Abstract
Objective
Positive HLA-B*5801 carriers are at greater risk of experiencing rare but severe allopurinol hypersensitivity syndrome (AHS) (i.e., Stevens-Johnson Syndrome [SJS] and Toxic Epidermal Necrolysis [TEN]); however, HLA-B*5801 prevalence and AHS risk vary by race/ethnicity. We evaluated the cost-effectiveness of HLA-B*5801 testing according to race/ethnicity in the US.
Methods
We determined the cost-effectiveness of universal testing for HLA-B*5801 compared to no testing prior to the initiation of allopurinol per US major race/ethnicity groups. Using US specific data, SJS/TEN risks and HLA-B*5801 prevalences were modeled per race/ethnicity (i.e., 1/3846 and 0.7% among Caucasians and Hispanics; 1/735 and 3.8% among African Americans; 1/336 and 7.4% among Asians, respectively). Those who tested positive for HLA-B*5801 received febuxostat. Costs, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs) were estimated over a lifetime.
Results
Compared to no testing, universal testing for HLA-B*5801 cost more and was more effective for all races/ethnicities. The ICERs varied substantially across racial/ethnic groups, following their HLA-B*5801 prevalences. HLA-B*5801 testing was cost-effective for African Americans (ICER $83,450) and Asians (ICER $64,190), but not for Caucasians or Hispanics (ICER $183,720), using accepted US willingness-to-pay threshold ($109,000/QALY). Results were robust in sensitivity analyses, except that reducing the risk of SJS/TEN by a half made testing not cost-effective for all races/ethnicities.
Conclusion
Testing for HLA-B*5801 prior to allopurinol initiation is cost-effective for Asians and African Americans, but not for Caucasians or Hispanics in the US. Reducing AHS risk by other predictive measures could make HLA-B*5801 testing not cost-effective even among Asians and Blacks.
Keywords: Gout, Outcomes Research, Economic Evaluations
INTRODUCTION
Although rare, the most feared adverse event associated with allopurinol, the most common urate-lowering therapy (ULT), is allopurinol hypersensitivity syndrome (AHS) (i.e., Stevens-Johnson Syndrome [SJS] and Toxic Epidermal Necrolysis [TEN]). SJS/TEN due to AHS almost exclusively occurs within the first few months of starting treatment, frequently involves major organs, and can be fatal in up to 32% of cases [1-6]. Those who survive SJS/TEN are often left with substantial sequelae of involved organs (e.g., corneal damage and renal insufficiency) [1,7]. The 2016 Agency for Healthcare Research and Quality review of the evidence on gout care has identified an important research gap about how to further minimize the rare but serious adverse events from ULT (e.g., by HLA typing for predisposition) in order to improve the benefit/risk profile of therapy and make more patients eligible for treatment [8]. As such, there is an unmet need to improve risk management and perception of allopurinol safety.
Pharmacogenomic studies have demonstrated that individuals with the human leukocyte antigen HLA-B*5801 are at a higher risk of developing SJS/TEN [9-13]. Odds ratios for the association between HLA-B*5801 and SJS/TEN have been reported as high as 580 [12,13]. The prevalence of HLA-B*5801 has been found to vary by race/ethnicity and geographic region. In the US, Caucasians and Hispanics have a low prevalence of HLA-B*5801 (<1%), whereas the prevalence is higher among African Americans (3.8%) and Asians (7.4%) [1,14].
The 2012 American College of Rheumatology (ACR) guidelines recommend testing for HLA-B*5801 prior to the initiation of allopurinol in high-risk patients such as those of Korean, Thai, and Han Chinese descent [1]. The guidelines do not address the utility of testing among other minority races/ethnic groups or the cost-effectiveness of any of their recommendations including HLA-B*5801 testing. To address this key knowledge gap, we evaluated the cost-effectiveness of testing for HLA-B*5801 prior to the initiation of allopurinol according to race/ethnicity in the US.
MATERIALS AND METHODS
Model Design and Study Population
We adapted a previously developed Markov model with a cycle length of 1 month to evaluate the cost-effectiveness of universal testing for HLA-B*5801 compared to no testing prior to the initiation of allopurinol according to race/ethnicity (i.e., Caucasians, Hispanics, African Americans, and Asians) in the US [15]. Both options allowed sequential use of febuxostat if allopurinol was ineffective or not tolerated. We did not include options with universal use of febuxostat as the first urate-lowering therapy, expect for positive HLA-B*5801 carriers in the testing strategy, as this was not cost-effective (dominated by current options) as a first-line treatment strategy in our previous analysis. As per the ACR guidelines, allopurinol was not considered as a treatment for positive HLA-B*5801 carriers in the testing strategy [1]. In addition, our base-case analysis focused on the use of fixed dose allopurinol (300mg) and febuxostat (80mg) as these are the most commonly prescribed doses and comparative efficacy data are available directly from source randomized trials [16-21]. However, in a sensitivity analysis we evaluated the potential effect of a reduction in AHS that could be achieved from using an allopurinol dose up-titration strategy. We evaluated costs and quality-adjusted life-years (QALYs) associated with each strategy over the life of a cohort of simulated gout patients aged 53 years; characteristics that are consistent with the study population of the source clinical trials [18-21].
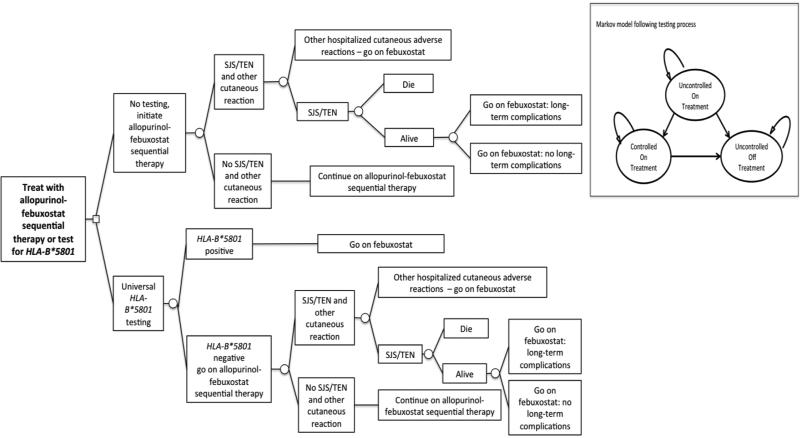
For each strategy, Figure 1 depicts the testing process, risk of SJS/TEN, and treatment assignment. Simulated gout patients in the testing strategy receive a polymerase chain reaction test for HLA-B*5801 prior to the initiation of therapy [1]. Patients who test positive for HLA-B*5801 are treated with febuxostat. Patients who test negative for HLA-B*5801 and all patients in the no testing strategy are treated with allopurinol-febuxostat sequential therapy. Regardless of strategy, once patients begin allopurinol, they can experience SJS/TEN [4]. Patients who experience SJS/TEN face an added risk of death [6]. Patients who do not die due to SJS/TEN are switched to febuxostat. Patients who do not die after SJS/TEN can develop long-term complications [7]. Finally, patients on allopurinol and febuxostat could experience related adverse events as reported in source clinical trials (detailed non-SJS/TEN adverse event rates are reported in our prior study) [15,18-21].
Figure 1. Decision Tree.
Figure Legend: Abbreviations: SJS, Stevens-Johnson Syndrome; TEN, Toxic Epidermal Necrolysis. The decision tree depicts the testing process, risk of SJS/TEN, and treatment assignment following SJS/TEN and other cutaneous reactions. The Markov model depicts the model states. Following the testing process, patients enter the model uncontrolled on therapy and can transition to controlled on therapy or uncontrolled off therapy.
Patients can die from any state.
As in our published study, [15] we assumed that all patients started with uncontrolled gout at the beginning of treatment (Markov model embed in Figure 1). With treatment they could become controlled (SUA<6 mg/dl), remain uncontrolled (SUA≥6 mg/dl), discontinue treatment and be uncontrolled, or die of age- and sex-related causes. Transitions between health states (e.g., moving between uncontrolled on treatment and controlled on treatment) were based on data obtained from source clinical trials (detailed transition probabilities are reported in our prior study) [15,18-21].
Prevalence of HLA-B*5801 According to Race/Ethnicity and SJS/TEN
Table 1 details model parameters for variables relevant to SJS/TEN. We estimated the prevalence of HLA-B*5801 specifically in the US by race/ethnicity (Caucasians and Hispanics 0.7%; African Americans 3.8%; and Asians 7.4%) using data from the Allele Frequency Net Database, a global repository for gene frequencies [14]. Allele frequencies, especially those for Africans and Asians, are reported to be higher in more homogenous nations [14]. Using data from Kim et al. we identified the probability of developing hospitalized severe cutaneous adverse reactions (ICD-9-CM 695.1x) in Caucasians (0.000294) [4]. We used 2009-2013 data from the National Inpatient Sample (NIS) to determine the proportion of patients who had been hospitalized with SJS/TEN and other hospitalized cutaneous adverse reactions (ICD-9-CM 695.13-15 and the rest of 695.1x, respectively) [22]. NIS provides nationally representative inpatient data on hospitalizations in the US, as reported to the Healthcare Costs and Utilization Project. Using the same NIS data, we estimated the risk in Hispanics, African Americans, and Asians using rate ratios of hospitalized SJS/TEN relative to Caucasians in the US (rate ratio for Hispanics 1.0; African Americans: 5.2; Asians: 11.4) [23]. We calculated positive and negative predictive values of SJS/TEN based on HLA-B*5801 test characteristics from a meta-analysis performed by the US Food and Drug Administration [12].
Table 1.
Model Inputs
| Model Estimate | Base-Case Estimate (Range) | Reference |
|---|---|---|
| Prevalence of HLA-B*5801 and Test Characteristics | ||
| Caucasians and Hispanics | 0.7% (0.4% – 1.0%) | [14] |
| African Americans | 3.8% (2.0% – 6.0%) | [14] |
| Asians | 7.4% (4.4% – 13.0%) | [14] |
| Sensitivity | 0.8417 (0.75 – 1.00) | [12] |
| Specificity | 0.9538 (0.85 – 1.00) | [12] |
| Probability and Complications of SJS/TEN | ||
| Caucasians and Hispanics | ||
| Probability of SJS/TENa | 0.00026 (0.00013 – 0.00039) | [4] |
| Risk of SJS/TEN if HLA-B*5801 Positive | 0.00472 (0.00129 – 1.00) | Calculated |
| Risk of SJS/TEN if HLA-B*5801 Negative | 0.00004 (0.00 – 0.00008) | Calculated |
| African Americans | ||
| Probability of SJS/TENb | 0.00136 (0.00068 - 0.00204) | [4,23] |
| Risk of SJS/TEN if HLA-B*5801 Positive | 0.02421 (0.00676 – 1.00) | Calculated |
| Risk of SJS/TEN if HLA-B*5801 Negative | 0.00022 (0.00 – 0.00040) | Calculated |
| Asians | ||
| Probability of SJS/TENc | 0.00298 (0.00149 – 0.00447) | [4,23] |
| Risk of SJS/TEN if HLA-B*5801 Positive | 0.05164 (0.01472 – 1.00) | Calculated |
| Risk of SJS/TEN if HLA-B*5801 Negative | 0.00050 (0.00 – 0.00087) | Calculated |
| Probability Death due to SJS/TENd | 0.30 (0.15 – 0.45) | [4] |
| Probability Long Term Complications due to SJS/TEN | 0.19 (0.10 – 0.29) | [7] |
| Utilitye | ||
| SJS/TEN | 0.35 (0.22 – 0.48) | [24] |
| Other Hospitalized Cutaneous Adverse Reactions | 0.53 (0.27 – 0.68) | Clinical Assumption |
| Long Term Complications from SJS/TEN | 0.68 (0.57 – 0.79) | [25] |
| Costs | ||
| HLA-B*5801 Testing | $129 ($65 - $258) | CPT Code 81381 |
| SJS/TEN | $45,661 ($22,830 - $68,491) | [22] |
| Other Hospitalized Cutaneous Adverse Reactions | $6,180 ($3,090 - $9,270) | [22] |
| Long Term Complications From SJS/TENf | $980 ($945 - $1,012) | [27] |
| Allopurinolg | $72 ($35 - $107) | [14] |
| Febuxostatg | $2,213 ($1,111 - $3,336) | [14] |
Abbreviations: SJS, Stevens-Johnson Syndrome; TEN, Toxic Epidermal Necrolysis
Risk of SJS/TEN and other hospitalißzed cutaneous adverse reactions is 0.000294 and based on NIS data it was estimated that 0.88 of events are SJS/TEN and 0.12 are other hospitalized cutaneous adverse reactions. In the first month of treatment the probability of SJS/TEN and other hospitalized cutaneous adverse reactions is 0.00013, between the 2nd and 6th month the probability is 0.00014, and in the last 6 months the probability is 0.00003.
Calculated based on a relative risk ratio (5.2) of SJS/TEN in African Americans compared to Caucasians
Calculated based on a relative risk ratio (11.4) of SJS/TEN in Asians compared to Caucasians
Kim et al. reports a mortality rate of 0.27 for SJS/TEN and other hospitalized cutaneous adverse reactions. We assumed only patients that experienced SJS/TEN events could die. The probability of death conditional on experiencing SJS/TEN is 0.30.
Multiplicative assumption was made when combing SJS/TEN utilities with gout symptoms. For example, the overall utility of a gout patient with long term complications (utility 0.68) and uncontrolled gout on medication (0.70) was 0.476 (0.68 * 0.70).
Annual cost of managing long-term complications is $980
Annual drug cost
Health-Related Quality-of-Life
We assigned a health-related quality-of-life weight of 0.35 and 0.53 for individuals who experienced SJS/TEN and other hospitalized cutaneous adverse reactions respectively (Table 1) [24]. These health-related quality-of-life weights were assumed to persist for the length of an SJS/TEN and other hospitalized cutaneous adverse reactions (1 month). After SJS/TEN and other hospitalized cutaneous adverse reactions, simulated patients switched to febuxostat, but patients who had a SJS/TEN could also experience long-term complications (e.g., dry eye syndrome, chronic kidney disease) [7]. We assumed that patients with long-term complications experienced a health utility of 0.68 [25]. As has been previously done, [25,26] we used a multiplicative approach to determine quality of life for controlled and uncontrolled patients with long-term complications. Patients who did not experience allopurinol SJS/TEN or other hospitalized cutaneous adverse events continued on their respective treatment strategy and experienced utilities associated with the effect of each strategy [15].
Costs
Costs were evaluated from the perspective of a US healthcare payer and adjusted to reflect 2016 US dollars using the medical care portion of the Consumer Price Index. The cost of testing for HLA-B*5801 by polymerase chain reaction was $129 (CPT code 81381 Centers for Medicare & Medicaid Services clinical laboratory fee schedule). We used 2009-2013 data from the NIS to determine hospital charges of patients who had been hospitalized with SJS/TEN and other hospitalized cutaneous adverse reactions [4,22]. We then calculated the cost associated with each type of event using cost-to-charge ratios supplied by the NIS. The costs of treating SJS/TEN and other hospitalized cutaneous adverse reactions were $45,661 and $6,180, respectively. Finally, it was estimated to cost $980 annually to manage long-term complications from SJS/TEN [7,27].
Cost-Effectiveness Analysis
To evaluate the incremental-cost effectiveness of testing for HLA-B*5801 we calculated the additional cost and benefit of testing compared to no testing (referent). We calculated the incremental cost-effectiveness ratio (ICER) by dividing the additional cost by the additional benefit to represent the cost per additional QALY gained from testing. All future costs and benefits were discounted by 3% annually to account for the time preference of money and health. ICERs with a value less than $109,000 per QALY were considered cost-effective, as it corresponds with recent recommendations for willingness-to-pay (WTP) thresholds in the US [28].
Sensitivity Analyses
To test for the effect of uncertainty on ICERs we conducted 1-way, 2-way, and structural sensitivity analyses. Furthermore, we conducted a probabilistic sensitivity analysis to evaluate the effect of simultaneous change of input variables. Probabilities and utilities were assigned beta distributions. Costs were assigned gamma distributions.
In particular, our sensitivity analyses addressed the impact of reducing the overall risk of developing SJS/TEN. For example, it may be possible to stratify the risk of SJS/TEN using available non-genetic predictors or slow up-titration of dose (e.g., starting patients on a dose of allopurinol 100 mg and then up titrating by 100 mg) [3,29,30]. Under this scenario we also examined the impact of up to a 5% increase in cost to account for potential additional resource utilization during the titration phase. As our analysis is over a lifetime time horizon, the titration phase is relatively short and the estimated cost is small (~1% of total lifetime costs). We also addressed the impact of natural variation in the prevalence of HLA-B*5801 and background SJS/TEN risk simultaneously. Finally, we conducted a structural sensitivity analysis assuming HLA-B*5801 predicted risk of SJS/TEN and other hospitalized cutaneous adverse reactions.
RESULTS
Base-Case Analyses
Compared to the no testing strategy, the strategy evaluating universal HLAB*5801 testing cost more and was more effective for all races/ethnicities that were analyzed (Table 2). However, the ICERs varied substantially across racial/ethnic groups depending on the prevalence of HLA-B*5801 (Table 2). For Caucasians and Hispanics (HLA-B*5801 prevalence 0.7%) the ICER of testing compared to no testing was $183,720 per QALY. As the prevalence of HLA-B*5801 and risk of SJS/TEN increased, the ICER of testing compared to no testing decreased. For African Americans (HLA-B*5801 prevalence 3.8%) the ICER was $83,450 per QALY and for Asians (HLA-B*5801 prevalence 7.4%) the ICER was $64,190 per QALY. Given a WTP threshold of $109,000 per QALY, testing for African Americans and Asians was cost-effective [1,28]
Table 2.
Results from the Base-Case Analysis
| Lifetime Costs, $ | Incremental Costs, $ | QALYs | QALYs Gained | ICER, $/QALY | |
|---|---|---|---|---|---|
| Caucasians and Hispanics (Prevalence HLA-B*5801 0.007, SJS/TEN Risk 0.00026) | |||||
| No testing, initiate allopurinol-febuxostat sequential therapy | $23,777 | 13.2248 | Reference | ||
| Universal HLA-B*5801 testing | $23,966 | $189 | 13.2258 | 0.0010 | $183,720 |
| African Americans (Prevalence HLA-B*5801 0.038, SJS/TEN Risk 0.00136) | |||||
| No testing, initiate allopurinol-febuxostat sequential therapy | $23,826 | 13.2205 | Reference | ||
| Universal HLA-B*5801 testing | $24,280 | $454 | 13.2259 | 0.0054 | $83,450 |
| Asians (Prevalence HLA-B*5801 0.074, SJS/TEN Risk 0.00298) | |||||
| No testing, initiate allopurinol-febuxostat sequential therapy | $23,898 | 13.2141 | Reference | ||
| Universal HLA-B*5801 testing | $24,648 | $750 | 13.2257 | 0.0117 | $64,190 |
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; SJS, Stevens-Johnson Syndrome; TEN, Toxic Epidermal Necrolysis
Sensitivity Analyses
For Caucasians, Hispanics, African Americans, and Asians overall results were robust to the variation of model parameters in the one-way sensitivity analyses, except for variation in background SJS/TEN risk and the cost of febuxostat (Table 3). Such reductions in the risk of SJS/TEN could be achieved from a titration strategy. For African Americans and Asians, as the risk of SJS/TEN decreased by half of the base-case risk, the ICER of testing approached $137,330 and $109,030 per QALY, respectively. However, for Caucasians and Hispanics ICERs were not materially different from that of the base-case analysis. Further, a 5% increase in cost combined with a 50% reduction in AHS risk did not alter our conclusions. Finally, when the cost of febuxostat increased by 50% the ICER of testing for African Americans approached $118,000. Change in the price of febuxostat did not substantially alter the ICERs for Caucasians and Hispanics or Asians. Results did not change materially with variation in health utilities or costs for SJS/TEN or long-term complications. Finally, results of our structural sensitivity analysis that assumed HLA-B*5801 predicted risk of SJS/TEN and other hospitalized cutaneous adverse reactions were similar to our base-case (ICERs for Caucasians/Hispanics $163,200; African Americans $72,010; Asians $54,870).
Table 3.
One-way Sensitivity Analysis
| Caucasians/Hispanics (base-case ICER of universal HLA-B*5801 testing $183,720) | African Americans (base-case ICER of universal HLA-B*5801 testing $83,450) | Asians (base-case ICER of universal HLA-B*5801 testing $64,190) | ||||
|---|---|---|---|---|---|---|
| Test Characteristics and Probability and Complications of SJS/TEN (base-case; low range, high range) | ||||||
| Sensitivity (0.8417; 0.74 , 1.00) | $201,790 | $158,930 | $91,800 | $71,930 | $70,680 | $55,150 |
| Specificity (0.9538; 0.85 , 1.00) | $187,250 | $182,420 | $85,080 | $82,850 | $65,470 | $63,720 |
| Death due to SJS/TEN (0.30; 0.15 , 0.45) | $261,170 | $124,030 | $117,470 | $65,040 | $91,310 | $49,840 |
| Probability of SJS/TEN (Caucasians and Hispanics: 0.00026; 0.00013 , 0.00039) (African Americans: 0.00136; 0.00068 , 0.00204) (Asians: 0.00298; 0.00149 , 0.00447) | $298,140 | $131,080 | $137,330 | $57,820 | $109,030 | $43,400 |
| Long Term Complications from SJS/TEN (0.19; 0.10 , 0.29) | $192,580 | $175,600 | $87,630 | $79,620 | $67,610 | $61,070 |
| Utility (base-case; low range, high range) | ||||||
| SJS/TEN (0.35; 0.22 , 0.48) | $183,490 | $183,900 | $83,350 | $83,550 | $64,110 | $64,270 |
| Other Hospitalized Cutaneous Adverse Reactions (0.53; 0.27 , 0.68) | $183,660 | $183,750 | $83,420 | $83,470 | $64,170 | $64,200 |
| Long Term Complications from SJS/TEN (0.68; 0.57 , 0.79) | $177,950 | $189,870 | $80,850 | $86,220 | $62,130 | $66,390 |
| Cost (base-case; low range, high range) | ||||||
| SJS/TEN ($45,661; $22,830 , $68,491) | $187,400 | $180,030 | $87,100 | $79,800 | $67,970 | $60,410 |
| Other Hospitalized Cutaneous Adverse Reactions ($6,180; $3,090 , $9,270) | $183,780 | $183,650 | $83,520 | $83,380 | $64,260 | $64,120 |
| Long Term Complications SJS/TEN ($980; $945 , $1,012) | $183,740 | $183,710 | $83,470 | $83,440 | $64,210 | $64,180 |
| Allopurinol ($72; $35 , $107) | $184,780 | $182,730 | $84,540 | $82,440 | $65,170 | $63,280 |
| Febuxostat ($2,213; $1,111 , $3,336) | $149,880 | $218,350 | $48,780 | $118,930 | $32,800 | $96,320 |
Abbreviations: ICER, incremental costeffectiveness ratio; SJS, Stevens-Johnson Syndrome; TEN, Toxic Epidermal Necrolysis
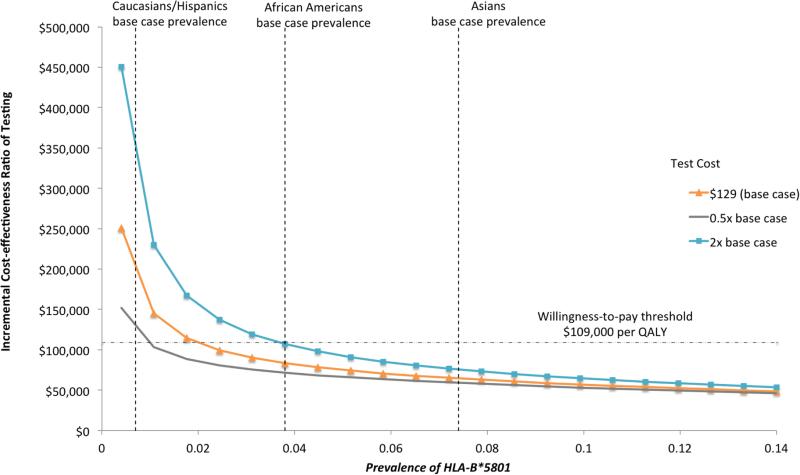
Results from the 2-way sensitivity analysis are presented in Figure 2 and illustrate the impact of a simultaneous change in the prevalence of HLA-B*5801 and the cost of testing (base-case value $129). As the prevalence of HLA-B*5801 increased, the ICER of testing decreased. In the base-case, universal testing was a cost-effective strategy when the prevalence of HLA-B*5801 was greater than 1.6% and WTP was $109,000 per QALY. As the cost of the test increased, universal testing became less cost-effective. For testing to be cost-effective in Caucasians and Hispanics, the test would have to cost less than $52.
Figure 2.
Two-way Sensitivity Analysis by Prevalence of HLA*5801 According to Race/Ethnicity and the Cost of HLA-B*5801 Test
Results from the probabilistic sensitivity analysis varying all input variables indicate that at a WTP of $109,000 per QALY testing was cost-effective in 9%, 77%, and 88% of simulations for Caucasians and Hispanics, African Americans, and Asians, respectively (eFigure 1). The 25th and 75th percentile ICERs of testing were $131,780 and $226,040 for Caucasians and Hispanics, $60,910 and $105,250 for African Americans, and $47,090 and $83,540 for Asians.
DISCUSSION
Our objective was to assess the cost-effectiveness of testing for HLA-B*5801 prior to the initiation of allopurinol in gout patients according to race/ethnicity in the US. As expected, cost-effectiveness varied substantially across racial/ethnic groups, following their HLA-B*5801 prevalences. HLA-B*5801 testing was cost-effective for African Americans and Asians, but not for Caucasians or Hispanics for a WTP of $109,000 per QALY. Overall, when the prevalence of HLA-B*5801 is greater than 1.6% testing is cost-effective. These cost-effectiveness results support the latest ACR guideline recommendations for Asians and Caucasians [1] and further extend to the two largest minority groups in the US, namely African Americans and Hispanics. Results also highlight the importance of taking a detailed clinical and demographic history to assess patients for increased AHS risk.
In the US the prevalence of HLA-B*5801 in African Americans is 3.8% and our analysis suggests that testing in this population is cost-effective. HLA-B*5801 frequency also varies within the same race by geographic region. For example, in Black Kenyans the population frequency of HLA-B*5801 is between 7 and 10% [14]. In populations with a high HLA-B*5801 prevalence, testing is expected to be even more cost-effective, although the results of the current analysis are specific to the US reflecting US-specific costs of testing and treatment and model parameters.
Similarly, the prevalence of HLA-B*5801 among Asians (i.e., 7.4%) [14] combines many diverse US Asian populations into one category. Thus, our results should be interpreted as the average effect of these diverse Asian groups, corresponding to the allele frequency estimates of HLA-B*5801 among US Asians. To that effect, it should be noted that the US Japanese population has a low allele frequency of HLA-B*5801 (0.8%), similar to the Japanese in Japan (0.6%)[14]. Thus, although we did not specifically analyze US Japanese individuals, we expect that their cost-effectiveness would be similar to that of Caucasians and Hispanics.
We are not aware of any previous studies that addressed the cost-effectiveness of HLA-B*5801 in the US; however, several Asian studies have addressed the topic in their specific Asian settings. Testing for HLA-B*5801 was found to be cost-effective in a relatively young Thai gout population (base-case age = 30 years) and Korean population with renal impairment [25,31]. Although both the Thai and Korean studies did not account for the benefit of ULT on gout morbidity in their modeling, unlike our analysis. In contrast, a Singapore study using an ethnically weighted prevalence of HLA-B*5801 frequency (Chinese, Malays, and Indians) and a sensitivity and specificity of HLA-B*5801 from a Chinese study (i.e., 100% and 85%, respectively) reported that the testing is not cost-effective [32]. Notably, our analyses employed summary sensitivity and specificity estimates from a US Food and Drug Administration systematic review and meta-analysis [12]. Furthermore, the Singapore study used probenecid as the alternative ULT whenHLA-B*5801 was positive, whereas our analysis used febuxostat. These differences and a higher WTP threshold in our study could explain the differing conclusions.
Overall, our sensitivity analysis results were robust except that of reducing background risk of SJS/TEN and increasing the cost of febuxostat. While the risk of SJS/TEN in our base-case reflected the current average practice, [1,16,17] our sensitivity analyses addressed the impact of lowering background SJS/TEN risk through different measures. For example, better risk prediction using various markers before engaging in genetic testing can lower the overall background risk of SJS/TEN [3,29]. Also, there is further indication that the risk of SJS/TEN in all races/ethnicities can be avoided through a slow up-titration of allopurinol dose [30]. While this suggests a potential strategy to mitigate SJS/TEN, uric acid achieved with this protocol was above the therapeutic level (>7 mg/dL) and urate saturation point. Nevertheless, our sensitivity analysis for these scenarios found that halving the risk of SJS/TEN increased the ICER associated with testing such that even the ICER exceeded the WTP threshold of $109,000 per QALY for African Americans and Asians. These findings suggest that if we can lower background SJS/TEN risk enough in a practically implementable way, without additional cost incurrence, universal HLA-B*5801 testing could become less or even not cost-effective at some point for all races. Future studies should quantify the magnitude of potential AHS risk mitigation associated with an up-titration strategy.
Finally, it should be noted that our model results are specific to patients where allopurinol is considered as a first-line treatment option. In clinical practice, some physicians may already bypass allopurinol due specific patient characteristic (e.g., renal failure) and/or the perceived risk of AHS. In such cases, HLA-B*5801 testing would not be needed as the physician has made an internal calculation regarding the risk of allopurinol.
Our analysis is not without limitations. Our main objective was to address the most commonly used urate-lowering therapy (allopurinol-febuxostat sequential therapy). However, several other options and strategies of urate-lowering therapy are available, including probenecid, combination urate-lowering therapy (for example, allopurinol or febuxostat combined with probenecid), and pegloticase. The choice of alterative urate-lower therapy and its efficacy relative to allopurinolfebuxostat sequential therapy may impact cost-effectiveness ratios. Although in the US these options are less commonly prescribed in current practice, future comprehensive cost-effectiveness analyses could incorporate them. While our study is focused on evaluating the cost-effectiveness of testing for HLA-B*5801 according to race/ethnicity, there are other potentially important non-genetic risk factors, including chronic kidney disease, perhaps female sex, and comorbid cardiovascular disease [3-5,29]. In our analyses, the effect of these other risk factors is modeled as a part of the underlying background SJS/TEN risk, whose impact is discussed above. Future studies should investigate the interaction between multiple risk factors and SJS/TEN risk, as those specific source data become available. Finally, this study, like most cost-effectiveness studies, is applicable to the population analyzed (i.e., the US); results may not be extrapolated to other countries, where costs for testing and treatment differ.
In conclusion, our race/ethnicity-stratified cost-effectiveness analysis suggests that testing for HLA-B*5801 prior to allopurinol initiation in the US is cost effective among African Americans and Asians, but not among Caucasians or Hispanics. If the overall SJS/TEN risk can be reduced, testing for HLA-B*5801 becomes less cost-effective.
Supplementary Material
Acknowledgements
Funding/Support: Dr. Dubreuil is supported by the Arthritis Foundation CRTA. Dr. Choi is supported by NIH (NIAMS) grants R01-AR056291, R01-AR065944, and R21 AR056042.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: Dr. Choi has received a research grant from Astra-Zeneca and served as a research consultant for Takeda, both unrelated to this manuscript. All other authors have declared no conflicts of interest.
References
- 1.Khanna D, FitzGerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: Systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care & Research. 2012;64:1431–46. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McInnes GT, Lawson DH, Jick H. Acute adverse reactions attributed to allopurinol in hospitalised patients. Ann Rheum Dis. 1981;40:245–9. doi: 10.1136/ard.40.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stamp LK, Taylor WJ, Jones PB, Dockerty JL, Drake J, Frampton C, et al. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: A proposed safe starting dose of allopurinol. Arthritis & Rheumatism. 2012;64:2529–36. doi: 10.1002/art.34488. [DOI] [PubMed] [Google Scholar]
- 4.Kim SC, Newcomb C, Margolis D, Roy J, Hennessy S. Severe cutaneous reactions requiring hospitalization in allopurinol initiators: A population-based cohort study. Arthritis Care & Research. 2013;65:578–84. doi: 10.1002/acr.21817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang C-Y, Chen C-H, Deng S-T, Huang C-S, Lin Y-J, Chen Y-J, et al. Allopurinol use and risk of fatal hypersensitivity reactions: a nationwide population-based study in Taiwan. JAMA Intern Med. 2015;175:1550–7. doi: 10.1001/jamainternmed.2015.3536. [DOI] [PubMed] [Google Scholar]
- 6.Arellano F, Sacristan JA. Allopurinol hypersensitivity syndrome: a review. Ann Pharmacother. 1993;27:337–43. doi: 10.1177/106002809302700317. [DOI] [PubMed] [Google Scholar]
- 7.Gueudry J, Roujeau J-C, Binaghi M, Soubrane G, Muraine M. Risk factors for the development of ocular complications of Stevens-Johnson syndrome and toxic epidermal necrolysis. Archives of Dermatology. 2009;145:157–62. doi: 10.1001/archdermatol.2009.540. [DOI] [PubMed] [Google Scholar]
- 8.Shekelle PG, FitzGerald J, Newberry SJ, Motala A, O'Hanlon CE, Okunogbe A, Tariq A, Han D, Dudley W, Shanman R, Booth M. Management of Gout. Comparative Effectiveness Review No. 176. (Prepared by the RAND Southern California Evidence-based Practice Center under Contract No. 290-2012-00006-I.) AHRQ Publication No.16-EHC017-EF. Agency for Healthcare Research and Quality; Rockville, MD: 2016. pp. 1–198. March 2016. www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 9.Yeo SI. HLA-B* 5801: utility and cost-effectiveness in the Asia-Pacific Region. International Journal of Rheumatic Diseases. 2013;16:254–7. doi: 10.1111/1756-185X.12050. [DOI] [PubMed] [Google Scholar]
- 10.Hung S-I, Chung W-H, Liou L-B, Chu C-C, Lin M, Huang H-P, et al. HLA-B* 5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4134–9. doi: 10.1073/pnas.0409500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tassaneeyakul W, Jantararoungtong T, Chen P, Lin P-Y, Tiamkao S, Khunarkornsiri U, et al. Strong association between HLA-B*5801 and allopurinol-induced Stevens–Johnson syndrome and toxic epidermal necrolysis in a Thai population. Pharmacogenetics and Genomics. 2009;19:704–9. doi: 10.1097/FPC.0b013e328330a3b8. [DOI] [PubMed] [Google Scholar]
- 12.Zineh I, Mummaneni P, Lyndly J, Amur S, La Grenade LA, Chang SH, et al. Allopurinol pharmacogenetics: assessment of potential clinical usefulness. Pharmacogenomics. 2011;12:1741–9. doi: 10.2217/pgs.11.131. [DOI] [PubMed] [Google Scholar]
- 13.Somkrua R, Eickman EE, Saokaew S, Lohitnavy M, Chaiyakunapruk N. Association of HLA-B*5801 allele and allopurinol induced stevens johnson syndrome and toxic epidermal necrolysis: a systematic review and meta-analysis. BMC Medical Genetics. 2011;12:118. doi: 10.1186/1471-2350-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Galarza FF, Takeshita LYC, Santos EJM, Kempson F, Maia MHT, Silva ALSD, et al. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Research. 2015;43:D784–8. doi: 10.1093/nar/gku1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jutkowitz E, Choi HK, Pizzi LT, Kuntz KM. Cost-Effectiveness of Allopurinol and Febuxostat for the Management of Gout. Annals of Internal Medicine. 2014;161:617. doi: 10.7326/M14-0227. [DOI] [PubMed] [Google Scholar]
- 16.Sarawate CA, Patel PA, Schumacher HR, Yang W, Brewer KK, Bakst AW. Serum urate levels and gout flares: analysis from managed care data. Journal of Clinical Rheumatology. 2006;12:61–5. doi: 10.1097/01.rhu.0000209882.50228.9f. [DOI] [PubMed] [Google Scholar]
- 17.Sarawate CA, Brewer KK, Yang W, Patel PA, Schumacher HR, Saag KG, et al. Gout medication treatment patterns and adherence to standards of care from a managed care perspective. Mayo Clin Proc. 2006;81:925–34. doi: 10.4065/81.7.925. [DOI] [PubMed] [Google Scholar]
- 18.Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450–61. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 19.Schumacher HR, Jr, Becker MA, Lloyd E, MacDonald PA, Lademacher C. Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology. 2009;48:188–94. doi: 10.1093/rheumatology/ken457. [DOI] [PubMed] [Google Scholar]
- 20.Schumacher HR, Jr, Becker MA, Wortmann RL, MacDonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: A 28- week, phase III, randomized, double-blind, parallel-group trial. Arthritis & Rheumatism. 2008;59:1540–8. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 21.Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis research & therapy. 2010;12:1–12. doi: 10.1186/ar2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP), Agency for Healthcare Research and Quality. [PubMed]
- 23.Lu N, Rai SK, Terkeltaub R, Kim SC, Menendez ME, Choi HK. Racial Disparities in the Risk of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis as Urate-Lowering Drug Adverse Events in the US. Seminars in Arthritis and Rheumatism. 2016 doi: 10.1016/j.semarthrit.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez J-LA, Pereperez SB, Bastida JL, Martinez MM. Cost-utility analysis applied to the treatment of burn patients in a specialized center. Archives of Surgery. 2007;142:50. doi: 10.1001/archsurg.142.1.50. [DOI] [PubMed] [Google Scholar]
- 25.Saokaew S, Tassaneeyakul W, Maenthaisong R, Chaiyakunapruk N. Cost-Effectiveness Analysis of HLA-B*5801 Testing in Preventing Allopurinol-Induced SJS/TEN in Thai Population. PLoS ONE. 2014;9:e94294. doi: 10.1371/journal.pone.0094294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ara R, Brazier J. Comparing EQ-5D scores for comorbid health conditions estimated using 5 different methods. Med Care. 2012;50:452–9. doi: 10.1097/MLR.0b013e318234a04a. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30:379–87. doi: 10.1097/ICO.0b013e3181f7f363. [DOI] [PubMed] [Google Scholar]
- 28.Owens DK, Qaseem A, Chou R, Shekelle P, Clinical Guidelines Committee of the American College of Physicians High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Annals of Internal Medicine. 2011;154:174–80. doi: 10.7326/0003-4819-154-3-201102010-00007. [DOI] [PubMed] [Google Scholar]
- 29.Hande KR, Noone RM, Stone WJ. Severe allopurinol toxicity: description and guidelines for prevention in patients with renal insufficiency. The American Journal of Medicine. 2003;76:47–56. doi: 10.1016/0002-9343(84)90743-5. [DOI] [PubMed] [Google Scholar]
- 30.Jung J-W, Kim D-K, Park H-W, Oh K-H, Joo KW, Kim Y-S, et al. An effective strategy to prevent allopurinol-induced hypersensitivity by HLA typing. Genet Med. 2015;17:807–14. doi: 10.1038/gim.2014.195. [DOI] [PubMed] [Google Scholar]
- 31.Park D-J, Kang J-H, Lee J-W, Lee K-E, Wen L, Kim T-J, et al. Cost-Effectiveness Analysis of HLA-B5801 Genotyping in the Treatment of Gout Patients With Chronic Renal Insufficiency in Korea. Arthritis Care & Research. 2015;67:280–7. doi: 10.1002/acr.22409. [DOI] [PubMed] [Google Scholar]
- 32.Dong D, Tan-Koi W-C, Teng GG, Finkelstein E, Sung C. Cost–effectiveness analysis of genotyping for HLA-B*5801and an enhanced safety program in gout patients starting allopurinol in Singapore. Pharmacogenomics. 2015;15:125. doi: 10.2217/pgs.15.125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.