Abstract
Infection with certain types of human papillomavirus (HPV) has been associated with the development of cervical and anal cancer. Worldwide, the incidence of anal cancer has increased markedly. The present study aimed to evaluate the prevalence of HPV infection of the uterine cervix and anal canal in human immunodeficiency virus (HIV)- and non-HIV-infected risk populations. Cervical and anal HPV swabs and cytology samples were collected from 287 patients at the University Hospital of Munich, Germany between 2011 and 2013. Patients were divided into HIV-negative controls (G1) and two risk groups, including HIV-negative patients with cytological abnormalities of the cervix (G2) and HIV-infected patients (G3). Data, including clinical parameters, were analysed. The risk groups had significantly more positive results for HPV in the anus (71.03 and 83.15% for G2 and G3, respectively), as compared with G1. The predominant HPV genotypes found in the anus were high-risk HPV genotypes, which were significantly correlated with concomittant cervical HPV findings. In the risk groups, a significant association between the cytological findings and HPV detection in the cervix was found, while the results of the anus revealed no significance. The results of the present study suggested that the prevalence of HPV infection in the anal canal of risk populations is high. Furthermore, patients with abnormal cervical cytology results and HIV-infected women, irrespective of their individual cervical findings, may have a risk of concomittant anal high-risk HPV infection. Based on the predominant HPV genotypes found in the study, HPV vaccination could reduce the incidence of anal cancer. Nevertheless, high-risk patients should be intensively screened for anal squamous intraepithelial abnormalities to avoid invasive cancer stages.
Keywords: human papillomavirus, human immunodeficiency virus, anal dysplasia, cancer screening, women
Introduction
Infection with certain types of human papillomavirus (HPV) plays an important role in the development of cervical and anal cancer. In the sexually active population, HPV infection of the anogenital region can be found in >60% of individuals (1). Screening for cervical cancer is well established, and is accompanied by a significant risk reduction in the incidence of that cancer type (2). During the pathogenesis of cervical cancer, precursor lesions, such as anal intraepithelial neoplasia (AIN), can develop into invasive squamous cell carcinoma of the anus (3). The majority of anal malignancies are associated with a persistent infection with HPV (4).
Over the past few decades, the incidence of anal cancer has increased significantly, particularly in women (5,6). In a previous study, coexisting HPV infection of the cervix and anal canal was detected in human immunodeficiency virus (HIV)-negative patients (7). Risk populations include men who have sex with men and transplant recipients. Furthermore, HIV-infected patients showed a higher prevalence of HPV-associated anal dysplasia or anal cancer compared with the HIV-negative population (8). In addition, the overall risk was ~28-fold higher in the female HIV-infected population (8). Whether treatment with combined antiretroviral therapy (cART) is able to reduce the risk of anal dysplasia and anal cancer in this patient group is controversial; however, its ability to reduce the occurrence of cervical and anal cancer has been poor to date (9–11).
The effectiveness of anal cancer screening has not been sufficiently evaluated. Furthermore, the question of whether the development of anal cancer can be prevented by the sufficient treatment of high-grade AIN alone has yet to be answered (2). At present, strategies for anal screening and the treatment of high-grade AINs are under investigation in randomized controlled studies (12,13). At the very least, the identification of risk factors, such as anal HPV infection, and the resulting short-term monitoring of high-risk populations, may lead to the early detection of the precancer and invasive stages.
Generally the prevalence of intra-anal HPV infection in the general HIV-negative female population, as compared with high-risk populations such as HIV-infected or HIV-negative patients with HPV-associated cervical abnormalities, is unknown. Therefore, the present prospective, cross-sectional study aimed to evaluate the prevalence of cervical and anal HPV infection, as well as clinical risk factors, in general controls, as compared with risk populations for anogenital dysplasia, including HIV-negative patients with abnormal cervical cytology attending the clinic for colposcopy evaluation and HIV-infected women.
Materials and methods
Study population
The prospective study included 287 patients who attended the Cervical Disease Screening and Treatment Unit or the specialized Gynaecological Outpatient Clinic for HIV-infected women at the Department of Gynecology and Obstetrics, Ludwig-Maximilian University of Munich (Munich, Germany) between 2011 and 2013. Patients were divided into three groups, as follows: G1, which included HIV-negative patients without a history of abnormal cytological findings who underwent routine cervical cytological screening (low-risk, n=93); G2, which included HIV-negative patients who were sent to our colposcopy unit with at least one preceding abnormal Papanicolaou-smear result (high-risk, n=90); and G3, which included HIV-infected patients who underwent routine cervical cytological screening at our outpatient department for HIV-infected women (high-risk, n=104). None of the patients had received the anti-HPV vaccination or were diagnosed with condyloma acuminata. All patients completed an anonymous, self-administered questionnaire, which collected information regarding their age, medical history, country of origin, smoking status, history of anal intercourse, number of sexual partners, age of first sexual intercourse and current marital status. According to the HIV-related history Centers for Disease Control and Prevention classification system, current cluster of differentiation (CD)4+ counts and nadir, information related to the viral load and ART were collected. Written informed consent was obtained from all patients. The present study was approved by the Local Ethics Committee of the Ludwig-Maximilian University of Munich (approval no. 273-10).
Specimen collection
Cervical and corresponding intra-anal cytology and HPV samples were obtained from each patient, according to the study protocol. Smears for cytology were performed in-house using a moistened cotton swab and cytobrush for cervical samples, and a moistened cotton swab for intraanal samples; they were fixed with M-Fix® spray fixative at room temperature (Merck KGaA, Darmstadt, Germany), stained according to the Papanicolaou protocol and evaluated using Munich nomenclature II (14) by an experienced cytologist using light microscopy. For further evaluation, the results were transferred to the Bethesda system (15). For HPV detection, a separate swab was used for cervical and intraanal probes.
Detection and genotyping of HPV in clinical specimens
DNA was isolated and purified from the specimens using a commercial kit (QIAamp DNA Mini kit; Qiagen GmbH, Hilden, Germany), according to the manufacturer's protocol. Amplification of the L1-open reading frame and genotyping of the HPVs were performed using the INNO-LiPA HPV Genotyping Extra Amp and the INNO-LiPA HPV Genotyping Extra kit (both from Fujirebio Europe, Gent, Belgium). Known HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68), as well as the genotypes 6, 11, 26, 40, 43, 44, 53, 54, 66, 73, 70 and 82 were covered.
Statistical analysis
Statistical analyses were performed by STAT-UP Statistical Consulting & Services (Munich, Germany) using the R statistical package software, version 2.14.0 for Windows. The threshold of significance was set as P=0.05. Groups were compared using Mann-Whitney U, Kruskal-Wallis, Fisher's exact and χ2 tests. Significance levels in post hoc tests were Bonferroni-Holm adjusted.
Results
Patient characteristics
A total of 287 patients were included in the study and divided into three groups. The baseline characteristics of the examined cohort are presented in Table I.
Table I.
Baseline patient characteristics.
| Characteristic | Whole cohort (n=287) | G1 (n=93) | G2 (n=90) | G3 (n=104) | P-values |
|---|---|---|---|---|---|
| Age, years | 37.59±9.04 | 39.74±9.78 | 33.61±7.52 | 39.11±8.51 | G1 vs. G2, P<0.05; G2 vs. G3, P<0.05; G1 vs. G3, P>0.05 |
| Origin | P<0.05 | ||||
| Western Europe | 61.8 | 65.6 | 95.4 | 37.2 | |
| Eastern Europe | 12.3 | 20.4 | 3.1 | 9.3 | |
| Central/South America | 4.0/10.9 | 1.5/4.7 | 1.5/0 | 2.3/38.4 | |
| Africa | 10.9 | 7.8 | 0 | 12.8 | |
| Smoking | P<0.05 | ||||
| Yes | 32.0 | 29.0 | 44.3 | 24.0 | |
| No | 68.0 | 71.0 | 55.7 | 76.0 | |
| Anal intercourse | P>0.05 | ||||
| Yes | 28.8 | 35.5 | 25.3 | 25.7 | |
| No | 71.2 | 64.5 | 74.7 | 74.3 | |
| Age at first sexual intercourse, years | P>0.05 | ||||
| ≤15 | 20.0 | 18.5 | 14.9 | 25.7 | |
| 16–19 | 56.1 | 53.3 | 66.7 | 49.5 | |
| 20–24 | 21.4 | 25.0 | 16.1 | 22.8 | |
| ≥25 | 2.5 | 3.2 | 2.3 | 2.0 | |
| Lifetime sex partners | P>0.05 | ||||
| 1 | 9.0 | 16.5 | 4.6 | 5.9 | |
| 2–5 | 46.2 | 42.9 | 46.0 | 49.5 | |
| 6–10 | 24.4 | 19.8 | 39.1 | 15.8 | |
| >10 | 20.4 | 20.9 | 10.3 | 28.8 | |
| Marital status/stable partner | P>0.05 | ||||
| Yes | 81.4 | 84.8 | 86.2 | 74.3 | |
| No | 18.6 | 15.2 | 13.8 | 25.7 | |
| History of worst cytological result of the cervix | P>0.05 | ||||
| Not specified | 44.3 | 92.9 | 21.4 | 19.6 | |
| PAP 2 | 8.6 | 1.2 | 1.4 | 19.6 | |
| PAP 3 | 3.9 | 2.4 | 4.3 | 4.9 | |
| PAP 3D | 33.1 | 3.5 | 65.7 | 35.3 | |
| PAP 4a | 0.4 | 0 | 0 | 1.0 | |
| PAP 4b | 9.7 | 0 | 7.2 | 19.6 |
Data are presented as the mean ± standard deviation or %. G1, HIV-negative patients who underwent routine cervical cytology screening; G2, HIV-negative patients with at least one abnormal cytological (Pap)-smear result of the cervix; G3, HIV-infected patients who underwent routine cervical cytology screening; PAP, Papanicolaou (Munich nomenclature II).
Anal HPV findings
The prevalence of anal HPV infection among the three analysed groups (G1-G3) was significantly different; 50, 71 and 83% of G1, G2 and G3, respectively, had a positive result for HPV in the anus. As compared with G1, G2 (P=0.011) and G3 (P<0.001) showed significantly more anal HPV infections of any type, while the difference between the G2 and G3 risk groups was not significantly different (P>0.05).
After dividing HPV genotypes into high-risk and low-risk anal HPV types, high-risk HPV genotypes were found significantly more often in the anal samples from G2 (65%; P<0.001) and G3 (62%; P<0.001), as compared with those from G1 (28%). The difference between G2 and G3 with regard to high-risk anal HPV genotypes, as well as the differences between the three groups with regard to low-risk anal HPV genotypes, were not significantly different.
Cervical and anal HPV findings
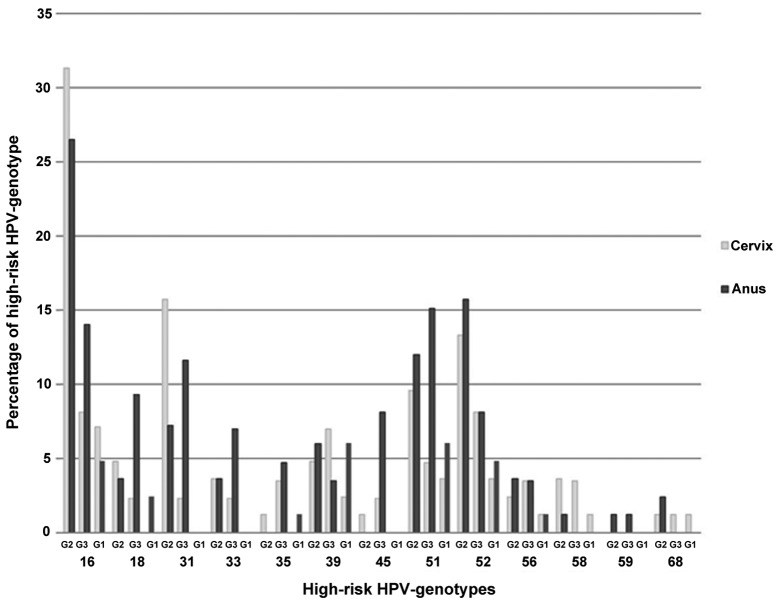
Regarding the prevalence of different HPV genotypes in the cervix and anus, 13 high-risk HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68) and 11 low-risk HPV genotypes (6, 11, 44, 53, 54, 66, 69, 70, 71, 74 and 82) were found. Fig. 1 illustrates the distribution of the different cervical and anal high-risk HPV genotypes in the analysed groups. In the risk groups (G2 and G3), the most prevalent anal high-risk HPV genotypes were genotypes 16 (G2, 27%; G3, 14%), 18 (G2, 4%; G3, 9%), 31 (G2, 7%; G3, 12%), 51 (G2, 12%; G3, 15%) and 52 (G2, 16%; G3, 8%).
Figure 1.
Illustration of the cervical and anal high-risk HPV genotypes in the analysed groups. G1, HIV-negative patients who underwent routine cervical cytology screening; G2, HIV-negative patients with at least one abnormal cytological (Pap)-smear result of the cervix; G3, HIV-infected patients who underwent routine cervical cytology screening; HPV, human papillomavirus; HIV, human immunodeficiency virus.
In all groups, a positive HPV result was associated with significant concomitant cervical and anal HPV infection of any type (all P<0.05). A positive result for HPV in samples from the cervix and anus was found in 29% of the analysed patients in G1, 68% in G2 and 56% in G3. The significant association between cervical and anal samples persisted even after dividing HPV genotypes into high-risk and low-risk groups (both P<0.05).
With regard to individual HPV genotypes, there was a significant association between several high- and low-risk HPV genotypes and cervical and anal findings in the three analysed groups. There was a significant association between the cervical HPV genotype and the anal HPV genotype for 4 of the 5 predominant high-risk HPV genotypes found in G2 and G3 (G2: HPV 16, P<0.001; HPV 18, P<0.001; HPV 31, P<0.001; HPV 51, P=0.006; G3: HPV 16, P<0.001; HPV 18, P<0.001; HPV 31, P=0.012; HPV 52, P=0.001). Overall 36% of the analysed patients had a negative HPV result in the cervix after being tested positive for any HPV in the anus. In G2, 19% of the patients tested positive for any HPV genotype in the anus (high-risk, 33%), while 67% of G3 tested positive for any HPV genotype in the anus (high-risk, 43%). The difference between G2 and G3 was statistically significant (P<0.05).
Cervical and anal cytology and HPV findings
Analysing the results of the cervical and anal cytological analysis for cervical and anal HPV detection, a significant association between the cervical cytology result and cervical HPV was detected in all groups (all P<0.05). The worse the results of the cervical cytology, the more patients in that group tested positive for any HPV genotype in the cervix. Regarding the association between the anal cytology results and anal HPV detection, the analysed groups revealed no significance (Table II).
Table II.
Percentage of patients who tested positive for HPV in association with the corresponding cytology results of the cervix and anus.
| Cytology classification | n | % HPV | P-value |
|---|---|---|---|
| G1 | |||
| Cervix | P<0.05 | ||
| PAP 2 (NILM) | 78 | 24 | |
| PAP 3 (ASCUS) | 12 | 58 | |
| PAP 3D (LSIL) | 2 | 100 | |
| Anus | P>0.05 | ||
| PAP 2 (NILM) | 64 | 45 | |
| PAP 3 (ASCUS) | 6 | 100 | |
| PAP 3D (LSIL) | 10 | 60 | |
| G2 | |||
| Cervix | P<0.05 | ||
| PAP 2 (NILM) | 15 | 56 | |
| PAP 3 (ASCUS) | 9 | 82 | |
| PAP 3D (LSIL) | 14 | 93 | |
| PAP 4a (HSIL) | 25 | 96 | |
| Anus | P>0.05 | ||
| PAP 2 (NILM) | 27 | 73 | |
| PAP 3 (ASCUS) | 3 | 75 | |
| PAP 3D (LSIL) | 17 | 71 | |
| PAP 4a (HSIL) | 1 | 100 | |
| G3 | |||
| Cervix | P<0.05 | ||
| PAP 2 (NILM) | 21 | 42 | |
| PAP 3 (ASCUS) | 15 | 63 | |
| PAP 3D (LSIL) | 13 | 100 | |
| PAP 4a (HSIL) | 6 | 100 | |
| PAP 4b (HSIL/cancer) | 1 | 100 | |
| PAP 5 (cancer) | 2 | 100 | |
| Anus | P>0.05 | ||
| PAP 2 (NILM) | 62 | 83 | |
| PAP 3 (ASCUS) | 4 | 67 | |
| PAP 3D (LSIL) | 5 | 100 | |
| PAP 4a (HSIL) | 1 | 100 |
G1, HIV-negative patients who underwent routine cervical cytology screening; G2, HIV-negative patients with at least one preceding abnormal cytological (PAP)-smear result of the cervix; G3, HIV-infected patients who underwent routine cervical cytology screening; PAP, Papanicolaou using Munich nomenclature II; HPV, human papillomavirus; HIV, human immunodeficiency virus; NILM, negative for intraepithelial lesion or malignancy; ASCUS, atypical squamous cells of undetermined significance; LSIL, low grade squamous intraepithelial lesion; HSIL, high grade squamous intraepithelial lesion.
Anal HPV findings and clinical parameters
A significant association was observed between the detection of anal HPV infection in the present study and the number of lifetime sex partners (P<0.001), history of abnormal cervical cytology (P=0.009) and the history of cervical HPV (P<0.001). None of the other analysed characteristics (age, origin, smoking, anal intercourse, age of first sexual intercourse or marital status) were significantly associated with anal HPV infection. Regarding the HIV-infected patients, only the CD4+ nadir was significantly associated with anal HPV (P=0.021). The current CD4+ count, current HIV viral load, and the use of cART were not significantly associated with anal HPV. The clinical aspects related to HIV infection in G3 are summarized in Table III.
Table III.
Specific characteristics of HIV-infected patients.
| Characteristic | G3 HIV-infected patients (n=104) |
|---|---|
| Current detectable viral load (copies/ml) | 2,772 (<20->100,000) |
| Taking cART | |
| Yes | 89 |
| No | 11 |
| cART duration (years) | |
| Range | 0–21 |
| Mean ± SD | 8.2±5.6 |
| Current CD4 count (cells/µl) | 540 (34–1650) |
| Nadir CD4 count (cells/µl) | 258 (1–994) |
Data are presented as the mean (interquartile range) or %. G3, HIV-infected patients who underwent routine cervical cytology screening; cART, combined antiretroviral therapy; HIV, human immunodeficiency virus; CD4, cluster of differentiation 4; SD, standard deviation.
Discussion
The incidence of HPV-related cancer of the anus has increased over the past several decades. Risk populations, including women with a history of genital neoplasia or HIV-infected patients, are known. Furthermore, methods for effective anal cancer screening are under investigation (13).
Consistently with a recent review by Stier et al (16), both high-risk groups in the present study (G2 and G3) tested positive for any HPV genotype in the anus more often than the controls (G1). Limited data exists regarding anal HPV infection in the non-immunosuppressed general population, with high variance in the prevalence of anal HPV detection itself, and the prevalence of anal HPV in HIV-uninfected high-risk patients compared with HIV-infected women (17,18). All predominant HPV genotypes found in the anus of the two risk groups in the present study were high-risk HPV genotypes. Compared with cervical cancer, the incidence of anal cancer in the general population is low (19). It seems that anal colonisation with HPV is less likely to lead to cell abnormalities than cervical colonisation, although the difference in the carcinogenesis of these mucosal sites has yet to be completely evaluated. At least chronological differences can be assumed (18). Nevertheless, due to high prevalence of anal HPV infection, the detection and possible treatment of anal precancerous lesions could decrease the incidence of anal cancer.
Sufficient cervical screening reduces the incidence of invasive cancer. At the same time, the prophylactic HPV vaccination reduces the incidence of HPV infection, assuming a reduction in the incidence of HPV-associated cancers (20). Prophylactic HPV vaccination is part of the individual immunisation schedule in many countries, although the country-specific performance varies considerably (21). Besides the effect on the uterine cervix, the quadrivalente HPV vaccine was demonstrated to prevent persistent anal HPV infection and anal intraepithelial lesions (22). The five most prevalent anal HPV genotypes found in the present study were HPV types 16, 18, 31, 52 and 51. With the administration of the 9-valent HPV vaccine (types 6, 11, 16, 18, 31, 33, 45, 52 and 58), the rising incidence of anal cancer could be prevented more effectively.
It was reported that the detection rate of HPV in simultaneously collected cervical and anal specimens was comparable or even higher in the anus (16). Consistent with the literature, a significant association between the prevalence of concomitant low- and high-risk cervical and anal HPV in all investigated patient groups was detected in the present study. One third of the analysed patients who had a negative result for HPV in the cervix tested positive in the anus. Of the HIV-infected patients with a negative result for HPV in the cervix, 67% tested positive for HPV in the anus. The risk for concomitant HPV infection of the cervix and anus appeared to be likely. However, a negative HPV result in the cervix should not discount the performance of an anal HPV screening, particularly in high-risk patients.
Screening for cervical cancer is well established. The benefits of cytology-based vs. HPV-based cancer screening of different mucosal sites are under discussion (23). A significant association between the cytology findings of the cervix and cervical HPV detection was found in the present study. Controversial results were published concerning the association between anal cytology and anal HPV prevalence. A previous study reported a suspicious anal cytology in <10% of women with lower genital tract dysplasia, while positive anal HPV results were detected in >50% (24). In the present study, a significant association between the cytological results of the cervix and HPV detection in the cervix was observed, while there was no significant association between the anal cytology results and positive anal HPV detection. Compared with the cervical findings, a correlation between anal HPV infection and a suspicious anal cytology is infrequently observed. To date, there have been no valid data concerning the efficacy on any type of anal cancer screening technique. Currently, the primary screening tool for anal HPV-associated disease is cytology. Although the performance of anal cytology is similar to cervical cytology, experience in interpreting anal samples is limited. Other techniques such as high-resolution anoscopy should be considered to verify the true rate of anal dysplasias, particularly in cytological-negative and HPV-positive anal samples from risk populations. Further studies on the evaluation and implementation of anal cancer screening are required (25).
Consistent with the literature, a significant association between the number of lifetime sex partners and the incidence of anal HPV infection was demonstrated in the present study. Anal intercourse itself was not a significant factor, as demonstrated previously (26,27). The prevalence of concomitant low- and high-risk cervical and anal HPV was significantly associated in all investigated patient groups. It is still unknown if there is a reservoir for HPV in the genito-anal area. Besides sexual transmission, the transfer of HPV between the different mucosal sites may occur as a result of autoinoculation or smear infection (28–30). Irrespective of their individual immune status, the majority of the analysed high-risk patients in the present study showed an comprehensive anogenital HPV colonisation at the time of specimen collection. It was postulated that some type of global immune dysregulation results in the persistence of HPV in the cervix and anus (31). Immunosuppression caused by HIV infection is associated with HPV-related malignancies and contributes to HIV pathogenesis (31). Regarding the HIV-infected patients in the present study, the current CD4 count, as well as the current HIV viral load, did not have a significant influence on anal HPV infection. The impact of CD4 count and HIV viral load was previously discussed controversially. The low number of HIV-infected women analysed in the present study could be an explanation for this discrepancy. However consistent with the findings of Hessol et al (17), in which women with lower CD4 cell counts were more likely to have detectable oncogenic and non-oncogenic HPV types, a low CD4 nadir was significantly associated with anal HPV detection in the present study. In a study by Cambou et al (32), a significant correlation between a low CD4 nadir and previous CD4 counts was detected, indicating an increased risk for anal dysplasia in HIV-infected women with a history of severe immune devastation. The increased prevalence of HPV infection in HIV-infected individuals seems to be associated with immunosuppression (33). A higher level of HPV replication in women with a compromised immune system due to HIV infection could be the reason for that fact (16). The exact mechanisms of HIV-HPV interactions are still under investigation. A previous study by Palefsky (9) evaluated whether the use of cART has an influence on the prevalence of anal HPV infection by immune recovery, finding that it lead to a limited reduction in HPV prevalence and the regression of cervical intraepithelial neoplasia. Subsequently, it was demonstrated that the risk of persistent HPV infection in HIV-infected women under long-term cART was reduced due to sustained viral suppression and increased CD4 counts (34,35). The relatively short duration of cART use in the present study did not have a significant effect on anal HPV reduction. Due to the increased lifespan of HIV-infected patients, a consistent ART seems to be important to reduce the incidence of acquired immunodeficiency syndrome-defining HPV-associated cancers, such as cervical or anal cancer.
The limitation of this study was the cross-sectional setting. It could not be determined how many of the HPV infections were transient. A type-specific clearance of high- and low-risk HPV genotypes in the majority of women over a 5-year mean follow-up detection period was reported (36). The exception was HPV type 16, and concomitant cervical infection with this genotype, which is found in the majority of cervical high-grade dysplasias, was associated with anal HPV persistence (36). Further studies are required to clear these facts.
In conclusion, the present study demonstrated that the prevalence of anal HPV infection in high-risk populations is high. Non-HIV-infected women with cervical dysplasia and HIV-infected women, irrespective of their individual cervical findings, had a high risk of concomitant anal HPV infection. Due to the increase in lifespan of HIV-infected women receiving cART, these patients should be seen as a lifetime risk population for HPV-associated anal cancer. Based on the predominant HPV genotypes found, the HPV vaccination could reduce the incidence of anal cancer. Concomitant intense screening for cervical and anal dysplasias in high-risk populations should be offered as a matter of routine to avoid invasive stages.
References
- 1.Nyitray A, Nielson CM, Harris RB, Flores R, Abrahamsen M, Dunne EF, Giuliano AR. Prevalence o fand risk factors for anal human papillomavirus infection in heterosexual men. J Infect Dis. 2008;197:1676–1684. doi: 10.1086/588145. [DOI] [PubMed] [Google Scholar]
- 2.Melbye M, Sprøgel P. Aetiological parallel between anal cancer and cervical cancer. Lancet. 1991;338:657–659. doi: 10.1016/0140-6736(91)91233-K. [DOI] [PubMed] [Google Scholar]
- 3.Watson AJ, Smith BB, Whitehead MR, Sykes PH, Frizelle FA. Malignant progression of anal intra-epithelial neoplasia. ANZ J Surg. 2006;76:715–717. doi: 10.1111/j.1445-2197.2006.03837.x. [DOI] [PubMed] [Google Scholar]
- 4.Gervaz P, Hirschel B, Morel P. Molecular biology of squamous cell carcinoma oft he anus. Br J Surg. 2006;93:531–538. doi: 10.1002/bjs.5376. [DOI] [PubMed] [Google Scholar]
- 5.Arbyn M, de Sanjosé S, Saraiya M, Sideri M, Palefsky J, Lacey C, Gillison M, Bruni L, Ronco G, Wentzensen N, et al. EUROGIN 2011 roadmap on prevention and treatment of HPV-related diesease. Int J Cancer. 2012;131:1969–1982. doi: 10.1002/ijc.27650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Cancer Society, corp-author. Cancer facts and figures. American Cancer Society; Atlanta, GA: 2012. [Google Scholar]
- 7.Guler T, Uygur D, Uncu M, Yayci E, Atacag T, Bas K, Gunay M, Yakicier C. Coexisting anal human papilloma virus infection in heterosexual women with cervical HPV infection. Arch Gynecol Obstet. 2013;288:667–672. doi: 10.1007/s00404-013-2821-0. [DOI] [PubMed] [Google Scholar]
- 8.Frisch M, Biggar RJ, Goedert JJ. Human papillomavirus-associated cancers in patients with human immunodeficiency virus infection and aquired immunodeficiency syndome. J Natl Cancer Inst. 2000;92:1500–1510. doi: 10.1093/jnci/92.18.1500. [DOI] [PubMed] [Google Scholar]
- 9.Palefsky JM. Cervical human papillomavirus infection and cervical intraepithelial neoplasia in women positive for human immunodeficiency virus in the era of highly active antiretroviral therapy. Curr Opin Oncol. 2003;15:382–388. doi: 10.1097/00001622-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Duncan KC, Chan KJ, Chiu CG, Montaner JS, Coldman AJ, Cescon A, Au-Yeung CG, Wiseman SM, Hogg RS, Press NM. HAART slows progression to anal cancer in HIV-infected MSM. AIDS. 2015;29:305–311. doi: 10.1097/QAD.0000000000000537. [DOI] [PubMed] [Google Scholar]
- 11.van der Snoek EM, van der Ende ME, den Hollander JC, Schutten M, Neumann HA, van Doornum GJ. Use of highly active antiretroviral therapy is associated with lower prevalence of anal intraepithelial neoplastic lesions and lower prevalence of human papillomavirus in HIV-infected men who have sex with men. Sex Transm Dis. 2012;39:495–500. doi: 10.1097/OLQ.0b013e31825aa764. [DOI] [PubMed] [Google Scholar]
- 12.ANCHOR Study, corp-author. https://anchorstudy.org. The Anal Cancer/HSIL Outcomes Research Study United States of America. 2016 [Google Scholar]
- 13.Gosens KC, Richel O, Prins JM. Human papillomavirus as a cause of anal cancer and the role of screening. Curr Opin Infect Dis. 2017;30:87–92. doi: 10.1097/QCO.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 14.https://leitlinien.net. Langfassung der Leitlinie: HPV-Infektion / präinvasive Läsionen des weiblichen Genitale: Prävention, Diagnostik und Therapie. 2008 [Google Scholar]
- 15.Wright TC, Jr, Cox JT, Massad LS, Twiggs LB, Wilkinson EJ. ASCCP-Sponsored Consensus Conference: 2001 Consensus Guidelines for the management of women with cervical cytological abnormalities. J Am Med Assoc. 2002;287:2120–2129. doi: 10.1001/jama.287.16.2120. [DOI] [PubMed] [Google Scholar]
- 16.Stier EA, Sebring MC, Mendez AE, Ba FS, Trimble DD, Chiao EY. Prevalence of anal human papillomavirus infection and anal HPV-related disorders in women: A systematic review. Am J Obstet Gynecol. 2015;213:278–309. doi: 10.1016/j.ajog.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hessol NA, Holly EA, Efird JT, Minkoff H, Weber KM, Darragh TM, Burk RD, Strickler HD, Greenblatt RM, Palefsky JM. Concomitant anal and cervical human papillomavirus infections and intraepithelial neoplasia in HIV-infected and uninfected women. AIDS. 2013;27:1743–1751. doi: 10.1097/QAD.0b013e3283601b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawford R, Grignon AL, Kitson S, Winder DM, Ball SL, Vaughan K, Stanley MA, Sterling JC, Goon PK. High prevalence of HPV in non-cervical sites of women with abnormal cervical cytology. BMC Cancer. 2011;11:473. doi: 10.1186/1471-2407-11-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Islami F, Ferlay J, Lortet-Tieulent J, Bray F, Jemal A. International trends in anal cancer incidence rates. Int J Epidemiol. 2016 Oct 27; doi: 10.1093/ije/dyw276. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 20.Mesher D, Panwar K, Thomas SL, Beddows S, Soldan K. Continuing reductions in HPV 16/18 in a population with high coverage of bivalent HPV vaccination in England: An ongoing cross-sectional study. BMJ Open. 2016;6:e009915. doi: 10.1136/bmjopen-2015-009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, Bosch FX, de Sanjosé S, Castellsagué X. Global estimates of human papillomavirus vaccination coverage by region and income level: A pooled analysis. Lancet Glob Health. 2016;4:e453–e463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 22.Stier EA, Chigurupati NL, Fung L. Prophylactic HPV vaccination and anal cancer. Hum Vaccin Immunother. 2016;12:1348–1351. doi: 10.1080/21645515.2016.1149274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herbert A. Primary HPV testing: A proposal for co-testing in initial rounds of screening to optimise sensitivity of cervical cancer screening. Cytopathology. 2016 Mar 23; doi: 10.1111/cyt.12334. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 24.Park IU, Ogilvie JW, Jr, Anderson KE, Li ZZ, Darrah L, Madoff R, L Jr Downs. Anal human papillomavirus infection and abnormal anal cytology in women with genital neoplasia. Gynecol Oncol. 2009;114:399–403. doi: 10.1016/j.ygyno.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Denny LA, Franceschi S, de Sanjosé S, Heard I, Moscicki AB, Palefsky J. Human papillomavirus, human immunodeficiency virus and immunosuppression. Vaccine. 2012;30(Suppl 5):F168–F174. doi: 10.1016/j.vaccine.2012.06.045. [DOI] [PubMed] [Google Scholar]
- 26.Beachler DC, D'Souza G, Sugar EA, Xiao W, Gillison ML. Natural history of anal vs oral HPV infection in HIV-infected men and women. J Infect Dis. 2013;208:330–339. doi: 10.1093/infdis/jit170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman MT, Shvetsov YB, McDuffie K, Wilkens LR, Zhu X, Thompson PJ, Ning L, Killeen J, Kamemoto L, Hernandez BY. Sequential acquisition of human papillomavirus (HPV) infection oft he anus and the cervix: The Hawaii HPV Cohort Study. J Infect Dis. 2010;201:1331–1339. doi: 10.1086/651620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sehnal B, Dusek L, Cibula D, Zima T, Halaska M, Driak D, Slama J. The relationship between the cervical and anal HPV infection in women with cervical intraepithelial neoplasia. J Clin Virol. 2014;59:18–23. doi: 10.1016/j.jcv.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Winer RL, Hughes JP, Feng Q, Xi LF, Cherne S, O'Reilly S, Kiviat NB, Koutsky LA. Detection of genital HPV types in fingertip samples from newly sexually active female univerity students. Cancer Epidemiol Biomarkers Prev. 2010;19:1682–1685. doi: 10.1158/1055-9965.EPI-10-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widdice LE, Breland DJ, Jonte J, Farhat S, Ma Y, Leonard AC, Moscicki AB. Human papillomavirus concordance in heterosexual couples. J Adolesc Health. 2010;47:151–159. doi: 10.1016/j.jadohealth.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brickman C, Palewsky JM. Human papillomavirus in the HIV-infected host: Epidemiology and pathogenesis in the antiretroviral era. Curr HIV/AIDS Rep. 2015;12:6–15. doi: 10.1007/s11904-014-0254-4. [DOI] [PubMed] [Google Scholar]
- 32.Cambou MC, Luz PM, Lake JE, Levi JE, Coutinho JR, de Andrade A, Heinke T, Derrico M, Veloso VG, Friedman RK, Grinsztejn B. Anal human papillomavirus (HPV) prevalences and factors associated with abnormal anal cytology in HIV-infected women in an urban cohort from Rio de Janeiro, Brazil. AIDS Patient Care STDS. 2015;29:4–12. doi: 10.1089/apc.2014.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palefsky J. Human papillomavirus-related disease in people with HIV. Curr Opin HIV AIDS. 2009;4:52–56. doi: 10.1097/COH.0b013e32831a7246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Konopnicki D, Manigart Y, Gilles C, Barlow P, de Marchin J, Feoli F, Larsimont D, Delforge M, De Wit S, Clumeck N. Sustained viral suppression and higher CD4+ T-cell count reduces the risk of persistent cervical high-risk human papillomavirus infection in HIV-positive women. J Infect Dis. 2013;207:1723–1729. doi: 10.1093/infdis/jit090. [DOI] [PubMed] [Google Scholar]
- 35.Zeier MD, Botha MH, Engelbrecht S, Machekano RN, Jacobs GB, Isaacs S, van Schalkwyk M, van der Merwe H, Mason D, Nachega JB. Combination antiretroviral therapy reduces the detection risk of cervical human papillomavirus infection in women living with HIV. AIDS. 2015;29:59–66. doi: 10.1097/QAD.0000000000000512. [DOI] [PubMed] [Google Scholar]
- 36.Moscicki AB, Ma Y, Farhat S, Jay J, Hanson E, Benningfield S, Jonte J, Godwin-Medina C, Wilson R, Shiboski S. Natural history of anal human papillomavirus infection in heterosexual women and risks associated with persistence. Clin Infect Dis. 2014;58:804–811. doi: 10.1093/cid/cit947. [DOI] [PMC free article] [PubMed] [Google Scholar]