Abstract
Introduction
Pulmonary emphysema is a common comorbidity in lung cancer, but its role in tumor prognosis remains obscure. Our aim was to evaluate the impact of the regional emphysema score (RES) on patient’s overall survival, quality of life (QOL), and pulmonary function recovery in stage I–II lung cancer.
Methods
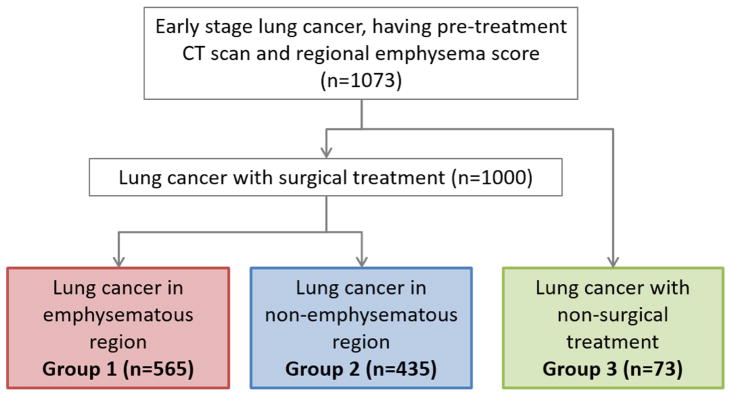
Between 1997 and 2009, 1,073 patients were identified and divided into two surgical groups (cancer in emphysematous [group 1, n=565] and non-emphysematous [group 2, n=435] region) and one non-surgical group (group 3, n=73). RES was derived from the emphysematous region and categorized into mild (≤5%), moderate (6–24%) and severe (25–60%).
Results
In group 1, patients with moderate and severe RES experienced slight decreases in postoperative FEV1, but increases in FEV1/FVC, compared to those with mild RES (p<0.01); however, this correlation was not observed in group 2. Post-treatment QOL was lower in patients with greater RES in all groups mainly due to dyspnea (p<0.05). Cox-regression analysis revealed that patients with higher RES had a significantly poorer survival in both surgical groups, with adjusted HRs of 1.41 and 1.43 for moderate RES and 1.63 and 2.04 for severe RES, respectively; however, this association was insignificant in the non-surgical group (adjusted HR of 0.99 for moderate/severe RES).
Conclusions
In surgically-treated patients with cancer in emphysematous region, RES is associated with postoperative changes in lung function. RES is also predictive of post-treatment QOL related to dyspnea in early-stage lung cancer. In both surgical groups, RES is an independent predictor of survival.
Keywords: Lung cancer, Emphysema, Survival, Quality of life, Pulmonary function
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) and lung cancer are leading causes of death worldwide.1 Emphysema is the major component of COPD and has been demonstrated to confer a higher risk of lung cancer, independent of tobacco smoking and airflow obstruction.2, 3 It is reported that more than half of patients with newly diagnosed lung cancer have emphysema,4, 5 but the prognostic role of emphysema in lung cancer remains unclear and conflicting.6–9 Our previous experience indicates that the local risk of lung cancer was related to regional emphysema severity;10 however, the prognostic significance of regional emphysema has not been well characterized. This paper investigates the impact of RES on patients with early-stage non-small cell lung cancer (NSCLC) in terms of overall survival (OS), health-related quality of life (QOL), and postoperative pulmonary function recovery.
METHODS
Subject recruitment
The study protocol was approved by the Mayo Clinic Institutional Review Board. Detailed procedures of patient enrollment, data collection, and follow-up have been described in previous publications.11, 12 In order to ensure patients with at least five-year follow-up appointments, the cohort diagnosed between 1997 and 2009 was considered; a total of 1,073 patients met our study inclusion criteria: pathologically-confirmed early-stage NSCLC (stage I–II), available standard-dose CT before treatment, and the provision of written informed consent.
Patient evaluation
The details of patient preoperative evaluations have been reported in previous work.13 The diagnosis of COPD was determined by patient’s medical records and/or documented irreversible airflow limitation (post-bronchodilator forced expiratory volume in 1 second [FEV1]/forced vital capacity [FVC] less than 70%14). Perioperative mortality was defined as death during hospitalization or within 30 days of operation.13 Postoperative complications included conditions such as atrial fibrillation, pneumonia, prolonged air leak (more than 7 days), and tracheostomy that occurred during hospitalization or within 30 days following surgery. A composite variable “any postoperative complications” was generated consisting of any complications recorded (including but not limited to the above listed) for each individual patient. Pulmonary function tests (PFT) were performed within half a year prior to lung cancer treatment and repeated within two years after pulmonary resection. The values of FEV1/FVC, FEV1%, residual volume (RV), total lung capacity (TLC), and diffusion capacity of lung for carbon monoxide (DLCO) were evaluated and expressed as the changes from preoperative to postoperative evaluation. QOL was assessed using the Lung Cancer Symptom Scales15 within two years after treatment and each item was scored on a scale of 0-worst to 10-best.
Emphysema evaluation
Emphysema evaluation was based on standard-dose CT before treatment. Patients with low-dose CT only were excluded. The methods of radiological diagnosis and quantification of emphysema have been reported.10, 16–18 In brief, the severity of emphysema was scored through direct interpretation by an experienced thoracic radiologist (SJS) under computer-aided quantitative standard images that were generated by using −950HU as the threshold for emphysema. The extent of emphysema was given to each of six lung zones: right and left lung, upper, middle (or lingula), and bilateral lower lobes. Individual RES were derived from the emphysematous region and classified as follows: mild (≤5%), moderate (6–24%) and severe (25–60%). Patients were then divided into three groups according to the tumor location and treatment modality (Figure 1): lung cancer in emphysematous (group 1, n=565) and non-emphysematous (group 2, n=435) region with surgical resection and lung cancer with non-surgical treatment (group 3, n=73). The frequency of mild, moderate and severe RES in each group is as follows. In group 1 (surgically treated cancer in emphysematous region) 63% had mild RES, 26.6% had moderate RES and a minority (10.4%) had severe RES. In group 2 (surgically treated cancer in non-emphysematous region), the frequency of RES was in a similar distribution (71.7% mild, 19.8% moderate and 8.5% severe). In group 3 (non-surgically treated cancer), the pattern was also similar (50.7% mild, 37% moderate and 12.3% severe) but the small numbers (total of 73 patients) meant that severe and moderate RES were combined for subsequent analysis.
Figure 1.
Study population
Statistical analysis
Clinical data was compared using the chi-square (x2) test, or the Fisher exact text (as appropriate) for categorical variables, and the unpaired t-test for continuous variables. Survival curves were generated by the Kaplan-Meier method and differences were assessed by the log-rank test. Cox proportional hazards models were used to evaluate the association between RES and OS after adjusting for patient’s demographics, tumor stage and treatment, and COPD status. The difference of QOL between groups was assessed using the x2 test and a clinically important difference was defined as a greater than 1 point. A p-value less than 0.05 was considered statistically significant. All statistical analyses were performed by SAS, v9.3 (SAS Institute).
RESULTS
Baseline clinical features
Patients with moderate and severe RES were noted more frequently in former or current smokers and had higher pack-years than those with mild RES in all groups (p<0.05). Coexistence of COPD was significantly more common in patients with higher RES (p<0.01). Of the 1,000 patients receiving surgical resection (group 1 and group 2), lobectomy was performed in 782 (78.2%) patients, segmentectomy in 68 (6.8%), and wedge resection in 150 (15.0%). No significant difference was found in the distribution of the type of surgical procedure between the two groups (p=0.73). In group 3, the patients with non-surgical treatment, 34 (46.6%) received radiation, 27 (37.0%) underwent chemotherapy/chemoradiotherapy, and the remaining 12 (16.4%) had other supportive treatment. More detailed association between RES, tumor location and clinical characteristics for each group can be found in Table 1.
Table 1.
Patient demographics and clinical characteristics by tumor location and regional emphysema score
| Regional emphysema score | group 1
|
group 2
|
group 3
|
|||||
|---|---|---|---|---|---|---|---|---|
| mild | moderate | severe | mild | moderate | severe | mild | moderate/severe | |
| Number of patients | 356 (63.0%) | 150 (26.6%) | 59 (10.4%) | 312 (71.7%) | 86 (19.8%) | 37 (8.5%) | 37 (50.7%) | 36 (49.3%) |
| Age (years) | 67.6±9.6 | 68.6±7.7 | 68.2±8.0 | 66.8±10.1 | 69.4±7.2 | 71.5±7.1** | 72.1±8.2 | 71.3±7.9 |
| Gender | ||||||||
| Male | 176 (49.4%) | 98 (65.3%) | 35 (59.3%)* | 147 (47.1%) | 56 (65.1%) | 19 (51.4%)* | 19 (51.4%) | 17 (47.2%) |
| Female | 180 (50.6%) | 52 (34.7%) | 24 (40.7%) | 165 (52.9%) | 30 (34.9%) | 18 (48.6%) | 18 (48.6%) | 19 (52.8%) |
| Body mass index | 27.8±5.0 | 26.5±4.6 | 25.0±5.0** | 28.4±5.7 | 26.4±4.7 | 25.5±4.4** | 28.6±5.6 | 26.6±5.5 |
| Smoking status | ||||||||
| Never | 63 (17.7%) | 1 (0.7%) | 0 (0.0%)** | 77 (24.7%) | 2 (2.3%) | 0 (0.0%)** | 5 (13.5%) | 0 (0.0%)* |
| Former | 207 (58.1%) | 80 (53.3%) | 35 (59.3%) | 177 (56.7%) | 57 (66.3%) | 27 (73.0%) | 26 (70.3%) | 25 (69.4%) |
| Current/ever | 86 (24.2%) | 69 (46.0%) | 24 (40.7%) | 58 (18.6%) | 27 (31.4%) | 10 (27.0%) | 6 (16.2%) | 11 (30.6%) |
| Pack-years | 46.0±31.1 | 59.5±32.2 | 66.6±33.0** | 45.0±31.4 | 58.1±27.4 | 52.8±27.6** | 50.6±39.8 | 65.4±33.4* |
| Histology | ||||||||
| adenocarcinoma | 240 (67.4%) | 84 (56.0%) | 22 (37.3%)** | 181 (58.0%) | 42 (48.8%) | 18 (48.6%)** | 16 (43.2%) | 11 (30.6%) |
| squamous | 79 (22.2%) | 55 (36.7%) | 28 (47.5%) | 59 (18.9%) | 36 (41.9%) | 13 (35.1%) | 13 (35.1%) | 15 (41.7%) |
| other NSCLC | 37 (10.4%) | 11 (7.3%) | 9 (15.3%) | 72 (23.1%) | 8 (9.3%) | 6 (16.2%) | 8 (21.6%) | 10 (27.8%) |
| Grade | ||||||||
| well | 138 (38.8%) | 35 (23.3%) | 8 (13.6%)** | 112 (35.9%) | 21 (24.4%) | 6 (16.2%)** | 7 (18.9%) | 4 (11.1%) |
| moderate | 152 (42.7%) | 86 (57.3%) | 31 (52.5%) | 113 (36.2%) | 53 (61.6%) | 23 (62.2%) | 14 (37.8%) | 15 (41.7%) |
| poorly | 66 (18.5%) | 29 (19.3%) | 20 (33.9%) | 87 (27.9%) | 12 (14.0%) | 8 (21.6%) | 16 (43.2%) | 17 (47.2%) |
| Stage | ||||||||
| Ia | 208 (58.4%) | 70 (46.7%) | 25 (42.4%)** | 160 (51.3%) | 39 (45.3%) | 24 (64.9%) | 11 (29.7%) | 13 (36.1%) |
| Ib | 88 (24.7%) | 44 (29.3%) | 14 (23.7%) | 73 (23.4%) | 21 (24.4%) | 9 (24.3%) | 6 (16.2%) | 8 (22.2%) |
| IIa | 26 (7.3%) | 19 (12.7%) | 6 (10.2%) | 46 (14.7%) | 13 (15.1%) | 3 (8.1%) | 4 (10.8%) | 3 (8.3%) |
| IIb | 34 (9.6%) | 17 (11.3%) | 14 (23.7%) | 33 (10.6%) | 13 (15.1%) | 1 (2.7%) | 16 (43.2%) | 12 (33.3%) |
| Comorbid COPD | ||||||||
| no | 206 (57.9%) | 33 (22.0%) | 5 (8.5%)** | 181 (58.0%) | 16 (18.6%) | 4 (10.8%)** | 13 (35.1%) | 2 (5.6%)** |
| yes | 150 (42.1%) | 117 (78.0%) | 54 (91.5%) | 131 (42.0%) | 70 (81.4%) | 33 (89.2%) | 24 (64.9%) | 34 (94.4%) |
Comparisons between different emphysema scores in three comparative groups:
p<0.05,
p<0.01
NSCLC, non-small cell lung cancer; COPD, chronic obstructive pulmonary disease.
In surgically-treated patients (group 1 and group 2), the preoperative PFT results are summarized in Supplementary Table S1. As expected, with higher RES, there was evidence of greater airflow obstruction, lower diffusion capacity and more marked hyperinflation. Postoperative complications increased in both surgical groups as the RES increased (Table 2). Postoperative pneumonia occurred more commonly in patients with severe RES than mild-moderate RES in both groups (both p<0.05). In group 1 (cancer in emphysematous region), the incidence of prolonged air leak was twice as high in moderate RES and three times as high in severe RES compared to that in patients with mild RES (p<0.01). Three patients died during the postoperative course, including 2 from pneumonia and 1 acute renal failure; however, there was no significant association between the RES and perioperative mortality in either group.
Table 2.
Postoperative complications in surgically treated patients (group 1 and group 2)
| Regional emphysema score | group 1
|
group 2
|
||||
|---|---|---|---|---|---|---|
| mild | moderate | severe | mild | moderate | severe | |
| atrial fibrillation | 35 (9.8%) | 20 (13.3%) | 5 (8.5%) | 25 (8.0%) | 11 (12.8%) | 6 (16.2%) |
| pneumonia | 6 (1.7%) | 7 (4.7%) | 5 (8.5%)* | 4 (1.3%) | 2 (2.3%) | 3 (8.1%)* |
| prolonged air leak (>7d) | 21 (5.9%) | 16 (10.7%) | 11 (18.6%)** | 16 (5.1%) | 8 (9.3%) | 4 (10.8%) |
| tracheostomy | 2 (0.6%) | 1 (0.7%) | 2 (3.4%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) |
| any postoperative complications | 88 (24.7%) | 62 (41.3%) | 29 (49.2%)** | 74 (23.7%) | 27 (31.4%) | 15 (40.5%)* |
| perioperative mortality | 0 (0.0%) | 1 (0.7%) | 1 (1.7%) | 1 (0.3%) | 0 (0.0%) | 0 (0.0%) |
Comparisons between different emphysema scores in group 1 and group 2:
p<0.05;
p<0.01
Operative mortality and complications were defined as the event (death or complications) that occurred during hospitalization or within 30 days of the operation. “Any postoperative complications” consisted of any complications recorded for each individual patient.
Changes in pulmonary function
After surgery, 152 (26.9%) patients in group 1 and 122 (28.0%) in group 2, had follow-up PFT, at a mean of 13.4±5.9 months after surgery (range, 3.2 to 23.8 months). In general, some decline in lung function was noticed in both groups. In group 1 (cancer in emphysematous region) there was a significantly greater decline in lung function (as measured by FEV1% and DLCO%) in patients with mild RES compared to those with moderate or severe RES; however, a significant improvement in FEV1/FVC was observed in patients with moderate and severe RES. These associations were not evident in group 2 (cancer in non-emphysematous region) (Supplementary Figure S1).
Post-treatment quality of life
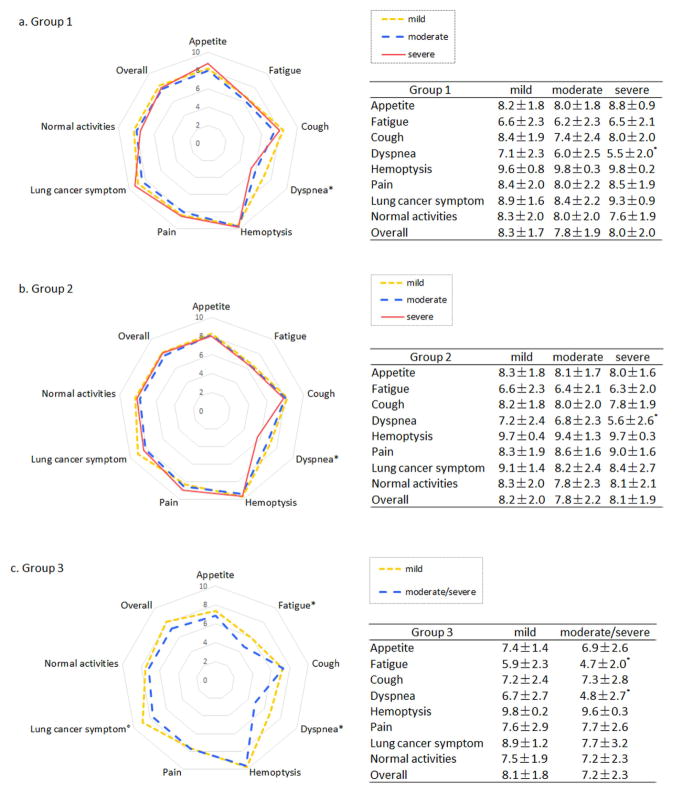
Within two years after treatment, 394 (69.7%) patients in group 1, 322 (74.0%) in group 2, and 54 (74.0%) in group 3 answered the QOL questionnaire. No striking difference in overall QOL was observed between different RES in each group (Figure 2). Among specific symptom subscales, dyspnea scores were worse in patients with severe RES in all groups (all p<0.05 and difference >1 point). In group 3 (cancer with non-surgical treatment), fatigue was worse in patients with moderate/severe RES than those with mild RES, which was independent of non-surgical treatment modalities (p=0.68).
Figure 2.
Quality of life within 2 years after treatment. (a) lung cancer in emphysematous region with surgical resection; (b) lung cancer in non-emphysematous region with surgical resection; (c) lung cancer with non-surgical treatment. (Outer circle representing a higher score and better quality of life; *p<0.05 and difference >1 point)
Overall survival
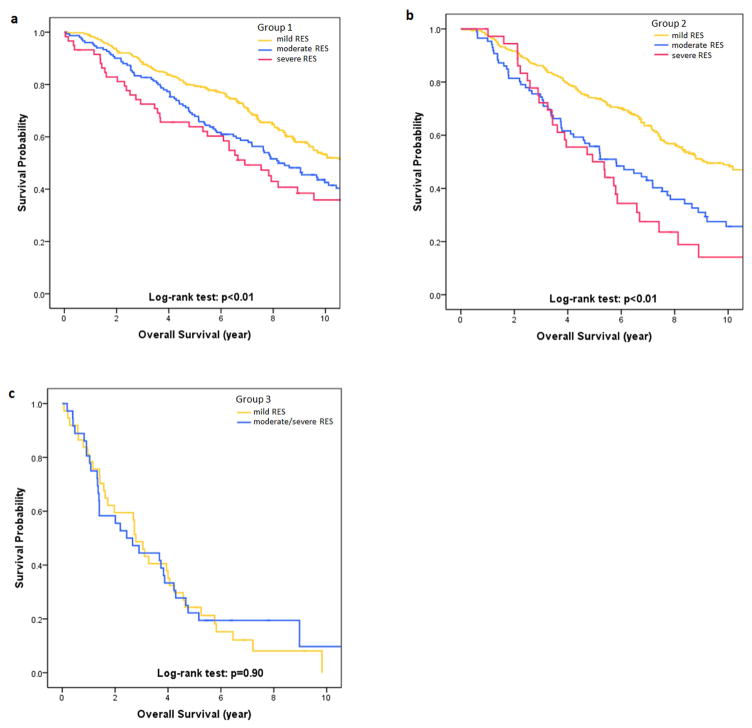
The 5-year OS rates in mild, moderate and severe RES were 79.6%, 67.8% and 63.8% in group 1, 74.1%, 55.8% and 50.0% in group 2, and 24.3% and 22.2% in patients with mild and moderate/severe RES in group 3. Kaplan-Meier survival analysis showed significant differences in OS among mild, moderate and severe RES in the surgical groups (both p<0.01), but no significant difference between mild and moderate/severe RES in the non-surgical group (p=0.90, Figure 3). In multivariate analysis, RES was significantly associated with reduced survival for moderate and severe RES compared with mild RES in group 1 (moderate: HR, 1.41[1.08, 1.84]; severe: HR, 1.63[1.11, 2.38]) and group 2 (moderate: HR, 1.43[1.04, 1.96]; severe: HR, 2.04[1.33, 3.12]). RES was not a prognostic factor in group 3 (Table 3, Supplementary Table S2).
Figure 3.
Kaplan-Meier curves for overall survival (OS) between different regional emphysema scores (RES) in three groups: (a) lung cancer in emphysematous region with surgical resection; (b) lung cancer in non-emphysematous region with surgical resection; (c) lung cancer with non-surgical treatment. There were significant differences in OS among patients with mild, moderate, and severe RES in surgically treated lung cancer, regardless of tumor location (p<0.01). This difference was insignificant between patients with mild RES and those with moderate/severe RES when lung cancer was treated non-surgically (p=0.90).
Table 3.
Univariate and multivariate analysis for overall survival in 3 comparative groups
| Regional emphysema score | No. | Median Survival (years) | univariate
|
multivariate#
|
||
|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||
| group 1 (tumor in emphysematous region with surgical resection) | <0.01 | <0.01 | ||||
| mild | 356 | 11.2 | -- | -- | ||
| moderate | 150 | 8.2 | 1.49 (1.17,1.90) | 1.41 (1.08,1.84) | ||
| severe | 59 | 6.9 | 1.73 (1.23,2.44) | 1.63 (1.11,2.38) | ||
| group 2 (tumor in non-emphysematous region with surgical resection) | <0.01 | <0.01 | ||||
| mild | 312 | 9.2 | -- | -- | ||
| moderate | 86 | 5.8 | 1.88 (1.41,2.51) | 1.43 (1.04,1.96) | ||
| severe | 37 | 5.1 | 2.61 (1.77,3.84) | 2.04 (1.33,3.12) | ||
| group 3 (tumor with non-surgical treatment) | 0.90 | 0.97 | ||||
| mild | 37 | 2.8 | -- | -- | ||
| moderate/severe | 36 | 2.5 | 0.97 (0.59,1.59) | 0.99 (0.55,1.78) | ||
Multivariate analysis was performed with the adjustment for patient demographics (age, gender, body mass index, smoking status), tumor histology and grade, lung cancer stage and treatment, and COPD status.
HR, hazard ratio; CI, confidence interval.
DISCUSSION
Emphysema is a common coexisting disease in lung cancer patients and increases postoperative pulmonary morbidities after lung resection.9, 19 In this retrospective analysis, we found that the RES was an independent predictor of OS in early-stage NSCLC after surgery regardless of tumor location, and was also associated with post-treatment dyspnea in severe RES versus mild-moderate RES. Lung function declined postoperatively in both surgical groups, with a significant increase in FEV1/FVC noted in moderate-severe RES in group 1 (cancer in emphysematous region).
To date, much attention has been focused on the difference in prognosis between lung cancer arising in emphysematous and non-emphysematous lungs.7 Several studies6, 8, 20 reported an association between the presence of emphysema and lung cancer mortality but other studies9, 21, 22 did not support such association after controlling for patient age, gender, smoking history, and cancer stage. In these previous studies, quantification of emphysema was based on whole lung evaluation, without accounting for regional distribution of emphysema. However, recent studies4, 10 suggest that primary lung cancers arise more frequently in regional areas of worse emphysematous change. We therefore hypothesized that RES may have prognostic value in lung cancer.
The multivariate analysis demonstrated that higher RES was significantly associated with worse OS in surgically-treated lung cancer, regardless of the tumor location and independent of smoking and COPD status. This finding was in line with previous studies where emphysema was quantified according to whole lung evaluation.4, 6, 8 Zulueta and colleagues revealed that patients with mild, moderate, and marked emphysema had a corresponding 1.4, 1.8 and 3.2 HR of death from lung cancer as compared with those free of emphysema.8 Oelsner and colleagues demonstrated the association between quantitatively assessed emphysema and lung cancer mortality where patients with worse emphysema had poorer outcomes.6 Our results indicated that RES had a similar impact on OS as generalized emphysema, and that a systemic effect of RES might underlie the prognostic association. Possible systemic mechanisms for the effect of regional emphysematous change on lung cancer survival in this study cohort could include the tumor-promoting effect of neutrophilic inflammation,23–25 enhanced angiogenesis secondary to chronic inflammation,26 and up-regulation of matrix metalloproteinase (MMP) in emphysematous lungs.7, 27 Up-regulation of MMP has been associated with lymphovascular tumor invasion and postoperative recurrence27 and could potentially contribute to poorer outcomes with more severe RES.
Health-related QOL has been increasingly emphasized in lung cancer.15 Previous studies found patient comorbidities, such as COPD, did not affect QOL for lung cancer patients;28, 29 however, the influence of emphysema is still uncertain. Our results showed that the overall QOL did not differ significantly between RES while dyspnea scores were worse in patients with severe RES in all groups. Dyspnea has been reported to constitute a major component of symptom burden in patients with lung cancer.30 Balduyck and colleagues found a significant correlation between comorbidity index and postoperative dyspnea.31 Fatigue is a common symptom of inoperable lung cancer.32 Our study found that fatigue was associated with RES in non-surgical group; however, these results are limited by the small number of patients.
Lung function is the main limiting factor when planning surgery for early-stage lung cancer. It has been recognized that lobectomy may lead to an improvement in postoperative ventilation capacity in patients with moderate to severe pulmonary emphysema.33, 34 Our findings highlighted the predictive value of RES in postoperative pulmonary function recovery. When tumor resection was performed in emphysematous region (group 1), patients with higher RES had less marked declines in FEV1% but greater reductions in RV, contributing to “volume reduction effect” on FEV1/FVC (and subsequent increase in FEV1/FVC ratio). This finding concurred with those of Ueda and colleagues,35 who reported an association between emphysematous lung tissue in resected lung and volume reduction effect on postoperative FEV1/FVC. In contrast, when lung cancer occurred away from emphysematous region (group 2), no obvious volume reduction effect was noted after surgical resection.
RES was significantly associated with postoperative pneumonia in both surgical groups. The incidence of prolonged air leak occurred more commonly in those patients with severe RES, and a statistically significant difference was noted in group 1 (cancer in emphysematous region) for severe RES compared with mild and moderate RES. Possible mechanisms include poorer postoperative healing due to more fragile, emphysematous tissue after.36
The current study has several limitations. First, although our patient cohort was prospectively followed in the past decade, this study was retrospective and observational in nature; thus, the potential bias could not be completely eliminated even with rigorous statistical analysis. Second, follow-up by lung function was not conducted routinely in postoperative course; rather, the PFT data were passively collected upon availability in medical records; only one-fourth of patients had follow-up lung function. Third, preoperative QOL was unavailable, which limits the ability of the study to assess the effects of therapy (and RES) on post-treatment change. Lastly, the relatively small number of early-stage patients who received non-surgical treatment in the present study limits interpretation of results for this group. Therefore, a larger-scale study is needed to confirm our results.
In conclusion, we explored the prognostic significance of regional emphysema severity in a retrospective cohort and found that RES was an independent predictor of OS in early-stage NSCLC after surgery regardless of tumor location, and was associated with post-treatment QOL related to dyspnea and postoperative pulmonary function recovery.
Supplementary Material
Supplementary Figure 1. Postoperative pulmonary function changes within 2 years. Values were expressed as the changes from preoperative evaluation to postoperative evaluation. Group 1, tumor in emphysematous region; Group 2, tumor in non-emphysematous region. (FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; TLC: total lung capacity; RV: residual volume; DLCO: diffusion capacity of lung for carbon monoxide)
Acknowledgments
Author contributions: Dr. Ping Yang had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Jie Dai and Dr. Ming Liu contributed substantially to the study design and the writing of the manuscript. Shawn M. Stoddard and Jason A. Wampfler contributed substantially to the data collection and analysis. Dr. Stephen J. Swensen, Dr. Andrew H. Limper, Dr. Gening Jiang and Dr. Ping Yang contributed substantially to the revision of the manuscript.
Funding support: This work is supported by NIH (#R01-CA-84354) and Mayo Foundation.
Abbreviations
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- DLCO
diffusion capacity of lung for carbon monoxide
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PFT
pulmonary function test
- QOL
quality of life
- RES
regional emphysema score
- RV
residual volume
- TLC
total lung capacity
Footnotes
Competing interests: none declared.
Other contributions: Ms. Pamela Neville contributed to the editing and technical assistance with the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132(6):1932–8. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez-Salcedo P, Wilson DO, De-Torres JP, Weissfeld JL, Berto J, Campo A, et al. Improving selection criteria for lung cancer screening. The potential role of emphysema. Am J Respir Crit Care Med. 2015;191(8):924–31. doi: 10.1164/rccm.201410-1848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishawi M, Moore W, Bilfinger T. Severity of emphysema predicts location of lung cancer and 5-y survival of patients with stage I non-small cell lung cancer. J Surg Res. 2013;184(1):1–5. doi: 10.1016/j.jss.2013.05.081. [DOI] [PubMed] [Google Scholar]
- 5.Ytterstad E, Moe PC, Hjalmarsen A. COPD in primary lung cancer patients: prevalence and mortality. Int J Chron Obstruct Pulmon Dis. 2016;11:625–36. doi: 10.2147/COPD.S101183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oelsner EC, Carr JJ, Enright PL, Hoffman EA, Folsom AR, Kawut SM, et al. Per cent emphysema is associated with respiratory and lung cancer mortality in the general population: a cohort study. Thorax. 2016;71(7):624–32. doi: 10.1136/thoraxjnl-2015-207822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ueda K, Murakami J, Sano F, Hayashi M, Suga K, Hamano K. Similar radiopathological features, but different postoperative recurrence rates, between Stage I lung cancers arising in emphysematous lungs and those arising in nonemphysematous lungs. Eur J Cardiothorac Surg. 2015;47(5):905–11. doi: 10.1093/ejcts/ezu311. [DOI] [PubMed] [Google Scholar]
- 8.Zulueta JJ, Wisnivesky JP, Henschke CI, Yip R, Farooqi AO, McCauley DI, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141(5):1216–23. doi: 10.1378/chest.11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SA, Sun JS, Park JH, Park KJ, Lee SS, Choi H, et al. Emphysema as a risk factor for the outcome of surgical resection of lung cancer. J Korean Med Sci. 2010;25(8):1146–51. doi: 10.3346/jkms.2010.25.8.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hohberger LA, Schroeder DR, Bartholmai BJ, Yang P, Wendt CH, Bitterman PB, et al. Correlation of regional emphysema and lung cancer: a lung tissue research consortium-based study. J Thorac Oncol. 2014;9(5):639–45. doi: 10.1097/JTO.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128(1):452–62. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 12.Sun Z, Aubry MC, Deschamps C, Marks RS, Okuno SH, Williams BA, et al. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: an analysis of 5018 hospital- and 712 population-based cases. J Thorac Cardiovasc Surg. 2006;131(5):1014–20. doi: 10.1016/j.jtcvs.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 13.Cassivi SD, Allen MS, Vanderwaerdt GD, Ewoldt LL, Cordes ME, Wigle DA, et al. Patient-centered quality indicators for pulmonary resection. Ann Thorac Surg. 2008;86(3):927–32. doi: 10.1016/j.athoracsur.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 15.Yang P, Cheville AL, Wampfler JA, Garces YI, Jatoi A, Clark MM, et al. Quality of life and symptom burden among long-term lung cancer survivors. J Thorac Oncol. 2012;7(1):64–70. doi: 10.1097/JTO.0b013e3182397b3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Swensen SJ, Karabekmez LG, Marks RS, Stoddard SM, Jiang R, et al. Effect of emphysema on lung cancer risk in smokers: a computed tomography-based assessment. Cancer Prev Res (Phila) 2011;4(1):43–50. doi: 10.1158/1940-6207.CAPR-10-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swensen SJ, Jett JR, Hartman TE, Midthun DE, Mandrekar SJ, Hillman SL, et al. CT screening for lung cancer: five-year prospective experience. Radiology. 2005;235(1):259–65. doi: 10.1148/radiol.2351041662. [DOI] [PubMed] [Google Scholar]
- 18.Kishi K, Gurney JW, Schroeder DR, Scanlon PD, Swensen SJ, Jett JR. The correlation of emphysema or airway obstruction with the risk of lung cancer: a matched case-controlled study. Eur Respir J. 2002;19(6):1093–8. doi: 10.1183/09031936.02.00264202. [DOI] [PubMed] [Google Scholar]
- 19.Na KJ, Kang CH, Jeon JH, Seong YW, Park IK, Goo JM, et al. Quantification of emphysema with preoperative computed tomography has stronger association with pulmonary complications than pulmonary function test results after pulmonary lobectomy. J Thorac Cardiovasc Surg. 2014;147(3):915–20. doi: 10.1016/j.jtcvs.2013.11.029. [DOI] [PubMed] [Google Scholar]
- 20.Gullon JA, Suarez I, Medina A, Rubinos G, Fernandez R, Gonzalez I. Role of emphysema and airway obstruction in prognosis of lung cancer. Lung Cancer. 2011;71(2):182–5. doi: 10.1016/j.lungcan.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 21.Mina N, Soubani AO, Cote ML, Suwan T, Wenzlaff AS, Jhajhria S, et al. The relationship between chronic obstructive pulmonary disease and lung cancer in African American patients. Clin Lung Cancer. 2012;13(2):149–56. doi: 10.1016/j.cllc.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumagai S, Marumo S, Yamanashi K, Tokuno J, Ueda Y, Shoji T, et al. Prognostic significance of combined pulmonary fibrosis and emphysema in patients with resected non-small-cell lung cancer: a retrospective cohort study. Eur J Cardiothorac Surg. 2014;46(6):e113–9. doi: 10.1093/ejcts/ezu384. [DOI] [PubMed] [Google Scholar]
- 23.Kadota K, Nitadori J, Ujiie H, Buitrago DH, Woo KM, Sima CS, et al. Prognostic Impact of Immune Microenvironment in Lung Squamous Cell Carcinoma: Tumor-Infiltrating CD10+ Neutrophil/CD20+ Lymphocyte Ratio as an Independent Prognostic Factor. J Thorac Oncol. 2015;10(9):1301–10. doi: 10.1097/JTO.0000000000000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ilie M, Hofman V, Ortholan C, Bonnetaud C, Coelle C, Mouroux J, et al. Predictive clinical outcome of the intratumoral CD66b-positive neutrophil-to-CD8-positive T-cell ratio in patients with resectable nonsmall cell lung cancer. Cancer. 2012;118(6):1726–37. doi: 10.1002/cncr.26456. [DOI] [PubMed] [Google Scholar]
- 25.Wu QD, Wang JH, Condron C, Bouchier-Hayes D, Redmond HP. Human neutrophils facilitate tumor cell transendothelial migration. Am J Physiol Cell Physiol. 2001;280(4):C814–22. doi: 10.1152/ajpcell.2001.280.4.C814. [DOI] [PubMed] [Google Scholar]
- 26.Dvorak HF. Tumors: wounds that do not heal-redux. Cancer Immunol Res. 2015;3(1):1–11. doi: 10.1158/2326-6066.CIR-14-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami J, Ueda K, Sano F, Hayashi M, Nishimoto A, Hamano K. Pulmonary emphysema and tumor microenvironment in primary lung cancer. J Surg Res. 2016;200(2):690–7. doi: 10.1016/j.jss.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Moller A, Sartipy U. Predictors of postoperative quality of life after surgery for lung cancer. J Thorac Oncol. 2012;7(2):406–11. doi: 10.1097/JTO.0b013e3182398e82. [DOI] [PubMed] [Google Scholar]
- 29.Pompili C, Brunelli A, Refai M, Xiume F, Sabbatini A. Does chronic obstructive pulmonary disease affect postoperative quality of life in patients undergoing lobectomy for lung cancer? A case-matched study. Eur J Cardiothorac Surg. 2010;37(3):525–30. doi: 10.1016/j.ejcts.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Poghosyan H, Sheldon LK, Leveille SG, Cooley ME. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2013;81(1):11–26. doi: 10.1016/j.lungcan.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Balduyck B, Hendriks J, Lauwers P, Sardari NP, Van Schil P. Quality of life evolution after lung cancer surgery in septuagenarians: a prospective study. Eur J Cardiothorac Surg. 2009;35(6):1070–5. 1075. doi: 10.1016/j.ejcts.2009.01.050. [DOI] [PubMed] [Google Scholar]
- 32.Tishelman C, Petersson LM, Degner LF, Sprangers MA. Symptom prevalence, intensity, and distress in patients with inoperable lung cancer in relation to time of death. J Clin Oncol. 2007;25(34):5381–9. doi: 10.1200/JCO.2006.08.7874. [DOI] [PubMed] [Google Scholar]
- 33.Kushibe K, Takahama M, Tojo T, Kawaguchi T, Kimura M, Taniguchi S. Assessment of pulmonary function after lobectomy for lung cancer--upper lobectomy might have the same effect as lung volume reduction surgery. Eur J Cardiothorac Surg. 2006;29(6):886–90. doi: 10.1016/j.ejcts.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 34.Choong CK, Meyers BF, Battafarano RJ, Guthrie TJ, Davis GE, Patterson GA, et al. Lung cancer resection combined with lung volume reduction in patients with severe emphysema. J Thorac Cardiovasc Surg. 2004;127(5):1323–31. doi: 10.1016/j.jtcvs.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 35.Ueda K, Murakami J, Sano F, Hayashi M, Kobayashi T, Kunihiro Y, et al. Assessment of volume reduction effect after lung lobectomy for cancer. J Surg Res. 2015;197(1):176–82. doi: 10.1016/j.jss.2015.03.064. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan T, Atac GK, Gunal N, Kocer B, Alhan A, Cubuk S, et al. Quantative computerized tomography assessment of lung density as a predictor of postoperative pulmonary morbidity in patients with lung cancer. J Thorac Dis. 2015;7(8):1391–7. doi: 10.3978/j.issn.2072-1439.2015.07.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Postoperative pulmonary function changes within 2 years. Values were expressed as the changes from preoperative evaluation to postoperative evaluation. Group 1, tumor in emphysematous region; Group 2, tumor in non-emphysematous region. (FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; TLC: total lung capacity; RV: residual volume; DLCO: diffusion capacity of lung for carbon monoxide)