Abstract
Considering mucin 1-variable number tandem repeat (MUC1-VNTRn) as a novel target for pancreatic cancer immunotherapy, the present study aimed to screen and identify the pVAX1-MUC1-VNTRn DNA vaccine with the strongest immunogenicity. Following construction of a pVAX1-MUC1-VNTRn plasmid, immature dendritic cells (DCs) were subjected to transfection, and mature DCs were then co-cultured with autologous T-cells. The numbers of cytotoxic T lymphocytes (CTLs) secreting interferon (IFN)-γ were determined using an enzyme-linked immunospot assay, and CytoTox® was also used to examine the MUC1-VNTRn-specific Lethal effect of CTLs on Capan2 cells. Additional in vivo experiments in mice were performed to confirm the antitumor effect of the DNA vaccine candidate. The present study successfully constructed the pVAX1-MUC1-VNTRn plasmid, which expresses the target protein in eukaryotic cells. Additionally, upon uptake of the pVAX1-MUC1-VNTRn plasmid, the immature DCs differentiated into mature DCs. The levels of the DC surface molecules cluster of differentiation (CD) 80, CD86, human leukocyte antigen-antigen D related, interleukin (IL)-12, IL-17 and IFN-γ were significantly higher, while the levels of IL-10 and IL-14 were lower, in mature DCs of the stimulated groups compared with the immature DCs of the non-stimulated groups (all P<0.01). In addition, the MUC1-VNTR6 and MUC1-VNTR9 groups, in which DCs were capable of activating autologous T-cells, showed increased IFN-γ-producing T-cells compared with the other groups (strong MUC1-VNTR1, weak VNTR1, VNTR3, VNTR4 and MUC1-cDNA groups; all P<0.001). In addition, the Lethal effect of CTLs on Capan2 cells in these two groups was stronger compared with the other groups (all P<0.001). Furthermore, the induced protective and therapeutic immune responses in mouse experiments showed that the pVAX1-MUC1-VNTR6DNA vaccine likely possessed the strongest immunogenicity, and its ability to inhibit panc02-MUC1 tumor growth was superior to other DNA vaccines (P<0.01). The present study provides compelling evidence that pVAX1-MUC1-VNTRn has the potential to express the target protein in eukaryotic cells, and thatpVAX1-MUC1-VNTR6 was characterized by the strongest Lethal effect in both in vivo and in vitro experiments.
Keywords: pancreatic cancer, dendritic cells, mucin 1-variable number tandem repeat, DNA vaccine, cytotoxic lymphocyte, interferon-γ
Introduction
Pancreatic cancer is an aggressive solid malignancy that occurs in the pancreas with clinical symptoms such as abdominal pain, back pain, sallow skin, unexplained weight loss and anorexia (1). In the United States, pancreatic cancer is the most common type of digestive cancer with an incidence of ~44,000 patients diagnosed per year, second only to colorectal cancer (2). With a morbidity rate of 2.8% in male and 3.2% in female patients, pancreatic cancer is the fifth leading cause of cancer-associated mortality in Europe; it is responsible for an estimated 70,000 mortalities annually in the Western world (3). The specific mechanisms underlying the initiation, development and maintenance of pancreatic cancer remain largely unknown, although it is considered that a variety of factors, such as tobacco, alcohol, coffee, diabetes and chronic pancreatitis, and even blood type, may contribute to the pathology of pancreatic cancer (2,4). Pancreatic cancer can also result from overexpression of oncogenes, inactivation of tumor suppressor genes or dysregulation of various signaling proteins (5). Current available treatments for pancreatic cancer are relatively inefficacious, and tumor resection surgery is the only choice that enables the possibility of long-term survival (6,7). The high 5-year mortality of pancreatic cancer is a combined consequence of delayed and inaccurate diagnostic techniques, and limited treatment options (4,8). All these clinical difficulties lead to an urgent requirement for an effective and accurate therapeutic tool for pancreatic cancer prevention and treatment.
Several previous studies have shown that mucin 1 (MUC1), a type of epithelial protein, is expressed in numerous malignant hematopoietic cell lines and is overexpressed in malignant tumor tissues, thus being implicated as a potential biomarker for cancers (9,10). Notably, the expression of MUC1 was observed in almost all examined pancreatic cancer cells, which suggests that MUC1 plays an instrumental role in the progression of pancreatic cancer. MUC1 represents a unique type of target that shows better efficacy when combined with immunotherapy rather than radiotherapies or chemotherapies; importantly, it allows specific targeting of the tumor without eliciting damage to adjacent normal tissues (11,12). Variable number of tandem repeat (VNTR) is defined as a variable number of connected repeats of a 20-amino acid sequence in the extracellular domain of MUC1. In normal cells, it is heavily glycosylated at the threonine and serine residues; however, it has underglycosylated VNTR domains that are overexpressed in 90% of pancreatic cancer (13). Previous studies have indicated that MUC1 exhibits abnormally high expression and incomplete glycosylation in pancreatic cancer epithelial cells, with exposed extracellular VNTRs that potentially become a new target for pancreatic cancer immunotherapy (14,15). DNA vaccines are a rapidly deployed next generation vaccination system that mainly contributes to the treatment of human and animal diseases by encoding antigenic proteins that initiate and mediate antibody- and cell-mediated immune responses (16). Increasing evidence suggests that the DNA vaccines of MUC1-VNTRn may influence cancer prognosis and treatment, although no previous studies have clarified the precise number of the repeat sequences of MUC1-VNTRn that allows for the optimum immunogenicity (17,18). Therefore, the current study aimed to screen for and identify the MUC1-VNTRn DNA vaccine with the strongest immunogenicity by constructing different extracellular repeats and assessing their effects in vitro and in vivo.
Materials and methods
Ethical issues
The present study was approved by the Animal Ethics Committee of the Affiliated Cancer Hospital of Guangzhou Medical University (Guangzhou, China). All experiments were conducted in strict accordance with the established Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Cell lines
The human pancreatic cancer cell strain Capan2 (MUC1-positive), mouse pancreatic cancer cell strain panc02 (MUC1-negative) and cervical carcinoma cell strain HeLa (MUC1-negative) were obtained from the laboratory center of the Affiliated Cancer Hospital of Guangzhou Medical University. The cells were cultured in an atmosphere containing 5% CO2 at 37°C. Cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (both Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Construction of the stable panc02-MUC1 cell line expression MUC1 was completed by Ryder Company (Guangzhou, China).
Construction of pVAX1-MUC1-VNTRn plasmid
The MUC1-VNTRn gene (one repeat of VNTR encoded 20 amino acids GVTSAPDTRPAPGSTAPPAH) (19) was synthesized and provided by Takara Biotechnology Co., Ltd. (Dalian, China). Two single repeat amino acid sequences of PDTRP were added close to the C-terminus (strong immunogenicity, strong MUC1-VNTR1) or N-terminus (weak immunogenicity, weak MUC1-VNTR1) of MUC1-VNTRn (Table I). The MUC1-VNTRn target gene sequence was obtained after n repeats of strong MUC1-VNTR1, and then cloned into pVAX1 (Invitrogen; Thermo Fisher Scientific, Inc.). The MUC1-VNTRn plasmid and pVAX1 vector were subjected to double enzyme digestion (NheI and KpnI) at 37°C for 2 h. The products of digestion were isolated by 1% agarose gel electrophoresis and verified using the UVP imaging system. Subsequently, the ligation reaction was prepared in a 10 µl volume containing: 3 µl VNTR gene, 2 µl pVAX1 vector, 1 µl 10X ligase buffer (Takara Biotechnology Co., Ltd.), 1 µl T4DNA ligase and 3 µl distilled H2O. This reaction was incubated at 16°C for 2 h. The ligation reaction product was subsequently transformed into Escherichia coli DH5α competent cells. The cells were cultured on Luria-Bertani agar medium containing 50 µg/ml kanamycin (Sigma-Aldrich; Merck Millipore, Darmstadt, Germany) to discriminate between recombinant and non-recombinant cells. The plates were incubated at 37°C for 16 h. The recombinant plasmids were digested using a double enzyme digest. The digested product was isolated by 1% agarose gel electrophoresis, and then sent for sequencing (Takara Biotechnology Co., Ltd.). The result was compared with the target sequence using BLAST. The HeLa cell strain (MUC1-negative) was transfected with pVAX1-MUC1-VNTRn. The blank pVAX1 was used as control group. At 48 h following transfection (X-tremeGENE HP DNA Transfection Reagent; Roche Diagnostics, Basel, Switzerland), expression of the target protein MUC1-VNTRn was examined using western blot analysis. Total protein was isolated from the HeLa cells using a radioimmunoprecipitation assay protein lysis buffer (Biyuntian, Shanghai, China). The concentration of protein was determined using a BCA protein quantification kit (Biyuntian), according to the manufacturer's protocol. A total of 20 µg protein was separated using a 12% (w/v) sodium dodecyl sulfate-poly-acrylamide gel electrophoresis (SDS-PAGE) according to the standard protocol, and then transferred to a polyvinylidene difluoride membrane. The membrane was incubated with the anti-MUC1-VNTR monoclonal antibody (cat. no. VU4H5; Cell Signaling Technology, Inc., Danvers, MA, USA; 1:1,000) at 4°C overnight. The membrane was subsequently incubated with the secondary goat anti-mouse immunoglobulin G antibody (Boster, China; 1:5,000) at room temperature for 1 h. Subsequently, the experiment results were obtained and analyzed by coloration using the SuperSignal West Pico kit (Thermo fisher Scientific, Inc.), development and fixing of the photographic materials.
Table I.
Fragments of MUC1-VNTR target gene.
| MUC1-VNTR1 | Sequence |
|---|---|
| Strong target gene | GCTAGCGCCACCATGCCGGGCTCCACCGCCCCCCCAGCCCACGGTGTCACCTCGGCCCCGGACACCAGGCCGGCCTGAGGTACC |
| Weak target gene | GCTAGCGCCACCATGCACGGTGTCACCCGGCCCCGGACACCAGGCCGGCCCCGGGCTCCACCGCCCCCCCAGCCTGAGGTACC |
The strong target gene shows strong immunogenicity, as the PDTRP sequence has been added to the C-terminal end of MUC1-VNTR. The weak target gene shows weak immunogenicity, as the PDTRP sequence has been added to the N-terminal end of MUC1-VNTR. GCCACC is a Kozak sequence and the italic part refers to MUC1-VNTR1. MUC1, mucin 1; VNTR, variable number of tandem repeats.
Dendritic cell (DC) culture and biological characteristics
Peripheral blood leukocyte suspensions from healthy subjects were collected from the Guangzhou Blood Bank (Guangzhou, China). Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll density gradient centrifugation. PBMCs was adjusted to 5×106/ml in RPMI-1640 culture medium containing 10% fetal bovine serum (both Gibco; Thermo Fisher Scientific, Inc.), 2 mmol/l L-glutamine and 100 U/ml penicillin-streptomycin, and then incubated at 37°C in 5% CO2 for 2 h to remove non-adherent cells. Adherent cells were cultured in RPMI-1640 culture medium supplemented with 1,000 U/ml granulocyte macrophage colony-stimulating factor (GM-CSF) and 500 U/ml interleukin (IL)-4, and approximately half of the medium was changed to supplement cell factors every other day. Immature DCs were obtained on the 6th day (control group) and divided into four groups: Group A, in which immature DCs were cultured with GM-CSF and IL-4; group B, in which immature DCs were treated with 1,000 U/ml tumor necrosis factor-α (TNF-α); group C, in which immature DCs were transfected with the VNTR1 plasmid; and group D, in which immature DCs were transfected with the VNTR1 plasmid and treated with 1,000 U/ml TNF-α. At 24 h following induction with TNF-α and plasmid, mature DCs were obtained, and the surface markers, cluster of differentiation CD80, CD86 and human leukocyte antigen-antigen D related (HLA-DR), were analyzed using flow cytometry (Cytomics FC 500MPL) performed by the Flow Cytometry Department of the Sun Yat-Sen Memorial Hospital Laboratory Center (Guangzhou, China). Additionally, the supernatant of DCs was collected to quantify the levels of IL-12, IL-4, IL-17, IL-10 and interferon (IFN)-γ using an ELISA kit (Neobioscience, Shenzhen, China).
Detection of CTLs producing IFN-γ using the enzyme-linked immunospot (ELISPOT) assay
The concentrations of DCs loaded with different MUC1-VNTRn antigens (MUC1-VNTRn antigens, n=3, 4, 6 and 9) in these four groups were adjusted to 1×105/ml. Autologous T-cells from the peripheral blood suspensions were filtrated using nylon wool columns were used as effector cells, and were adjusted to 1×106/ml. A total of 100 µl of DCs and T-cells were added to the ELISPOT plate (BD Biosciences, Franklin Lakes, NJ, USA) in the ratio of 1:10. Subsequently, 100 µl culture medium was added to one group of effector cells (3 wells), while 100 µl culture medium containing 2 µg/ml phytohemagglutinin was added to another group of effector cells (3 wells). Cells were then incubated at 37°C with 5% CO2 for 16 h. The IFN-γ ELISPOT kit (BD Biosciences) was used to detect IFN-γ-producing CTLs according to the manufacturer's protocol. The organization of the groups is presented in Table II.
Table II.
Organization of the groups in the present study.
| Determination of IFN-γ-producing CTLs by ELISPOT | Lethal effect of CTLs in vitro | Establishment of pancreatic cancer in mice | Treatment of tumor-bearing mice |
|---|---|---|---|
| Empty vector group | Empty vector group | Empty vector group | Empty vector group |
| Weak VNTR1 group | HeLa cells group | Panc02 control group | Panc02 control group |
| Strong VNTR1 group | Weak VNTR1 group | Weak VNTR1 group | Weak VNTR1 group |
| VNTR3 group | Strong VNTR1 group | Strong VNTR1 group | Strong VNTR1 group |
| VNTR4 group | VNTR3 group | VNTR3 group | VNTR3 group |
| VNTR6 group | VNTR4 group | VNTR4 group | VNTR4 group |
| VNTR9 group | VNTR6 group | VNTR6 group | VNTR6 group |
| MUC1-cDNA group | VNTR9 group MUC1-cDNA group | VNTR9 group | VNTR9 group |
Weak VNTR1, single VNTR repetitive sequence with weak immunogenicity; Strong VNTR1, single VNTR repetitive sequence with strong immunogenicity; VNTR3, strong VNTR1 with 3 repeats; VNTR4, strong VNTR1 with 4 repeats; VNTR6, strong VNTR1 with 6 repeats; VNTR9, strong VNTR1 with 9 repeats; MUC1, mucin 1; CTLs, cytotoxic T lymphocytes.
Lethal effect of CTLs in vitro
Mature DCs loaded with different MUC1-VNTRn antigens and autologous T-cells (ratio 1:10) were seeded into a 6-well plate supplemented with 100 U/ml IL-2 culture medium; approximately half of the medium was changed to supplement cell factors every other day. Subsequent to a 5-day culture, the cells were counted using a light microscope. A total of three groups were classified: MUC1-VNTRn group; pVAX1 without vector group; and HeLa cells group (negative control group). The organization of the groups is presented in Table II. MUC1-positive Capan2 cell strains were collected as the target cells, and the effector cells and target cells were seeded into a 96-well plate with the ratio of 40:1. The Lethal effect of CTLs was examined using the CytoTox Non-Radioactive Cytotoxicity assay kit (Promega Corporation, Madison, WI, USA), according to the manufacturer's protocol.
Establishment of pancreatic cancer in mice
A total of 120 female C57BL/6 mice were purchased from the laboratory animal center of Sun Yat-Sen University (Guangzhou, China). The experiments were performed in strict accordance with the requirements of biosafety rules and operated under the protocol of the laboratory animal center of Sun Yat-Sen University. The C57BL/6 mice were housed at a controlled temperature of between 20 and 26°C with a relative humidity of between 40 and 60%. The mice were subjected to a 12 h light-dark cycle and were fed and given water 2 times/week. The C57BL/6 mice were randomly divided into eight groups (n=10 per group): i) Empty vector group; ii) panc02 control group; iii) strong VNTR1 group; iv) weak VNTR1 group; v) VNTR3 group; vi) VNTR4; vii) VNTR6 group; and viii) VNTR9 group. The organization of the groups is presented in Table II. The mice in each group were injected into the right anterior tibial muscle with 100 µg/100 µl plasmid DNA inPBS3 times at 2-week intervals. At 1 week following the third injection, the C57BL/6 mice were treated with subcutaneous injection of 5×105/100 µl panc02-MUC1 or panc02 cells into the left leg. The tumor size (long and short diameter) was measured every 2–3 days for 4 weeks, and the survival time was recorded.
Treatment of tumor-bearing mice
The second batch of 60 C57BL/6 mice were randomly divided into the same eight groups (n=10 per group). The mice were subcutaneously injected with 5×105/100 µl panc02 or panc02-MUC1 cells. On the 4, 8 and 12th days following inoculation of the tumor cells, the mice were administered intramuscular injection of 100 µg/100 µl plasmid in PBS solution. Additionally, the tumor size (long/short diameter) was measured every 2–3 days for 4 weeks to generate the tumor growth curve. The survival time was recorded.
Statistical analyses
Measurement data are expressed as the mean ± standard deviation. The comparison between two independent samples was performed using Student's t-test. Additionally, measurement data were compared using analysis of variance, and the Fisher's least significant difference (LSD) test was applied for comparisons among multiple groups. SPSS 16.0 statistical software (SPSS, Inc., Chicago, IL, USA) was used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Plasmid construction
The present study successfully constructed 7 plasmids containing weak pVAX1-MUC1-VNTR1, strong VNTR1, VNTR3, VNTR4, VNTR6, VNTR9 and pVAX1-MUC1 (empty plasmid). Double digestion products of the plasmids were separated by agarose gel electrophoresis, and the present study was able to confirm the insertion of the target gene fragments into the pVAX1 plasmid vector in reference to the band size at the corresponding position in the gel. Sequencing results of the 7 plasmids were compared with the fragments of the target gene in the Basic Local Alignment Search Tool program (blast .ncbi.nlm.nih.gov/Blast.cgi), and showed a 100% overlap ratio. MUC1-negative HeLa cell lines were transfected with the pVAX1-MUC1-VNTRn plasmid, and expression of the target protein MUC1-VNTRn was detected by western blot analysis, confirming that the pVAX1-MUC1-VNTRn plasmid can be expressed in eukaryotic cells (data not shown).
Cultivation and biological characteristics of DCs
Under an inverted microscope (Fig. 1), mature DCs in suspension were found to be larger compared with leukomonocytes, with a spinous shape and numerous slender protrusions. As shown in Table III, expression of the surface markers CD80, CD86, and HLA-DR in mature DCs, as examined by flow cytometry, exhibited significant differences between the stimulated groups (groups B, C and D) and the control (all P<0.05). However, there was no significant difference among the DC phenotypes without stimulation on days 6 and 7 (P>0.05). Similarly, no significant differences were identified among the mature DC phenotypes subsequent to induction of the stimulated groups (all P>0.05). However, the pairwise comparisons between stimulated and non-stimulated groups showed significant differences (all P<0.05).
Figure 1.
Mature dendritic cells in suspension under an inverted microscope at (A) magnification, ×20 and (B) magnification, ×40, were larger compared with leukomonocytes, with spinous processes and a number of slender protrusions, as indicated by the black arrow.
Table III.
Dendritic cell phenotype, as determined by flow cytometry.
| Phenotype | |||
|---|---|---|---|
| Treatment group | CD80 (P-value) | CD86 (P-value) | HLA-DR (P-value) |
| Day 6 without stimulation (control group) | 10.12±4.15 (reference) | 19.25±8.76 (reference) | 70.89±8.21 (reference) |
| Day 7 without stimulation (group A) | 16.86±8.05 (P=0.267) | 24.85±11.64 (P=0.542) | 71.62±8.31 (P=0.919) |
| TNF-α-stimulated maturation (group B) | 49.92±9.36 (P=0.003) | 89.68±2.12 (P<0.001) | 89.45±2.81 (P=0.021) |
| Stimulation by the VNTR1 plasmid (group C) | 53.87±8.41 (P=0.001) | 91.29±3.93 (P<0.001) | 90.83±4.91 (P=0.023) |
| TNF-α and VNTR1 plasmid co-stimulation (group D) | 58.63±9.05 (P=0.001) | 92.24±3.27 (P<0.001) | 93.43±3.62 (P=0.012) |
CD, cluster of differentiation; HLA-DR, human leukocyte antigen-antigen D related; TNF-α, tumor necrosis factor-α.
The supernatants of immature DCs and cytokine/antigen-induced mature DCs were collected to determine the amounts of secreted IL-12, IL-10, IL-4, IL-17 and IFN-γ using double-antibody sandwich ELISAs, which indirectly reflected the degree of maturation in DCs. As shown in Table IV, there were no significant differences in the levels of IL-12, IL-10, IL-4, IL-17 and IFN-γ between days 6 and 7 without stimulation (all P>0.05). Additionally, no significant differences were found in the levels of IL-12, IL-10, IL-4, IL-17 and IFN-γ between the supernatants of TNF-α-induced, VNTR1 plasmid-stimulated, and TNF-α+VNTR1 plasmid-stimulated groups (all P>0.05), while the pairwise comparisons using the LSD test showed significant differences between the stimulated and non-stimulated groups (all P<0.01).
Table IV.
Secretion of IL-12, IL-10, IL-4, IL-17 and IFN-γ by dendritic cells, as determined using a double-antibody sandwich enzyme-linked immunosorbent assay.
| Factor, pg/ml | |||||
|---|---|---|---|---|---|
| Treatment group | IL-12 (P-value) | IL-10 (P-value) | IL-4 (P-value) | IL-17 (P-value) | IFN-γ (P-value) |
| Day 6 without stimulation (control group) | 7.18±2.63 (reference) | 64.42±3.59 (reference) | 49.58±2.84 (reference) | 16.38±6.94 (reference) | 70.12±3.25 (reference) |
| Day 7 without stimulation (group A) | 5.86±3.54 (P=0.632) | 65.23±4.10 (P=0.810) | 50.41±2.94 (P=0.743) | 16.21±6.86 (P=0.977) | 69.25±3.42 (P=0.765) |
| TNF-α-stimulated maturation (group B) | 57.01±12.54 (P=0.003) | 14.33±2.39 (P<0.001) | 8.29±2.05 (P<0.001) | 60.01±5.96 (P=0.001) | 218.69±7.51 (P<0.001) |
| Stimulation by the VNTR1 plasmid (group C) | 46.18±9.93 (P=0.003) | 13.96±2.77 (P<0.001) | 9.17±2.16 (P<0.001) | 68.26±7.01 (P<0.001) | 224.31±7.61 (P<0.001) |
| TNF-α and VNTR1 plasmid co-stimulation (group D) | 52.47±11.26 (P=0.003) | 13.24±2.48 (P<0.001) | 9.53±2.44 (P<0.001) | 61.95±7.12 (P=0.001) | 228.95±7.81 (P<0.001) |
IL, interleukin; IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α.
Determination of IFN-γ-producing CTLs by ELISPOT
As shown in Fig. 2, the MUC1-VNTR6- and MUC1-VNTR9-transfectedDCsinducedsignificantly more IFN-γ-producing T-cells compared with the DCs in the other groups (MUC1-VNTR6, 105.2±8.7; MUC1-VNTR9, 93.7±7.3; strong MUC1-VNTR1, 56.4±6.2; weak MUC1-VNTR1, 47.5±5.4; MUC1-VNTR3, 69.3±7.2; MUC1-VNTR4, 71.5±6.7; MUCI-cDNA, 81.2±7.4; empty plasmid, 16.8±4.8; P<0.001). However, there was no significant difference in the number of T-cells between the VNTR6 and VNTR9 groups (P=0.350).
Figure 2.
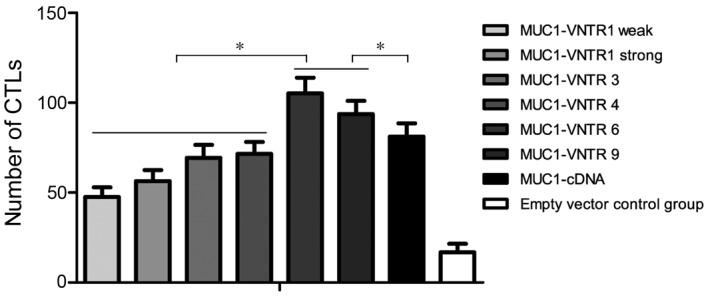
Number of CTLs producing IFN-γ in the different groups, as determined by an enzyme-linked immunospot assay. The MUC1-VNTR6 and MUC1-VNTR9 groups showed increased IFN-γ-producing T-cells compared with the other groups (VNTR1, weak VNTR1, VNTR3, VNTR4 and MUC1-cDNA). *P<0.001. IFN, interferon; CTL, cytotoxic T lymphocyte; MUC1, mucin 1; VNTR, variable number tandem repeat.
pVAX1-MUC1-VNTRn enhances the cytotoxicity of CTLs
As shown in Fig. 3, a stronger Lethal effect on Capan2 cells was observed for the T-cells stimulated with VNTR6 and VNTR9DCs compared with the other groups (VNTR6,41.25±3.34%; VNTR9, 37.18±3.61%; strong VNTR1,15.32±2.71%; weak VNTR1,12.25±2.35%; VNTR3, 21.24±1.89%; VNTR4, 23.28±3.27%; MUC1-cDNA, 29.85±2.89%; all P<0.001). The Lethal effect of CTLs on Capan2 cells was significantly different between the empty vector group (2.46±1.87%) and all pVAX1-MUC1-VNTRn DNA vaccine groups (P<0.001). Additionally, there were significant differences in the Lethal effect of CTLs on MUC1-negative HeLa cells between the strong VNTR1 group (2.17±1.98%) and the other groups (all P<0.001).
Figure 3.
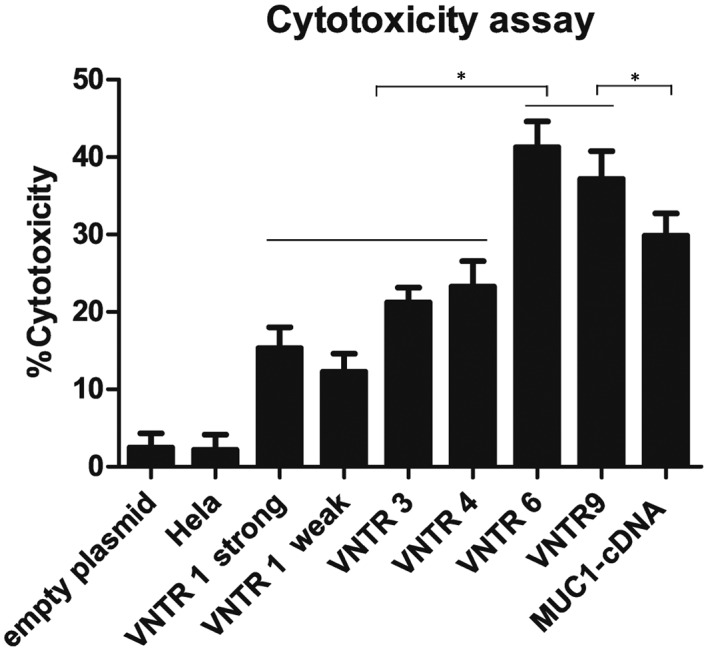
Lethal effect of MUC1-VNTRn-stimulatedcytotoxic T lymphocytes on Capan2 cells. VNTR6 and VNTR9groups exhibited stronger Lethal effects on Capan2 cells compared with the other groups (strong VNTR1, weak VNTR1, VNTR3, VNTR4 and MUC1-cDNA). *P<0.001. VNTR, variable number tandem repeat; MUC1, mucin 1.
pVAX1-MUC1-VNTRn DNA vaccine suppresses panc02-MUC1 tumor growth in vivo
Compared with the empty vector and panc02 control groups, the strong pVAX1-MUC1-VNTR1, VNTR3, VNTR4, VNTR6 and VNTR9 groups showed significantly increased inhibitory effects on panc02-MUC1 cell tumor growth from day 12 following tumor challenge (all P<0.001; Fig. 4). In addition, the inhibitory effect of the weak VNTR1 DNA plasmid on panc02-MUC1 tumor growth was significantly milder compared with the other p-VAX1-MUC1-VNTRn groups (all P<0.05). LSD analysis indicated that the pVAX1-MUC1-VNTR6 DNA vaccine showed a stronger in vivo suppressive effect on panc02-MUC1 tumor growth compared with the strong pVAX1-MUC1-VNTR1, VNTR3, VNTR4 and VNTR9 vaccines (all P<0.01), while there was no significant difference in the suppressive effects among the strong pVAX1-MUC1-VNTR1, VNTR3 and VNTR4vaccines (all P>0.05; Fig. 4). pVAX1-MUC1-VNTRn DNA vaccines showed no detectable inhibitory effects on panc02 tumor cells (Fig. 4), indicating robust MUC1 specificity.
Figure 4.
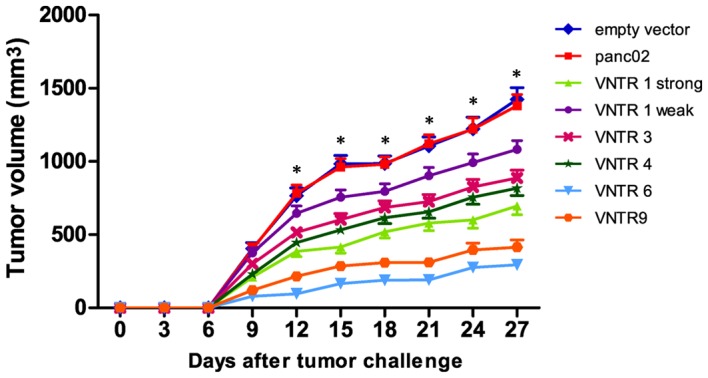
Effect of pVAX1-MUC1-VNTRn DNA vaccine immunization on panc02-MUC1 tumor growth in mice. The pVAX1-MUC1-VNTR6 DNA vaccine showed a stronger inhibitory effect on panc02-MUC1 tumor growth in vivo compared with the strong pVAX1-MUC1-VNTR1, VNTR3, VNTR4 and VNTR9 groups. pVAX1-MUC1-VNTRn DNA vaccines showed no evident inhibitory effect on panc02 tumor cells, indicating MUC1 specificity. *P<0.001. MUC1, mucin 1; VNTR, variable number tandem repeat.
Treatment with p-VAX1-MUC1-VNTRn suppresses panc02-MUC1 tumor growth in tumor-bearing mice
The mice were administered subcutaneous injections of panc02 or panc02-MUC1 cells and, after 4, 8 and 12 days, received DNA vaccination. As shown in Fig. 5, compared with the empty vector and panc02 negative groups, growth of panc02-MUC1 tumors was significantly inhibited from day 9 in the strong pVAX1-MUC1-VNTR1, VNTR3, VNTR4, VNTR6 and VNTR9 groups (all P<0.001). In addition, the suppressive effect of the weak VNTR1 vaccine on panc02-MUC1 tumor growth was reduced compared with the other p-VAX1-MUC1-VNTRn groups (all P<0.05). According to the LSD analysis, the pVAX1-MUC1-VNTR6 DNA vaccine showed a stronger inhibitory effect on panc02-MUC1 tumor growth compared with the strong pVAX1-MUC1-VNTR1, VNTR3, VNTR4 and VNTR9 vaccines (all P<0.01), while there was no significant difference among the strong pVAX1-MUC1-VNTR1, VNTR3, VNTR4 and VNTR9 groups (all P>0.05; Fig. 5). No evident inhibitory effects on panc02 tumor cells were found for all pVAX1-MUC1-VNTRn DNA vaccines (Fig. 5), indicating MUC1 specificity.
Figure 5.
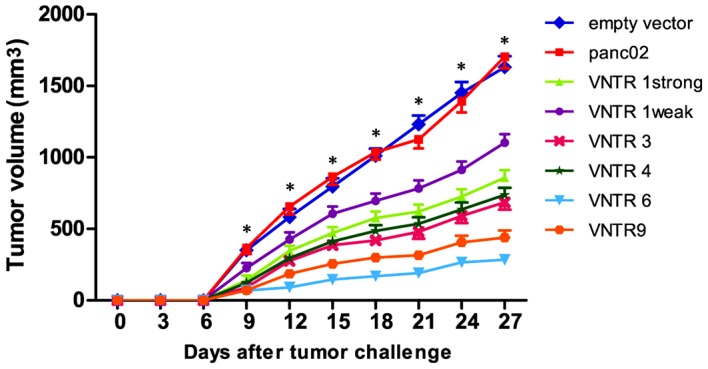
Effect of treatment with thepVAX1-MUC1-VNTR n DNA vaccines on in vivo growth of panc02-MUC1 tumors. The pVAX1-MUC1-VNTR6 DNA vaccine showed a stronger inhibitory effect on panc02-MUC1 tumor growth compared with the strong pVAX1-MUC1-VNTR1, VNTR3, VNTR4 and VNTR9 groups. No evident inhibitory effects on panc02 tumor cells were observed for all pVAX1-MUC1-VNTRn DNA vaccines, indicating MUC1 specificity. *P<0.001. MUC1, mucin 1; VNTR, variable number tandem repeat.
Discussion
The present study, utilizing MUC1-VNTRn as a potentially novel target for pancreatic cancer immunotherapy, screened for and identified the pVAX1-MUC1-VNTRn DNA vaccine with the strongest immunogenicity. As a highly malignant carcinoma with the poorest prognosis of all cancers, pancreatic cancer is one of the leading causes of cancer-associated mortality in the USA (2). Therapeutic modalities used to treat pancreatic cancer include chemotherapy, immunotherapy, surgical resection and radiotherapy; however, the survival outcome for patients with pancreatic cancer remains poor (4,6). As a transmembrane mucin glycoprotein that is aberrantly overexpressed in a variety of adenocarcinomas, including pancreatic cancer, MUC1 provides a reliable target for immunotherapy (20). Importantly, the present results demonstrated successful construction of pVAX1-MUC1-VNTRn, which was shown to enable expression of the target protein in eukaryotic cells. Generally, the peptide backbone of MUC1 is controlled by a region that constitutes 80–200 repetitions of 20-amino-acid repeats (21,22). It is well documented that the MUC1 region, which possesses long branched O-linked carbohydrates, is heavily glycosylated in healthy epithelial cells. By contrast, enhanced expression of MUC1 with heavy glycosylation occurs in unhealthy epithelial cells in the majority of human adenocarcinomas (23). A previous study reported that the majority of pancreatic cancer patients have compromised MUC1-specific immune responses, and are thus unable to inhibit and kill tumor cells (24). The present study presumed that MUC1-VNTR, as a new target for immunotherapy, may effectively enhance the immune response to MUC1-positive tumor cells, eliciting and boosting efficacious antigen-specific immune responses.
Another significant finding in the current study was that co-stimulation of immature DCs with the VNTR1 antigen and TNF-α was able to robustly induce DC maturation, thereby strongly activating the proliferation of allogeneic T-cells due to the high antigen-presenting ability of mature DCs (25). It has previously been reported that DCs, known as a potent type of antigen-presenting cell, are distributed widely throughout the human body. DCs have been identified in the majority of tissues, including the cornea, heart and brain tissues. However, the total quantity of DCs is estimated to be low in the body, only accounting for 1% of peripheral white blood cells and 0.5% of the total number of spleen cells (26). Previous studies have indicated that DCs are capable of activating T lymphocytes, thereby inducing cellular immune responses including CD4+T helper type 1cells and CD8+T-cells (11,27). In addition, DCs can activate memory B lymphocytes to induce humoral immune responses. Furthermore, DCs are also able to activate natural killer (NK) cells and NKT-cells, which play a unique role in the induction of immune responses (26).
The present study also demonstrated that the amount of IFN-γ-producing CTLs in the MUC1-VNTR6 group and the MUC1-VNTR9 group were significantly increased compared with the other groups. In addition, the Lethal effect of CTLs in these two groups on Capan2 cells was significantly stronger compared with the other groups. The extracellular domain, particularly the VNTR region, of MUC1 undergoes marked alterations in posttranslational modifications when normal cells are transformed into tumor cells, and this may lead to variable interactions with the extracellular environment (27). Previous studies in animal models have demonstrated that CTLs alleviate disease severity and promote viral clearance rather than providing sterilizing immunity (28,29). In addition, CTLs induced by DNA vaccination play a key role in eradicating tumors, because the CTLs can directly kill tumor cells and indirectly elicit IFN-γ-mediated antitumor responses (30). The present study adopted DCs transfected with the MUCI-VNTR DNA vaccine as antigen presenting cells to stimulate autologous T lymphocytes and induce the amplification of MUCI-specific CTLs.
The current study determined that the pVAX1-MUC1-VNTR6DNA vaccine had the strongest Lethal effect in both in vivo and in vitro experiments, and demonstrated that this vaccine possessed MUC1 specificity. DNA vaccines are reported to be potentially safer compared with traditional vaccines, in addition to their stability and cost effectiveness (31). Additionally, the immune system responses activated by anticancer DNA vaccines effectively prevented the occurrence and recurrence of cancer in patients in a previous study (32). A variety of vaccines utilizing MUC1 as the target antigen were shown to induce MUC1-specific humoral and cellular immunity to varying extents, thereby eradicating MUC1-expressingtumor cells (33). Snyder et al (34) indicated that the use of a certain number of tandem repeat sequences in the MUC1-VNTRn DNA vaccine was able to induce a robust and strong immune response to target and kill pancreatic cancer cells. In addition, Quinlin et al (35) found that the peptide vaccine, VNTR1, with its PDTRP sequence adjacent to the C-terminus, was characterized by an increased immunogenicity compared with VNTR3, with its PDTRP sequence adjacent to the N-terminus. In the present study, protective and therapeutic immune response experiments in mice were performed to further assess the immunogenicity of the DNA vaccines. It was found that the ability of the pVAX1-MUC1-VNTR6 DNA vaccine to inhibit panc02-MUC1 tumor growth in vivo was significantly superior to that of the pVAX1-MUC1-VNTR1, VNTR3 and VNTR9 DNA vaccines.
In summary, the present study demonstrated that pVAX1-MUC1-VNTRn has the potential to express target proteins in eukaryotic cells, and that pVAX1-MUC1-VNTR6 was characterized by a strong Lethal effect on MUC1-positive tumor cells in both in vivo and in vitro experiments.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (grant nos. 81370059 and 81000917), Guangzhou Science and Technology Research Project (grant no. 2014J4100173) and the Scientific Study Project Foundation of Guangzhou Medical University (grant nos. 2013A43 and 2015C38). The authors would like to acknowledge the reviewers for their helpful comments on this paper.
References
- 1.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 2.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–1403. doi: 10.1016/j.ejca.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 4.Buanes TA. Pancreatic cancer-improved care achievable. World J Gastroenterol. 2014;20:10405–10418. doi: 10.3748/wjg.v20.i30.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarkar FH, Banerjee S, Li Y. Pancreatic cancer: Pathogenesis, prevention and treatment. Toxicol Appl Pharmacol. 2007;224:326–336. doi: 10.1016/j.taap.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bilimoria KY, Bentrem DJ, Ko CY, Tomlinson JS, Stewart AK, Winchester DP, Talamonti MS. Multimodality therapy for pancreatic cancer in the US Utilization, outcomes, and the effect of hospital volume. Cancer. 2007;110:1227–1234. doi: 10.1002/cncr.22916. [DOI] [PubMed] [Google Scholar]
- 7.Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS. National failure to operate on early stage pancreatic cancer. Ann Sur. 2007;246:173–180. doi: 10.1097/SLA.0b013e3180691579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida Y, Raina D, Kharbanda S, Kufe D. Inhibition of the MUC1-C oncoprotein is synergistic with cytotoxic agents in the treatment of breast cancer cells. Cancer Biol Ther. 2013;14:127–134. doi: 10.4161/cbt.22634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant S. Bortezomib resistance and MUC1 in myeloma. Blood. 2014;123:2910–2912. doi: 10.1182/blood-2014-03-563882. [DOI] [PubMed] [Google Scholar]
- 11.Shindo Y, Hazama S, Maeda Y, Matsui H, Iida M, Suzuki N, Yoshimura K, Ueno T, Yoshino S, Sakai K, et al. Adoptive immunotherapy with MUC1-mRNA transfected dendritic cells and cytotoxic lymphocytes plus gemcitabine for unresectable pancreatic cancer. J Transl Med. 2014;12:175. doi: 10.1186/1479-5876-12-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidiyoor A, Schettini J, Besmer DM, Rego SL, Nath S, Curry JM, Roy LD, Dréau D, Mukherjee P. Pancreatic cancer cells isolated from Muc1-Null tumors favor the generation of a mature less suppressive MDSC population. Front Immunol. 2014;5:67. doi: 10.3389/fimmu.2014.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang S, Zhang H, Shi H, Yu X, Kong W, Li W. Induction of immune response and anti-tumor activities in mice with a DNA vaccine encoding human mucin 1 variable-number tandem repeats. Hum Immunol. 2008;69:250–258. doi: 10.1016/j.humimm.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Chen X, Tang M. Quantitative assessment of the diagnostic role of MUC1 in pancreatic ductal adenocarcinoma. Tumour Biol. 2014;35:9101–9109. doi: 10.1007/s13277-014-2186-4. [DOI] [PubMed] [Google Scholar]
- 15.Nath S, Mukherjee P. MUC1: A multifaceted oncoprotein with a key role in cancer progression. Trends Mol Med. 2014;20:332–342. doi: 10.1016/j.molmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams JA. Improving DNA vaccine performance through vector design. Curr Gene Ther. 2014;14:170–189. doi: 10.2174/156652321403140819122538. [DOI] [PubMed] [Google Scholar]
- 17.Weng Y, Shao L, Ouyang H, Liu Y, Yao J, Yang H, Luo Y, Wang H, Zhao Z, Mou H, et al. A unique MUC1-2-VNTR DNA vaccine suppresses tumor growth and prolongs survival in a murine multiple myeloma model. Oncol Rep. 2012;27:1815–1822. doi: 10.3892/or.2012.1707. [DOI] [PubMed] [Google Scholar]
- 18.Roulois D, Grégoire M, Fonteneau JF. MUC1-specific cytotoxic T lymphocytes in cancer therapy: Induction and challenge. Biomed Res Int. 2013;2013:871936. doi: 10.1155/2013/871936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rong Y, Jin D, Wu W, Lou W, Wang D, Kuang T, Ni X, Qin X. Induction of protective and therapeutic anti-pancreatic cancer immunity using a reconstructed MUC1 DNA vaccine. BMC Cancer. 2009;9:191. doi: 10.1186/1471-2407-9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueda M, Miura Y, Kunihiro O, Ishikawa T, Ichikawa Y, Endo I, Sekido H, Togo S, Shimada H. MUC1 overexpression is the most reliable marker of invasive carcinoma in intraductal papillary-mucinous tumor (IPMT) Hepatogastroenterology. 2005;52:398–403. [PubMed] [Google Scholar]
- 21.Engelmann K, Baldus SE, Hanisch FG. Identification and topology of variant sequences within individual repeat domains of the human epithelial tumor mucin MUC1. J Biol Chem. 2001;276:27764–27769. doi: 10.1074/jbc.M103187200. [DOI] [PubMed] [Google Scholar]
- 22.Baldus SE, Engelmann K, Hanisch FG. MUC1 and the MUCs: A family of human mucins with impact in cancer biology. Crit Rev Clin Lab Sci. 2004;41:189–231. doi: 10.1080/10408360490452040. [DOI] [PubMed] [Google Scholar]
- 23.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 24.Curry JM, Thompson KJ, Rao SG, Besmer DM, Murphy AM, Grdzelishvili VZ, Ahrens WA, McKillop IH, Sindram D, Iannitti DA, et al. The use of a novel MUC1 antibody to identify cancer stem cells and circulating MUC1 in mice and patients with pancreatic cancer. J Surg Oncol. 2013;107:713–722. doi: 10.1002/jso.23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pei Q, Pan J, Zhu H, Ding X, Liu W, Lv Y, Zou X, Luo H. Gemcitabine-treated pancreatic cancer cell medium induces the specific CTL antitumor activity by stimulating the maturation of dendritic cells. Int Immunopharmacol. 2014;19:10–16. doi: 10.1016/j.intimp.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Hon H, Jacob J. Tracking dendritic cells in vivo: Insights into DC biology and function. Immunol Res. 2004;29:69–80. doi: 10.1385/IR:29:1-3:069. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Vlad A, Milcarek C, Finn OJ. Human mucin MUC1 RNA undergoes different types of alternative splicing resulting in multiple isoforms. Cancer Immunol Immunother. 2013;62:423–435. doi: 10.1007/s00262-012-1325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tu W, Mao H, Zheng J, Liu Y, Chiu SS, Qin G, Chan PL, Lam KT, Guan J, Zhang L, et al. Cytotoxic T lymphocytes established by seasonal human influenza cross-react against 2009 pandemic H1N1 influenza virus. J Virol. 2010;84:6527–6535. doi: 10.1128/JVI.00519-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hillaire ML, Osterhaus AD, Rimmelzwaan GF. Induction of virus-specific cytotoxic T lymphocytes as a basis for the development of broadly protective influenza vaccines. J Biomed Biotechnol. 2011;2011:939860. doi: 10.1155/2011/939860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagata T, Aoshi T, Uchijima M, Suzuki M, Koide Y. Cytotoxic T-lymphocyte-, and helper T-lymphocyte-oriented DNA vaccination. DNA Cell Biol. 2004;23:93–106. doi: 10.1089/104454904322759902. [DOI] [PubMed] [Google Scholar]
- 31.Lu S, Wang S, Grimes-Serrano JM. Current progress of DNA vaccine studies in humans. Expert Rev Vaccines. 2008;7:175–191. doi: 10.1586/14760584.7.2.175. [DOI] [PubMed] [Google Scholar]
- 32.Choi Y, Jeon YH, Jang JY, Chung JK, Kim CW. Treatment with mANT2 shRNA enhances antitumor therapeutic effects induced by MUC1 DNA vaccination. Mol Ther. 2011;19:979–989. doi: 10.1038/mt.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kimura T, McKolanis JR, Dzubinski LA, Islam K, Potter DM, Salazar AM, Schoen RE, Finn OJ. MUC1 vaccine for individuals with advanced adenoma of the colon: A cancer immunoprevention feasibility study. Cancer Prev Res (Phila) 2013;6:18–26. doi: 10.1158/1940-6207.CAPR-12-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snyder LA, Goletz TJ, Gunn GR, Shi FF, Harris MC, Cochlin K, McCauley C, McCarthy SG, Branigan PJ, Knight DM. A MUC1/IL-18 DNA vaccine induces anti-tumor immunity and increased survival in MUC1 transgenic mice. Vaccine. 2006;24:3340–3352. doi: 10.1016/j.vaccine.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 35.Quinlin IS, Burnside JS, Dombrowski KE, Phillips CA, Dolby N, Wright SE. Context of MUC1 epitope: Immunogenicity. Oncol Rep. 2007;17:453–456. doi: 10.3892/or.17.2.453. [DOI] [PubMed] [Google Scholar]