Abstract
PARP inhibitors are used in the treatment of gynecological malignancies and it has been demonstrated in preclinical studies that PARP inhibition sensitizes cancer cells to cytotoxic agents. In the present study, PARP expression was detected in different endometrial cancer cell lines by western blot analysis, and PARP activity was measured using an enzymatic assay. In addition, the endometrial cancer cell lines were treated with paclitaxel or carboplatin in combination with the PARP inhibitor PJ34 prior to a cell viability assay and apoptotic nuclei measurement. PARP protein was detected in all four cell lines examined, although its activity varied between the cell lines. Treatment with PJ34 in combination with paclitaxel decreased endometrial cancer cell viability compared with treatment with paclitaxel alone. These results indicate that the inhibition of PARP with PJ34 sensitizes endometrial cancer cells to cytotoxic treatment with paclitaxel.
Keywords: endometrial cancer, poly (ADP-ribose) polymerase inhibition, paclitaxel, apoptosis, proliferation
Introduction
Endometrial cancer is the most common gynecological malignancy in females, with a peak incidence in those between the ages of 55 and 65 years old (1). Worldwide ~142,000 women are diagnosed with endometrial cancer annually (1). Endometrial cancer is frequently diagnosed at an early stage, as women affected typically present with abnormal vaginal bleeding. At this early stage, endometrial cancer can be treated surgically with the intention to cure (2). In patients with high-risk endometrial cancer, postoperative pelvic radiotherapy, adjuvant radiation therapy and adjuvant chemotherapy have been demonstrated to improve patient outcome (3).
In total, ~13% of all patients with endometrial cancer exhibit disease recurrence (2). In the treatment of recurrent endometrial cancer, therapeutic modalities consisting of radiotherapy, surgery and systemic therapies, such as chemotherapy and hormone therapy, are in use (3). Clinical trials evaluating chemotherapeutic regimens for patients with endometrial cancer include combinations of doxorubicin and cisplatin, and cyclophosphamide/paclitaxel and carboplatin; however, the majority of these are administered in a palliative situation (3). These systemic treatment options are typically highly toxic; thus, the development of targeted treatments with fewer side effects is warranted.
A loss-of-function mutation in phosphatase and tensin homolog (PTEN), a tumor suppressor gene, is observed in ~80% of endometrioid adenocarcinoma cases (4). PTEN is known to serve a role in cell signaling and to be involved in the maintenance of genomic stability. Loss of PTEN function causes defects in the repair of DNA double-strand breaks, sensitizing cells to PARP inhibition (5). PARP inhibitors are currently used in the treatment of various gynecological malignancies, including breast and ovarian cancer. Numerous preclinical and clinical trials have identified that PARP inhibitors are toxic to cancer cells with a defect in homologous recombination DNA repair mechanisms, which are frequently caused by breast cancer (BRCA) gene mutations (6,7). The PARP inhibitor olaparib is used for the treatment of BRCA-mutated ovarian cancer in patients suffering from platinum-sensitive recurrent disease (8). Several other preclinical studies (9,10) have identified a sensitization of cancer cells to cytotoxic agents when they are combined with PARP inhibitors in vitro. Magan et al (9) demonstrated that the PARP inhibitor PJ34 enhances doxorubicin-mediated cell death in HeLa cells. In combination with PARP inhibitors, lower concentrations of cytotoxic agents could be used and therefore side effects would be decreased. Gambi et al (10) reported that PARP inhibition potentiates the cytotoxic effects of cisplatin in tumor protein p53 mutated carcinoma cell lines.
In the present study, the effect of the PARP inhibitor PJ34 in combination with carboplatin or paclitaxel was evaluated in endometrial cancer cell lines, in order to determine whether PARP inhibition sensitizes endometrial cancer cells to the effects of chemotherapeutic agents.
Materials and methods
Endometrial cancer cell lines
Endometrial cancer cells (HEC-1A, KLE, RL95-2 and AN3CA) were obtained from American Type Culture Collection (Manassas, VA, USA). HEC-1A cells were cultured in McCoy's 5A (Modified) medium (Biochrom GmbH, Berlin, Germany). KLE and RL95-2 cells were cultured in Dulbecco's modified Eagle medium: Nutrient Mixture F12, and AN3CA cells were cultured in Minimum Essential Medium with Earle's salts (all Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). All media contained 10% fetal bovine serum and 50 µg/ml gentamycin (both Invitrogen; Thermo Fisher Scientific, Inc.), and cells were cultivated at 37°C in a humidified atmosphere with 5% CO2. The cell lines chosen differed in grading and the pattern of metastatic spread. HEC-1A and RL95-2 cell lines are moderately differentiated cells lines of endometrial adenocarcinoma and originate from the epithelial layer of the endometrium (11,12). KLE and AN3CA are poorly differentiated cell lines of an endometrial adenocarcinoma, with AN3CA originating from a lymph node metastasis (11,12). Cell lines were cultured for 48 h prior to each experiment and for each cell line a different number of cells were used as follows: RL95-2, 50,000; HEC-1A, 30,000; KLE, 15,000; and AN3CA, 40,000.
Total protein isolation, SDS-PAGE and western blotting for PARP
Total cellular protein of all four mentioned untreated cell lines was isolated using RIPA lysis buffer, which included protease and phosphatase inhibitors (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) according to the manufacturer's protocol. Subsequently, the whole lysate was used for western blot analysis. Lysates were separated using SDS-PAGE on a 9% gel and the proteins transferred to a nitrocellulose membrane (Whatman GmbH, Dassel, Germany). The membranes were incubated with 5% non-fat milk for 1 h at room temperature, followed by washing with Tris-buffered saline with Tween®-20. The membranes were incubated with a primary antibody directed against PARP (rabbit anti-PARP monoclonal antibody; dilution, 1:1,000; cat. no. 9532; Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C overnight, followed by incubation with a IRDye® 680-conjugated secondary antibody (donkey anti-rabbit IgG; dilution, 1:10,000; cat. no. P/N 925-68073) for 1 h at room temperature and visualized using the Odyssey® CLx imaging system (both LI-COR Biosciences, Ltd., Lincoln, NE, USA). β-actin was used as a loading control and detected with a rabbit anti-β-actin primary antibody (dilution, 1:10,000; cat. no. ab8227; Abcam, Cambridge, UK) through incubation overnight at 4°C. The secondary antibody mentioned above was used for 1 h at room temperature. The experiments were performed under normoxic conditions (95% ambient air containing 21% O2 and 5% CO2) or under hypoxic conditions (1–5% O2).
Treatment with paclitaxel or carboplatin
Endometrial cancer cell lines (HEC-1A, KLE, RL95-2 and AN3CA) were incubated for different durations (0–120 h) with different concentrations of paclitaxel or carboplatin. Cells were cultured for 48 h until they reached 90% confluency, followed by treatment with different doses of paclitaxel or carboplatin, in order to identify the subtoxic and toxic doses of the two drugs prior to further experiments. The doses of the drugs used were as follows: Paclitaxel subtoxic, 0.001, 0.01 and 0.1 nM; paclitaxel toxic, 1, 10 and 100 nM; carboplatin subtoxic, 0.01, 0.1 and 1 µM; and carboplatin toxic, 10, 25, 50, 75 and 100 µM.
Enzymatic PARP activity assay
PARP activity in the extracts of four different endometrial cancer cell lines (HEC-1A, KLE, RL95-2 and AN3CA) was measured using the PARP Universal Colorimetric assay kit (R&D systems GmbH, Heidelberg, Germany) according to the manufacturer's protocol. Untreated cells and cells treated with PJ34 (10 µM) alone were used as controls. The extracts used in this assay were unfractioned cell lysates, which were obtained using the M-PER Mammalian Protein Extraction reagent (cat. no. 78503) containing a protease inhibitor cocktail (cat. no. 78410) (both Pierce; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol.
Cell viability assay
The number of viable endometrial cancer cells was measured relative to the untreated control cells using the CellTiter-Blue® assay (Promega Corporation, Madison, WI, USA) according to the manufacturer's instructions. Fluorescence was recorded using the FLUOstar OPTIMA system (BMG Labtech GmbH, Ortenburg, Germany). Excitation was measured at 560 nm and emission was measured at 590 nm.
Apoptotic nuclei measurement
The apoptotic nuclei were measured as described previously by Nicoletti et al (13). Briefly, apoptotic nuclei were prepared by lysing cells in a hypotonic buffer (0.1% sodium citrate and 0.1% Triton X-100) containing 50 mg/ml propidium iodide (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) and subsequently analyzed using flow cytometry. Nuclei to the left of the peak containing hypodiploid DNA were considered apoptotic. Measurements were performed on a FACSCanto™ Flow Cytometer using FACSDiva™ software (version 4.1.2; both BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Statistical analysis was performed using one-way ANOVA followed by Dunnett's and Bonferroni multiple comparison tests or unpaired Mann-Whitney U tests using GraphPad Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Treatment with paclitaxel or carboplatin
Endometrial cancer cells (HEC-1A, KLE, RL95-2 and AN3CA) were cultured for 48 h prior to paclitaxel treatment. A notable difference in cell viability was observed in cells treated with 0.1 nM (subtoxic dose) compared with 100 nM (toxic dose) paclitaxel (data not shown). For carboplatin, a notable difference in cell viability was detected in cells treated with 1 µM (subtoxic dose) compared with 100 µM (toxic dose) (data not shown).
Western blot analysis for PARP, PTEN and AKT serine/threonine kinase (Akt) proteins
Endometrial cancer cell expression of PARP, PTEN and Akt in untreated cells was analyzed using western blotting (Fig. 1). PARP and Akt protein was detected in all four endometrial cancer cell lines. However, PTEN was only detected in HEC-1A and KLE cells under hypoxic conditions. Furthermore, in normoxic conditions weak bands were observed in RL95-2 and AN3CA cell lines. Phosphorylated Akt was detected in AN3CA and RL95-2 cell lines with little or without PTEN.
Figure 1.
Western blot analysis of PARP, PTEN and Akt expression. PTEN was detected in HEC1-A and KLE cells, furthermore weak bands were observed in AN3CA and RL95-2 cell lines. PARP and Akt were detected in all cell lines, and p-Akt was detected in cell lines without or little PTEN. Lanes: A, AN3CA; H, HEC-1A; K, KLE; and R, RL95-2. PARP, poly (ADP-ribose) polymerase; PTEN, phosphatase and tensin homolog; Akt, AKT serine/threonine kinase; p, phosphorylated.
PARP activity assay
The PARP activity assay demonstrated different levels of PARP activity between the four different endometrial cancer cell lines. PARP activity was significantly decreased in KLE and RL95-2 cells following treatment with 10 µM PJ34 compared with the untreated control groups (P<0.05; Fig. 2).
Figure 2.
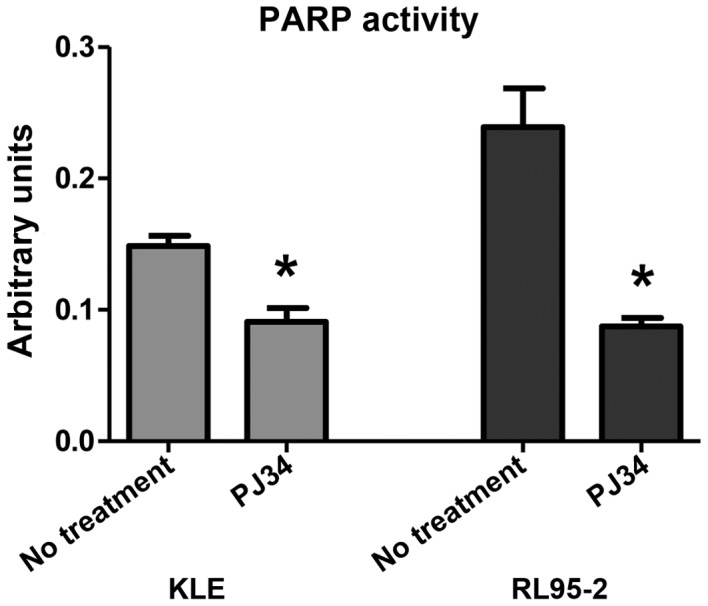
PARP activity assay. A significant decrease in PARP activity was detected following treatment with PJ34 in KLE and RL95-2 cells. *P<0.05 vs. untreated cells. PARP, poly (ADP-ribose) polymerase.
Cell viability assay
The viability of endometrial cancer cell lines following treatment with paclitaxel, PJ34 or a combination of paclitaxel and PJ34 was evaluated (Fig. 3). Combined treatment of 0.1 nM paclitaxel and 10 µM PJ34 decreased AN3CA and HEC-1A cell viability compared with cells treated with 0.1 nM paclitaxel alone (Fig. 3).
Figure 3.

Viability of endometrial cancer cells following treatment with paclitaxel and/or PJ34. The combination of 0.1 nM paclitaxel and 10 µM PJ34 decreased AN3CA and HEC-1A cell viability compared with the treatment with paclitaxel alone. PTX, paclitaxel.
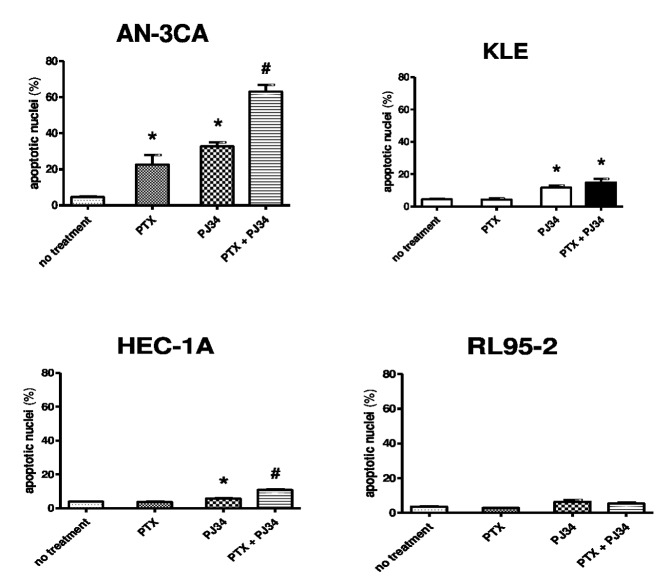
Apoptotic nuclei measurements
The number of apoptotic nuclei in the endometrial cancer cell lines increased following treatment with paclitaxel at subtoxic doses in combination with 10 µM PJ34 under normoxic conditions (Fig. 4). This increase was significant in AN3CA (P<0.05 vs. treatment with paclitaxel alone), HEC-1A (P<0.05 vs. treatment with paclitaxel alone) and KLE (P<0.05 vs. the untreated control group) cells (Fig. 4). Similar results were identified under hypoxic conditions (data not shown). However, no significant differences in the number of apoptotic nuclei were identified in cells treated with carboplatin at subtoxic doses in combination with 10 µM PJ34 compared with untreated cells (data not shown).
Figure 4.
Detection of apoptotic nuclei. There was a significant increase in apoptotic nuclei in the endometrial cancer cell lines AN3-CA and HEC-1A following treatment with a combination of paclitaxel and PJ34. *P<0.05 vs. untreated cells; #P<0.05 vs. PTX treatment alone. PTX, paclitaxel.
Discussion
The present study demonstrated that the PARP inhibitor PJ34 sensitizes endometrial cancer cells to paclitaxel-induced apoptosis. This is in agreement with the results of several previous preclinical studies that reported that PARP inhibition increased the cytotoxic effects of various chemotherapeutics (9,10).
In the current study, the combination of the subtoxic dose of paclitaxel with PJ34 decreased AN3CA and HEC-1A cell viability compared with treatment with paclitaxel alone. AN3CA is a poorly differentiated cell line and in western blot analysis little PTEN was observed. It is known that PTEN-deficient cells are sensitive to PARP inhibitors (5,14,15), a fact that could explain the findings in AN3CA cells. However, HEC-1A cells showed similar results in the cell viability assay following treatment with paclitaxel and/or PJ34, but in these cells PTEN was detected. Miyasaka et al (16) reported a similar effect of the PARP inhibitor olaparib in endometrial cancer cells. In this study the association between PTEN expression and sensitivity to olaparib of 16 endometrial cancer cell lines was evaluated; however, the hypothesis that PTEN deficiency was associated olaparib sensitivity could not be confirmed. In this study, siRNA knockdown of PTEN was also performed in cell lines with wild-type PTEN and the sensitivity to olaparib did not change (16). The authors of this study concluded that PTEN inactivation may be a biomarker of endometrial cancer and that olaparib is a promising therapeutic option (16).
In the present study, treatment of endometrial cancer cell lines with a combination of carboplatin and PJ34 did not significantly affect cell viability assay or the number of apoptotic nuclei. In these assays cell numbers were counted at a single time point, so conclusions about cell turnover can be made. In addition, the cell viability assay does not measure apoptosis, necrosis or proliferation, so a decrease in cell proliferation cannot be excluded as a cause of the cell viability results observed.
The results of the present study may aid in the development of novel therapeutic strategies for the treatment of endometrial cancer. Patients frequently present with early stage endometrial cancer and can be cured at this stage with surgical treatment or surgery and radiation. However, novel therapeutic options for recurrent or metastatic disease, in addition to rare histological types, such as undifferentiated endometrial cancer, are required. For these patients, new systemic treatment options with fewer side effects are warranted, as current treatments are highly toxic and typically produce short responses (17,18).
In conclusion, the results of the present study indicate that the combination of PARP inhibition with cytotoxic agents is a promising therapeutic option for the systemic therapy of advanced or recurrent endometrial cancer and will reduce the side effects of chemotherapy.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.van Wijk FH, van der Burg MEL, Burger CW, Vergote I, van Doorn HC. Management of surgical stage III and IV endometrioid endometrial carcinoma: An overview. Int J Gynecol Cancer. 2009;19:431–446. doi: 10.1111/IGC.0b013e3181a1a04f. [DOI] [PubMed] [Google Scholar]
- 3.Ray M, Fleming G. Management of advanced-stage and recurrent endometrial cancer. Semin Oncol. 2009;36:145–154. doi: 10.1053/j.seminoncol.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, Weng LP, Eng C. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000;92:924–930. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 5.Dedes KJ, Wetterskog D, Mendes-Pereira AM, Natrajan R, Lambros MB, Geyer FC, Vatcheva R, Savage K, Mackay A, Lord CJ, et al. PTEN deficiency in endometrioid endometrial adenocarcinomas predicts sensitivity to PARP inhibitors. Sci Transl Med. 2010;2:53ra75. doi: 10.1126/scitranslmed.3001538. [DOI] [PubMed] [Google Scholar]
- 6.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, Giavara S, O'Connor MJ, Tutt AN, Zdzienicka MZ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–8115. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 7.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 8.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott C, Meier W, Shapira-Frommer R, Safra T, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366:1382–1392. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 9.Magan N, Isaacs RJ, Stowell KM. Treatment with the PARP-inhibitor PJ34 causes enhanced doxorubicin-mediated cell death in HeLa cells. Anticancer Drugs. 2012;23:627–637. doi: 10.1097/CAD.0b013e328350900f. [DOI] [PubMed] [Google Scholar]
- 10.Gambi N, Tramontano F, Quesada P. Poly(ADPR)polymerase inhibition and apoptosis induction in cDDP-treated human carcinoma cell lines. Biochem Pharmacol. 2008;75:2356–2363. doi: 10.1016/j.bcp.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Dawe CJ, Banfield WG, Morgan WD, Slatick MS, Curth HO. Growth in continuous culture, and in hamsters, of cells from a neoplasm associated with acanthosis nigricans. J Natl Cancer Inst. 1964;33:441–456. [PubMed] [Google Scholar]
- 12.Kuramoto H. Studies of the growth and cytogenetic properties of human endometrial adenocarcinoma in culture and its development into an established line. Acta Obstet Gynaecol Jpn. 1972;19:47–58. [PubMed] [Google Scholar]
- 13.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-O. [DOI] [PubMed] [Google Scholar]
- 14.Mendes-Pereira AM, Martin SA, Brough R, McCarthy A, Taylor JR, Kim JS, Waldman T, Lord CJ, Ashworth A. Synthetic lethal targeting of PTEN mutant cells with PARP inhibitors. EMBO Mol Med. 2009;1:315–322. doi: 10.1002/emmm.200900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEllin B, Camacho CV, Mukherjee B, Hahm B, Tomimatsu N, Bachoo RM, Burma S. PTEN loss compromises homologous recombination repair in astrocytes: Implications for glioblastoma therapy with temozolomide or poly(ADP-ribose) polymerase inhibitors. Cancer Res. 2010;70:5457–5464. doi: 10.1158/0008-5472.CAN-09-4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyasaka A, Oda K, Ikeda Y, Wada-Hiraike O, Kashiyama T, Enomoto A, Hosoya N, Koso T, Fukuda T, Inaba K, et al. Anti-tumor activity of olaparib, a poly (ADP-ribose) polymerase (PARP) inhibitor, in cultured endometrial carcinoma cells. BMC Cancer. 2014;14:179. doi: 10.1186/1471-2407-14-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellinger TH, Monk BJ. Systemic therapy for recurrent endometrial cancer: A review of North American trials. Expert Rev Anticancer Ther. 2009;9:905–916. doi: 10.1586/era.09.54. [DOI] [PubMed] [Google Scholar]
- 18.Fleming GF. Systemic chemotherapy for uterine carcinoma: Metastatic and adjuvant. J Clin Oncol. 2007;25:2983–2990. doi: 10.1200/JCO.2007.10.8431. [DOI] [PubMed] [Google Scholar]