitor cells in healthy young women. Atherosclerosis. 2009;204:544–548. doi: 10.1016/j.atherosclerosis.2008.09.035. [DOI] [PubMed] [Google Scholar]
- Martinez-Villaluenga C, Dia VP, Berhow M, Bringe NA, Gonzalez de Mejia E. Protein hydrolysates from beta-conglycinin enriched soybean genotypes inhibit lipid accumulation and inflammation in vitro. Mol Nutr Food Res. 2009;53:1007–1018. doi: 10.1002/mnfr.200800473. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126(9):913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Masuda T, Hidaka K, Shinohara A, et al. Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. J. Agric. Food Chem. 1999;47:71–77. doi: 10.1021/jf9805348. [DOI] [PubMed] [Google Scholar]
- Medjakovic S, Mueller M, Jungbauer A. Potential health-modulating effects of isoflavones and metabolites via activation of PPAR and AhR. Nutrients. 2010;2:241–279. doi: 10.3390/nu2030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: The benefits of caloric restriction, exercise, and mimetics. Ageing Res. Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita K, Nishikawa S, Beppu F, Tsukui T, Abe M, Hosokawa M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J Sci Food Agric. 2011;91(7):1166–1174. doi: 10.1002/jsfa.4353. [DOI] [PubMed] [Google Scholar]
- Miura K, Okuda N, Turin TC. Dietary salt intake and blood pressure in a representative Japanese population: baseline analyses of NIPPON DATA80. J Epidemiol. 2010;20(Suppl 3):S524–S530. doi: 10.2188/jea.JE20090220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N, Armada F, Willcox DC. Walking to school in Japan and childhood obesity prevention: new lessons from an old policy. Am. J. Public Health. 2012;102:2068–2073. doi: 10.2105/AJPH.2012.300913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris BJ. A forkhead in the road to longevity: the molecular basis of lifespan becomes clearer. J. Hypertens. 2005;23:1285–1309. doi: 10.1097/01.hjh.0000173509.45363.dd. [DOI] [PubMed] [Google Scholar]
- Morya VK, Kim J, Kim EK. Algal fucoidan: structural and size-dependent bioactivities and their perspectives. Appl Microbiol Biotechnol. 2012;93(1):71–82. doi: 10.1007/s00253-011-3666-8. [DOI] [PubMed] [Google Scholar]
- Murakami A, Ishida H, Kubo K, et al. Suppressive effects of Okinawan food items on free radical generation from stimulated leukocytes and identification of some active constituents: implications for the prevention of inflammation-associated carcinogenesis. Asian Pacific J. Cancer Prev. 2005;6:437–448. [PubMed] [Google Scholar]
- Nagata C, Takatsuka N, Shimizu N, Shimizu H. Sodium intake and risk of death from stroke in Japanese men and women. Stroke. 2004;35:1543–1547. doi: 10.1161/01.STR.0000130425.50441.b0. [DOI] [PubMed] [Google Scholar]
- Nagarajan S, Burris RL, Stewart BW, Wilkerson JE, Badger TM. Dietary soy protein isolate ameliorates atherosclerotic lesions in apolipoprotein E-deficient mice potentially by inhibiting monocyte chemoattractant protein-1 expression. J Nutr. 2008;138:332–337. doi: 10.1093/jn/138.2.332. [DOI] [PubMed] [Google Scholar]
- Naguib YM. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000;48:1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- Ogura C, Nakamoto H, Uema T, Yamamoto K, Yonemori T, Yoshimura T. Prevalence of senile dementia in Okinawa, Japan. COSEPO Group. Study group of epidemiology for psychiatry in Okinawa. Int. J. Epidemiol. 1995;24:373–380. doi: 10.1093/ije/24.2.373. [DOI] [PubMed] [Google Scholar]
- Okinawa Prefecture, Department of Health and Welfare. 2011 Health and Nutrition Status of Okinawan People. 2013 www.kenko-okinawa21.jp_kankobutu_eiyo_H23eiyo_H23eiyo. [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, et al. A potential decline in life expectancy in the United States in the 21st century. N. Eng. J. Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Perry D, Miller RA, Butler RN. Pursuing the longevity dividend: scientific goals for an aging world. Ann. N.Y. Acad. Sci. 2007;1114:11–13. doi: 10.1196/annals.1396.050. [DOI] [PubMed] [Google Scholar]
- Papadakis S, Moroz I. Population-level interventions for coronary heart disease prevention: what have we learned since the North Karelia project? Curr Opin Cardiol. 2008 Sep;23(5):452–461. doi: 10.1097/HCO.0b013e32830c217e. [DOI] [PubMed] [Google Scholar]
- Pashkow FJ, Watumull DG, Campbell CL. Astaxanthin: a novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008;101:58D–68D. doi: 10.1016/j.amjcard.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Pomin VH, Mourao PA. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology. 2008;18:1016–1027. doi: 10.1093/glycob/cwn085. [DOI] [PubMed] [Google Scholar]
- Popkin BM. Urbanization, lifestyle changes and the nutrition transition. World Dev. 1999;27:1905–1916. [Google Scholar]
- Popkin BM. The nutrition transition and obesity in the developing world. J. Nutr. 2001;131:871S–873S. doi: 10.1093/jn/131.3.871S. [DOI] [PubMed] [Google Scholar]
- Poulain M, Pes GM, Grasland C, Carru C, Ferrucci L, Baggio G, Franceschi C, Deiana L. Identification of a geographic area characterized by extreme longevity in the Sardinia island: the AKEA study. Exp Gerontol. 2004 Sep;39(9):1423–1429. doi: 10.1016/j.exger.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Pawlikowska L, Hu D, Huntsman S, et al. Study of osteoporotic fractures: association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8:460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priyadarsini KI, Guha SN, Rao MN. Physicochemical properties and antioxidant activities of methoxy phenols. Free Radic. Biol. Med. 1998;24:933–941. doi: 10.1016/s0891-5849(97)00382-1. [DOI] [PubMed] [Google Scholar]
- Puska P. From Framingham to North Karelia: from descriptive epidemiology to public health action. Prog Cardiovasc Dis. 2010 Jul-Aug;53(1):15–20. doi: 10.1016/j.pcad.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Qin LQ, Xu JY, Wang PY, Hoshi K. Soy food intake in the prevention of breast cancer risk in women: a meta-analysis of observational epidemiological studies. J. Nutri. Sci. Vitaminol. (Tokyo) 2006;52:428–436. doi: 10.3177/jnsv.52.428. [DOI] [PubMed] [Google Scholar]
- Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-κB and pro-inflammatory gene expression. Biochem. Pharmacol. 2004;68:1255–1267. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Hormesis in aging. Ageing Res. Rev. 2008;7:63–78. doi: 10.1016/j.arr.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Rattan SI. Rationale and methods of discovering hormetins as drugs for healthy ageing. Expert Opin. Drug Disc. 2012;7:439–448. doi: 10.1517/17460441.2012.677430. [DOI] [PubMed] [Google Scholar]
- Rietjens IM, Sotoca AM, Vervoort J, Louisse J. Mechanisms underlying the dualistic mode of action of major soyisoflavones in relation to cell proliferation and cancer risks. Mol Nutr Food Res. 2013;57(1):100–113. doi: 10.1002/mnfr.201200439. [DOI] [PubMed] [Google Scholar]
- Robine JM, Herrmann FR, Arai Y, Willcox DC, Gondo Y, Hirose N, Suzuki M, Saito Y. Exploring the impact of climate on human longevity. Exp Gerontol. 2012;47(9):660–671. doi: 10.1016/j.exger.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N. Engl. J. Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M American Heart Association Nutrition Committee. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of dietary approaches to stop hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases--incidence: a systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29:611–618. doi: 10.1016/j.nut.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Savica V, Bellinghieri G, Kopple JD. The effect of nutrition on blood pressure. Annu Rev Nutr. 2010;21;30:365–401. doi: 10.1146/annurev-nutr-010510-103954. [DOI] [PubMed] [Google Scholar]
- Sanmukhani J, Satodia V, Trivedi J, et al. Efficacy and Safety of Curcumin in Major Depressive Disorder: A Randomized Controlled Trial. Phytother. Res. 2013 doi: 10.1002/ptr.5025. [DOI] [PubMed] [Google Scholar]
- Sathishsekar D, Subramanian S. Antioxidant properties of Momordica Charantia (bitter gourd) seeds on Streptozotocin induced diabetic rats. 2005;14:153–158. [PubMed] [Google Scholar]
- Scapagnini G, Colombrita C, Amadio M, et al. Curcumin activates defensive genes and protects neurons against oxidative stress. Antioxid. Redox. Signal. 2006;8:395–403. doi: 10.1089/ars.2006.8.395. [DOI] [PubMed] [Google Scholar]
- Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Galvano F. Modulation of nrf2/are pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 2011;44:202. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scapagnini G, Davinelli S, Drago F, De Lorenzo A, Oriani G. Antioxidants as antidepressants: facts or fiction? CNS drugs. 2012;26:477–490. doi: 10.2165/11633190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Schröder H, Schmelz E, Marrugat J. Relationship between diet and blood pressure in a representative Mediterranean population. Eur. J. Nutr. 2002;41:161–167. doi: 10.1007/s00394-002-0372-4. [DOI] [PubMed] [Google Scholar]
- Senni K, Pereira J, Gueniche F, Delbarre-Ladrat C, Sinquin C, Ratiskol J, Godeau G, Fischer AM, Helley D, Colliec-Jouault S. Marine polysaccharides: a source of bioactive molecules for cell therapy and tissue engineering. Mar Drugs. 2011;9(9):1664–1681. doi: 10.3390/md9091664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan Q, Lu J, Zheng Y, Li J, Zhou Z, Hu B, Zhang Z, Fan S, Mao Z, Wang YJ, Ma D. Purple sweet potato color ameliorates cognition deficits and attenuates oxidative damage and inflammation in aging mouse brain induced by d-galactose. J Biomed Biotechnol. 2009;2009:564737. doi: 10.1155/2009/564737. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LR, Parnell LD, Ordovas JM, Lai CQ. Curcumin an aging. Biofactors. 2013;39:133–140. doi: 10.1002/biof.1086. [DOI] [PubMed] [Google Scholar]
- Shikata K, Kiyohara Y, Kubo M, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196–201. doi: 10.1002/ijc.21822. [DOI] [PubMed] [Google Scholar]
- Shirani F, Salehi-Abargouei A, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH) diet on some risk for developing type 2 diabetes: a systematic review and meta-analysis on controlled clinical trials. Nutrition. 2013;29:939–947. doi: 10.1016/j.nut.2012.12.021. [DOI] [PubMed] [Google Scholar]
- Sho H. History and characteristics of Okinawan longevity food. Asia Pac. J. Clin. Nutr. 2001;10:159–164. doi: 10.1111/j.1440-6047.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- Speciale A, Chirafisi J, Saija A, et al. Nutritional antioxidants and adaptive cell responses: an update. Current Molecular Medicine. 2011;11:770–789. doi: 10.2174/156652411798062395. [DOI] [PubMed] [Google Scholar]
- Singh S, Aggarwal BB. Activation of transcription factor NF-kB is suppressed by curcumin (diferuloylmethane) J. Biol. Chem. 1995;270:24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- Sreejayan A, Rao MN. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- Sung B, Prasad S, Yadav VR, Aggarwal BB. Cancer cell signaling pathways targeted by spice-derived nutraceuticals. Nutr. Cancer. 2012;64:173–197. doi: 10.1080/01635581.2012.630551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Ju YS, Yasuoka J, Jimba M. Rapid increase in Japanese life expectancy after World War II. Biosci Trends. 2010;4:9–16. [PubMed] [Google Scholar]
- Suzuki M, Willcox BJ, Willcox DC. Implications from and for food cultures for cardiovascular disease: longevity. Asia Pac. J. Clin. Nutr. 2001;10:165–171. doi: 10.1111/j.1440-6047.2001.00219.x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Willcox DC, Rosenbaum MW, Willcox BJ. Oxidative stress and longevity in Okinawa: an investigation of blood lipid peroxidation and tocopherol in Okinawan centenarians. Curr. Gerontol. Geriatr. Res. 2010:380460. doi: 10.1155/2010/380460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata H, Suzuki M, Ishii T, Sekiguchi S, Iri H. Influence of major histocompatibility compatibility complex region genes on human longevity among Okinawan-Japanese centenarians and nonagenarians. Lancet. 1987;10:824–826. doi: 10.1016/s0140-6736(87)91015-4. [DOI] [PubMed] [Google Scholar]
- Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 2007;85:1148–1156. doi: 10.1093/ajcn/85.4.1148. [DOI] [PubMed] [Google Scholar]
- Todoriki H, Willcox DC, Willcox BJ. The effects of post-war dietary change on longevity and health in Okinawa. Oki. J. Amer. Studies. 2004;1:55–64. [Google Scholar]
- Tsugane S, Sasazuki S. Diet and the risk of gastric cancer: review of epidemiological evidence. Gastric Cancer. 2007;10:75–83. doi: 10.1007/s10120-007-0420-0. [DOI] [PubMed] [Google Scholar]
- Tuekpe MK, Todoriki H, Sasaki S, Zheng K-C, Ariizumi M. Potassium excretion in healthy Japanese women was increased by a dietary intervention utilizing home-parcel delivery of Okinawan vegetables. Hypertension Res. 2006;29:389–396. doi: 10.1291/hypres.29.389. [DOI] [PubMed] [Google Scholar]
- Umesawa M, Iso H, Date C, et al. Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: the Japan collaborative cohort study for evaluation of cancer risks. Am J Clin Nutr. 2008;88:195–202. doi: 10.1093/ajcn/88.1.195. [DOI] [PubMed] [Google Scholar]
- University of Hawaii, College of Tropical Agriculture and Human Resources. Hawaii Foods, Nutrition with Aloha.; http://hawaiifoods.hawaii.edu/index.asp. [Google Scholar]
- US Burden of Disease Collaborators et al. The State of US Health: 1990-2010: Burden of Diseases, Injuries, and Risk Factors. JAMA. 2013 doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen E, Laatikainen T, Peltonen M, Juolevi A, Männistö S, Sundvall J, Jousilahti P, Salomaa V, Valsta L, Puska P. Thirty-five-year trends in cardiovascular risk factors in Finland. Int J Epidemiol. 2010 Apr;39(2):504–518. doi: 10.1093/ije/dyp330. [DOI] [PubMed] [Google Scholar]
- Valsta LM, Tapanainen H, Sundvall J, Laatikainen T, Männistö S, Pietinen P, Vartiainen E. Explaining the 25-year decline of serum cholesterol by dietary changes and use of lipid-lowering medication in Finland. Public Health Nutr. 2010 Jun;13(6A):932–938. doi: 10.1017/S1368980010001126. [DOI] [PubMed] [Google Scholar]
- Vasto S, Candore G, Balistreri CR, Caruso M, Colonna-Romano G, Grimaldi MP, Listi F, Nuzzo D, Lio D, Caruso C. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Vijg J, Campisi J. Puzzles, promises and a cure for ageing. Nature. 2008;454:1065–1071. doi: 10.1038/nature07216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale DC, Piazza C, Melilli B, Drago F, Salomone S. Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet. 2013;38(1):15–25. doi: 10.1007/s13318-012-0112-y. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Q, Zhang Z, Li Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int J Biol Macromol. 2008;42:127–132. doi: 10.1016/j.ijbiomac.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Zheng YL, Lu J, et al. Purple sweet potato color suppresses lipopolysaccharide-induced acute inflammatory response in mouse brain. Neurochem. Int. 2010;56:424–430. doi: 10.1016/j.neuint.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Willcox B, Willcox DC, Suzuki M. The Okinawa Diet Plan. New York, NY: Three Rivers Press, A Division of Random House; 2004. [Google Scholar]
- Willcox BJ, He Q, Chen R, et al. Midlife risk factors and healthy survival in men. JAMA. 2006;296:2343–2350. doi: 10.1001/jama.296.19.2343. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Willcox DC, Todoriki H, et al. Caloric restriction, the traditional Okinawan diet, and healthy aging: the diet of the world’s longest-lived people and its potential impact on morbidity and life span. Ann. N.Y. Acad. Sci. 2007;1114:454–455. doi: 10.1196/annals.1396.037. [DOI] [PubMed] [Google Scholar]
- Willcox BJ, Donlon TA, He Q, et al. FOXO3A genotype is strongly associated with human longevity. Proc. Natl. Acad. Sci. U.S.A. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox DC, Willcox BJ, Todoriki H, Suzuki M. The Okinawan diet: health implications of a low-calorie, nutrient-dense, antioxidant-rich dietary pattern low in glycemic load. J. Am. Coll. Nutr. 2009;28(Suppl):500S–516S. doi: 10.1080/07315724.2009.10718117. [DOI] [PubMed] [Google Scholar]
- Willcox DC, Willcox BJ, Yasura S, Ashitomi I, Suzuki M. Gender gap in healthspan and life expectancy in Okinawa: health behaviours. Asian J Gerontol Geriatr. 2012;7:49–58. [Google Scholar]
- Willcox BJ, Suzuki M, Donlon TA, He Q, Grove JS, Masaki K, Willcox DC. Optimizing Human Health Span and Life Span: Insights from Okinawa and Hawaii. Ann Rev Gerontol Geriatr. 2013;33(1):133–170. [Google Scholar]
- World Health Organization. Joint WHO/FAO Expert Consultation on Diet, Nutrition and the Prevention of the Chronic Diseases. Geneva: 2003. [Google Scholar]
- World Health Organization. Preventing chronic diseases : a vital investment : WHO global report. Geneva: Who Press; 2005. [Google Scholar]
- Wu AH, Ziegler RG, Horn-Ross PL, et al. Tofu and risk of breast cancer in Asian-Americans. Cancer Epidemiol. Biomarkers Prev. 1996;5:901–906. [PubMed] [Google Scholar]
- Xiang L, Nakamura Y, Lim YM, et al. Tetrahydrocurcumin extends life span and inhibits the oxidative stress response by regulating the FOXO forkhead transcription factor. Aging. 2011;3:1098–1109. doi: 10.18632/aging.100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YX, Pindolia KR, Janakiraman N, Chapman RA, Gautam SC. Curcumin inhibits IL1α and TNFα induction of AP-1 and NF-kB DNA-binding activity in bone marrow stromal cells. Hematopathol. Mol. Hematol. 1997;11:49–62. [PubMed] [Google Scholar]
- Yaffe K, Tocco M, Petersen RC, et al. The epidemiology of Alzheimer's disease: Laying the foundation for drug design, conduct, and analysis of clinical trials. Alzheimers Dement. 2012;8:237–242. doi: 10.1016/j.jalz.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Yan L, Spitznagel EL. Meta-analysis of soy food and risk of prostate cancer in men. Int. J. Cancer. 2005;117:667–669. doi: 10.1002/ijc.21266. [DOI] [PubMed] [Google Scholar]
- Yazaki K, Yoshikoshi C, Oshiro S, Yanase S. Supplemental cellular protection by a carotenoid extends lifespan via Ins/IGF-1 signaling in Caenorhabditis elegans. Oxid Med Cell Longev. 2011;2011:596240. doi: 10.1155/2011/596240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D, Ibuki M, Nakamori T, Fan M, Mine Y. Soy-derived di- and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. J Nutr. 2012;142:363–368. doi: 10.3945/jn.111.149104. [DOI] [PubMed] [Google Scholar]
- Zhang ZF, Fan SH, Zheng YL, et al. Purple sweet potato color attenuates oxidative stress and inflammatory response induced by d-galactose in mouse liver. Food Chem. Toxicol. 2009;47:496–501. doi: 10.1016/j.fct.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li Q, Zhou D, Chen H. Genistein, a soya isoflavone, prevents azoxymethane-induced up-regulation of WNT/β-catenin signalling and reduces colon pre-neoplasia in rats. Br J Nutr. 2013;109:33–42. doi: 10.1017/S0007114512000876. [DOI] [PubMed] [Google Scholar]
- Zhao BL, Li XJ, He RG, et al. Scavenging effect of extracts of green tea and natural antioxidants on active oxygen radicals. Cell Biophys. 1989;14:175–185. doi: 10.1007/BF02797132. [DOI] [PubMed] [Google Scholar]
- Zhou BF, Stamler J, Dennis B, Moag-Stahlberg A, Okuda N, Robertson C, Zhao L, Chan Q, Elliott P INTERMAP Research Group. Nutrient intakes of middle-aged men and women in China, Japan, United Kingdom, and United States in the late 1990s: the INTERMAP study. J Hum Hypertens. 2003 Sep;17(9):623–630. doi: 10.1038/sj.jhh.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]


