Abstract
Objectives
To determine if selected socio-demographic and HBV-specific clinical factors are associated with health-related quality of life (HRQoL) among pediatric patients chronically infected with hepatitis B virus (HBV).
Methods
Children with chronic HBV enrolled in the Hepatitis B Research Network completed the Child Health Questionnaire (CHQ) at study entry. Caregivers of children 5–<10 years completed the parent-reported PF50; youth 10–< 18 years completed the child-reported CF87. We examined univariable associations of CHQ scores with selected independent variables: sex, adoption status, maternal education, alanine aminotransferase (ALT, U/L), aspartate aminotransferase-to-platelet ratio index (APRI), and HBV-specific symptom count.
Results
244 participants (83 young children 5–<10y, 161 youth 10–<18y) were included, all HBV treatment naïve. Among young children, increased ALT level was negatively associated with PF50 psychosocial summary T-Score (r=−0.28, p=0.01). No other subscale comparisons for young children were statistically significant. Among youth, adoption was associated with better physical functioning and general health (p<0.01). Higher maternal education was associated with better role/functioning-physical and -emotional scores (p<0.05). Maternal education and adoption status were linked, with adoption associated with higher maternal education. Increased symptom count in youth was associated with worse HRQoL in subscales measuring bodily pain, behavior, mental health, and self-esteem (p<0.01).
Conclusions
Although overall HRQoL is preserved in children with chronic HBV, some socio-demographic and HBV-related clinical factors were associated with impaired HRQoL in our pediatric patients at baseline. Measurement of HRQoL can focus resources on education and psychosocial support in children and families most in need.
Keywords: Child Health Questionnaire, adoption, refugee, hepatitis B, quality of life, chronic medical condition, children
Introduction
Chronic hepatitis B (HBV) infection is associated with significant morbidity and mortality, with 25% of infected individuals developing cirrhosis and/or hepatocellular carcinoma (1, 2). Universal vaccination and prenatal screening in the US and Canada have decreased the incidence of chronic HBV in children born in these countries. However, new cases of childhood hepatitis B are seen in North America in children of immigrants and children adopted internationally (3–5). On average, 53,800 chronic HBV cases were imported to the US annually during 2004 – 2008 (4).
Adults with chronic HBV experience decreased health-related quality of life (HRQOL) (6) but there is little information about HRQOL in children with HBV. HRQoL is a complex and inherently subjective measure that is an important outcome of disease management. Impaired HRQoL has been associated with poor medication adherence, depression, anxiety and worse health outcomes (7). Importantly, patients and their families may make treatment decisions for chronic disease management based upon how treatments affect HRQoL and life functioning (8). The measurement of HRQoL in children with chronic HBV has the potential to allow early interventions that may reduce adult depression and increase adherence to therapies for HBV.
The Hepatitis B Research Network (HBRN) (9) provides a unique opportunity to study a large, well-characterized cohort of children with chronic HBV infection in the US and Canada (5). Using this cohort, we assessed HRQoL in children and youth with chronic HBV using an age-appropriate instrument, the Child Health Questionnaire (CHQ) (10). In this exploratory study, our aim was to describe the HRQoL of children with chronic HBV and their families, and to examine selected socio-demographic and disease-related factors associated with HRQoL in our cohort. This will contribute to the overarching goal of the Pediatric HBRN, to improve the lives of children with chronic HBV.
Methods
Seven pediatric hepatology centers in the US and Canada participated in the HBRN Pediatric Cohort study (5, 9). Subject inclusion criteria included age 6 months to <18 years and hepatitis B surface antigen (HBsAg)-positive. Exclusion criteria included history of hepatic decompensation, hepatocellular carcinoma, solid organ or bone marrow transplant, co-infection with human immunodeficiency virus, current HBV anti-viral therapy, or social or medical factors that would make follow-up difficult.
The HRQoL sub-study included pediatric HBRN participants ≥5 to <18 years of age who were HBV treatment naive. English literacy was required for caregivers of children <10 years of age and for children ≥10 years of age because translations were not available for several of the more common languages in the HBRN cohort. Participants for this analysis were enrolled between December 2010 and September 2015.
All sites had institutional review board approval. Written informed consent was obtained from parents or legal guardians; assent was obtained from children according to the requirements of the local institutional review board.
Measures
Socioeconomic and demographic characteristics
Demographic variables collected at entry into the study included age, sex, race, adoption status and measures of family socioeconomic status (e.g. maternal education).
Disease characteristics
Serum alanine aminotransferase (ALT, U/L) was recorded within 6 months of the baseline study visit. HBV DNA (IU/mL) and hepatitis e antigen (HBeAg) status were tested by a central laboratory. When central laboratory results were unavailable (e.g. due to inadequate sample), the most recent clinical results obtained within 1 year (HBV DNA) or 2 years (HBeAg) of baseline were used. Other laboratory values, including international normalized ratio (INR), albumin (g/dL), and direct bilirubin (mg/dL), were based on the latest values obtained within 1 year of baseline. On average, labs were taken <1.5 months prior to the baseline visit. Liver biopsies were not required as part of the cohort study; AST-to-platelet ratio index (APRI (11)) was calculated for each subject, with an AST of 40 U/L used as the upper limit of normal for this calculation to facilitate comparison with other studies using this index (12, 13). Symptom counts (0, 1, 2, 3+) were based on the following HBV-specific symptoms during the last month: fatigue, pain over liver, nausea, poor appetite, weight loss, itching, irritability, depression/sadness, jaundice, and dark urine.
Child Health Questionnaire (CHQ)
The CHQ was completed at the baseline visit. The CHQ is a validated instrument that measures generic HRQoL in the physical, psychological and social domains in children, including physical functioning (PF), bodily pain/discomfort (BP), general health (GH), general behavior (BE), mental health (MH), self-esteem (SE); role/social limitations as a result of physical (RP), emotional (RE), and behavioral (RB) problems; and the impact on parental time (PT), parental emotion (PE), and family activities (FA) (Table 1, Supplemental) (10). The CHQ-Parent Report Form (PF50) includes 11 subscales, while the CHQ-Child Report Form (CF87) includes ten subscales. Each subscale is calculated by summing items to a total score of 0–100; higher scores reflect better HRQoL. Specifically for the PF50, two additional summary T-scores (physical and psychosocial) were derived by aggregating 10 individual scales (omitting the family activities subscale), and were standardized using a linear T-score transformation (mean 50, standard deviation 10) from the combined general U.S. sample (N=391) and six clinical samples (N=563) (14). The PF50 was administered to biological or adoptive parents or legal guardians, henceforth referred to as caregivers of young children 5–<10 years of age. Children who were at least 10 years of age (identified below as “youth”) completed the CF87 themselves.
Table 1.
Demographic and Clinical Characteristics
| Characteristics | All N=244 |
Young children 5–<10 years N=83 |
Youth 10–<18 years N=161 |
|---|---|---|---|
| Age, in yearsa | N=244 | N=83 | N=161 |
| 11.89 (3.58) | 7.68 (1.49) | 14.05 (2.10) | |
| Sexb | N=244 | N=83 | N=161 |
| Male | 95 (38.9%) | 21 (25.3%) | 74 (46.0%) |
| Female | 149 (61.1%) | 62 (74.7%) | 87 (54.0%) |
| Raceb | N=242 | N=82 | N=160 |
| White | 15 (6.2%) | 3 (3.7%) | 12 (7.5%) |
| Black/African-American | 30 (12.4%) | 7 (8.5%) | 23 (14.4%) |
| Asian | 188 (77.7%) | 68 (82.9%) | 120 (75.0%) |
| Other | 9 (3.7%) | 4 (4.9%) | 5 (3.1%) |
| Adoptedb | N=244 | N=83 | N=161 |
| 131 (53.7%) | 57 (68.7%) | 74 (46.0%) | |
| Maternal Educationb,e | N=217 | N=71 | N=146 |
| Less than high school | 26 (12.0%) | 0 (0.0%) | 26 (17.8%) |
| High school or equivalent | 25 (11.5%) | 4 (5.6%) | 21 (14.4%) |
| Some college or equivalent | 45 (20.7%) | 17 (23.9%) | 28 (19.2%) |
| Bachelor’s degree or higher | 121 (55.8%) | 50 (70.4%) | 71 (48.6%) |
| ALT (U/L)c | N=240 | N=82 | N=158 |
| 40 (28, 54) | 38 (26, 51) | 40 (30, 54) | |
| (12, 2067) | (12, 365) | (13, 2067) | |
| HBeAgb | N=228 | N=76 | N=152 |
| Negative | 54 (23.7%) | 7 (9.2%) | 47 (30.9%) |
| Positive | 174 (76.3%) | 69 (90.8%) | 105 (69.1%) |
| HBV DNA (log10 IU/ml)d | N=229 | N=75 | N=154 |
| Median (25th percentile, 75th percentile) | 8.1 (5.3, 8.5) | 8.2 (7.8, 8.7) | 7.9 (4.0, 8.4) |
| < 3 | 32 (14.0%) | 6 (8.0%) | 26 (16.9%) |
| 3 – < 6 | 31 (13.5%) | 2 (2.7%) | 29 (18.8%) |
| 6 – < 8 | 40 (17.5%) | 13 (17.3%) | 27 (17.5%) |
| ≥8 | 126 (55.0%) | 54 (72.0%) | 72 (46.8%) |
| International Normalized Ratio (INR) c | N=189 | N=56 | N=133 |
| 1.0 (1.0, 1.1) | 1.0 (1.0, 1.0) | 1.00 (1.0, 1.1) | |
| (0.8, 4.1) | (0.9, 1.2) | (0.8, 4.1) | |
| Albumin (g/dL)c | N=218 | N=74 | N=144 |
| 4.4 (4.2, 4.6) | 4.4 (4.2, 4.6) | 4.3 (4.2, 4.6) | |
| (1.1, 5.4) | (1.1, 5.4) | (3.4, 5.2) | |
| Direct Bilirubin (mg/dL)c | N=180 | N=59 | N=121 |
| 0.08 (0.00, 0.10) | 0.10 (0.0, 0.10) | 0.00 (0.00, 0.12) | |
| (0.00, 0.99) | (0.00, 0.99) | (0.00, 0.82) | |
| APRI (AST-platelet-ratio index)c | N=208 | N=69 | N=139 |
| 0.34 (0.26, 0.52) | 0.37 (0.30, 0.52) | 0.33 (0.25, 0.50) | |
| (0.14, 12.31) | (0.15, 2.31) | (0.14, 12.31) | |
| Total Symptom Count | N=244 | N=83 | N=161 |
| 0 | 135 (55.3%) | 49 (59.0%) | 86 (53.4%) |
| 1 | 51 (20.9%) | 22 (26.5%) | 29 (18.0%) |
| 2 | 25 (10.2%) | 7 (8.4%) | 18 (11.2%) |
| 3+ | 33 (13.5%) | 5 (6.0%) | 28 (17.4%) |
Mean (SD)
The first row gives the number of non-missing observations, followed by N (%) in each category.
The first row gives the number of non-missing observations. The second row gives median (25th percentile, 75th percentile), and the third row gives the (minimum, maximum).
The first row gives the number of non-missing observations. The second row gives median (25th percentile, 75th percentile), and the remaining rows give N (%) for each category.
For one child with two adoptive mothers, maternal education for the mother with ‘some college or equivalent’ was randomly selected for inclusion in this analysis; the other mother had a Master’s Degree. Maternal education level was set to missing for one child with a mother who was deceased.
Statistical Analysis
Summary statistics were used to describe demographic and disease-related characteristics. Mean with standard deviation (SD), or median with (25th percentile, 75th percentile) and (minimum, maximum) were reported for continuous variables. Frequencies and proportions were reported for categorical variables. Mean (SD) and 95% confidence intervals (CI) were calculated for each CHQ subscale and summary T-score. CHQ subscales were presented as radar plots to allow easy visualization of the range of mean scores across multiple subscales. The plot radius indicates the score (much like a y-axis); points farther out on the radar plot indicate higher scores (i.e. higher quality of life). Descriptive characteristics are presented for the overall sample and separately by age group. For descriptive comparison, we included summary scores from a group of adolescent liver transplant recipients (7.5 ± 5.7 years post-transplant) (7) and a healthy pediatric population (15).
We examined univariable associations of CHQ scores with selected independent variables: sex, adoption status, maternal education, ALT (U/L), APRI, and HBV-specific symptom count. For categorical independent variables, CHQ summary T-scores were presented as mean (SD) by category; since subscale scores were skewed, median (25th percentile, 75th percentile) were presented. Moreover, because >75% of participants obtained the maximum score (100) on several subscales, we also presented the frequency and percent of participants with subscale scores below 100. Kruskal-Wallis non-parametric tests were used to compare the distribution of CHQ scores across levels of categorical variables. Spearman correlation coefficients, with corresponding p-values, were used to assess the correlation of CHQ scores with continuous independent variables (ALT and APRI).
Sensitivity Analysis
Because flares are associated with increased liver enzymes, and may be associated with increased symptoms and decreased HRQoL, we repeated our analysis after excluding 1 younger and 5 older children with ALT flares, which was defined as an ALT more than 10 times the ULN using the following age- and sex-specific ULN cut-points: 25 U/L for males and females 5–<13 year-olds, 25 U/L for males ≥13 years, and 22 U/L for females ≥13 years (16, 17). Results for these sensitivity analyses were similar to those obtained with the full sample, and thus are not reported.
Statistical significance was determined as p<0.05. For subscale score comparisons, Bonferroni-Holm adjusted p-values were calculated to indicate statistical significance after accounting for multiple comparisons across the 11 and 10 subscales for the PF50 and CF87, respectively. SAS 9.3 (SAS Institute, Cary NC, USA) was used for statistical analyses.
Results
Demographic and clinical characteristics
As of September 2015, 395 children were enrolled in the HBRN Pediatric Cohort study; 330 were at least 5 years of age. Of these, 280 were treatment naïve, and thus eligible to participate in the HRQoL study. 244 of these had CHQ data and were included in the analysis. The PF50 was available for 83 children 5–<10 years old, and the CF87 was available for 161 youth 10–<18 years old (see Table 1).
Demographic characteristics of 5–<10 year olds are found in Table 1.. Serum ALT levels ranged from 12–365 (median 39) U/L. The majority were HBeAg positive (91%), and 72% had HBV DNA levels ≥108 U/mL. INR ranged from 0.9–1.2, albumin 1.1–5.4 g/dL, and direct bilirubin 0.0–0.99 mg/dL. APRI median score was 0.37; four subjects (5.8%) had values above 1.0. Presumed mode of HBV transmission was vertical in 97%. Two or more of the queried symptoms were reported in 14% within the past 4 weeks of the baseline visit, most commonly itching (n=15), irritability (n=11), fatigue (n=7), depression/sadness (n=6), and nausea (n=6). None of the queried comorbid conditions were noted; although 6 children were currently taking medications, including bronchodilators (n=1), antihistamines (n=1), and ADHD medications (n=4).
Demographic characteristics of youth ≥10 years of age are found in Table 1.. Median ALT level was 41 (range: 13–2067) U/L. 69% were HBeAg positive, with high HBV DNA levels (≥108 IU/mL or above limits of quantification) in 47%. INR ranged from 0.8–4.1, albumin 3.4–5.2 g/dL, and direct bilirubin 0.0–0.82 mg/dL. Median APRI was 0.33, with values above 1.0 in six participants (4.3%). Vertical transmission was suspected in 95%. Nearly one-third (29%) reported 2 or more of the queried symptoms within the past 4 weeks, most commonly fatigue (n=44), irritability (n=37), itching (n=24), depression/sadness (n=20), poor appetite (n=20), nausea (n=16), pain over liver (n=13), and weight loss (n=10). Queried comorbid conditions were noted in 5 children: anemia (n=2), anemia and cytopenia (n=1), diabetes (n=1), and non-alcoholic fatty liver disease (n=1). Medication use was noted in 28 (4 of whom used multiple medications): bronchodilators (n=9), antihistamines (n=7), antidepressants/anxiolytics/antipsychotics (n=5), ADHD medication (n=4), acne medication (n=4), estrogen/birth control pills (n=2), and analgesic/pain medications (n=1).
HRQoL among children with chronic HBV
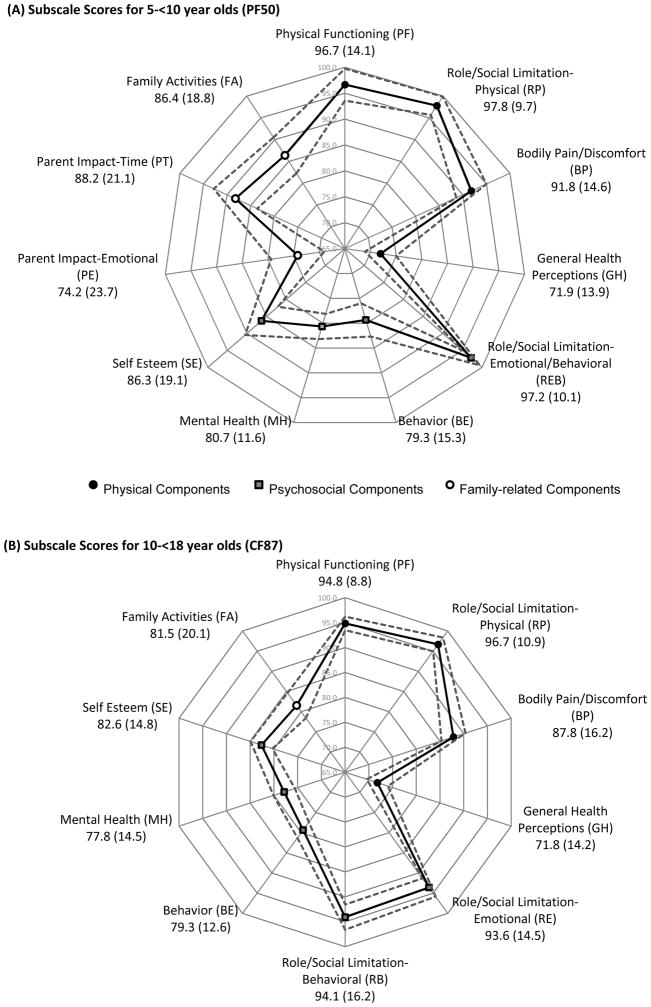
Among 5–<10 year-olds, mean T-scores for the two summary scales (physical and psychosocial) were 54.1 (SD 6.3) for the PHS, and 52.8 (SD 8.2) for the PSS (not shown). Mean subscale scores for subscales related to physical HRQoL ranged from a low of 71.9 (SD 13.9) for GH, to 97.8 (SD 9.7) for RP (Figure 1A). For subscales related to psychosocial HRQoL, mean scores ranged from 79.3 (SD 15.3) to 97.2 (SD 10.1) for the BE and REB subscales, respectively. Family-related components ranged from a mean of 74.2 (SD 23.7) to 88.2 (SD 21.1) on the PE and PT subscales, respectively.
Figure 1.
Mean scores (standard deviation) and 95% confidence interval of (A) subscale scores for 5–<10 year olds (PF50) and (B) subscale scores for 10–<18 year olds (CF87). Solid lines demonstrate mean scores and dashed lines indicate 95% confidence intervals.
In children ≥10 years old, mean physical HRQoL subscale scores ranged from 71.8 (SD 14.2) (GH) to 96.7 (SD 10.9) (RP), whereas mean psychosocial HRQoL subscale scores ranged from 77.8 (SD 14.5) for MH, to 94.1 (SD 16.2) for RB (Figure 1B). The FA subscale had a mean of 81.5 (SD 20.1).
Table 2a and b present mean (95% CI) PF 50 (a) and CF87 (b) subscale scores for the HBRN Pediatric Cohort, side-by-side with scores from other clinical pediatric populations. Scores from these populations cannot be compared statistically because of important differences in demographic characteristics between the groups. Instead, they provide an understanding of the range of scores in normative and chronically ill populations.
Table 2a.
Parent-reported HRQoL (PF50) in young children with chronic hepatitis B, chronic hepatitis C, and normative sample
| Population | Clinic Based Chronic Hepatitis B | Clinic Based Chronic Hepatitis C | Normative Sample |
| Country | United States and Canada (HBRN, current study) | United States (24) | United States (15) |
| Sample size | 83 | 114 | 391 |
| Mean Age (Range) | 7.7 (5–<10) years | 11 years | 11.5 years (5–<18) years |
| Race/Ethnicity of the sample | 83% Asian, 6% White | 4% Asian, 75% White | 1% Asian, 83% White |
|
| |||
| CHQ-PF50 | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) |
| Physical Summary Score | 54 (53–55) | 52 (51–53) | 53 (52–54) |
| Psychosocial Summary Score | 53 (51–55) | 52 (50–54) | 51 (50–52) |
| Physical Components | |||
| Physical Functioning | 97 (94–100) | 96 (94–98) | 96 (95–97) |
| Role Functioning-Physical | 98 (96–100) | 97 (95–99) | 94 (92–96) |
| Bodily Pain | 92 (89–95) | 82 (79–85) | 82 (80–84) |
| General Health | 72 (69–75) | 66 (63–69) | 73 (71–75) |
| Psychosocial Components | |||
| Role Functioning-Emotional/Behavior | 97 (95–99) | 95 (92–98) | 93 (91–95) |
| Behavior | 79 (76–82) | 79 (76–82) | 76 (74–78) |
| Mental Health | 81 (78–84) | 80 (78–82) | 79 (78–80) |
| Self-Esteem | 86 (82–90) | 83 (80–86) | 80 (78–82) |
| Family Related Components | |||
| Parent-Impact-Emotional | 74 (69–79) | 65 (60–70) | 80 (78–82) |
| Parent-Impact-Time | 88 (83–93) | 88 (85–91) | 88 (86–90) |
| Family Activities | 86 (82–90) | 85 (82–88) | 90 (88–92) |
Table 2b.
Child-reported HRQoL (CF87) in youth with chronic hepatitis B, youth after liver transplantation, and normative sample
| Population | Clinic Based Chronic Hepatitis B | Clinic Based Liver transplant recipients | School based |
| Country | United States and Canada (HBRN, current study) | United States (7) | United States (10) |
| Sample size | 161 | 28 | 232 |
| Mean Age (Range) | 14.1 (10–<18) years | 15.1 (12–<18) years | 13.0 (10–15) years |
| Race/Ethnicity of the sample | 75% Asian, 7.5% White | 72% White | Predominantly African-American |
|
| |||
| CHQ-CF87 | Mean (95% CI) | Mean (95% CI) | Mean (95% CI) |
| Physical Components | |||
| Physical Functioning | 94.8 (93.5–96.2) | 90.2 (85.0–95.4) | 88.8 (87.0–90.6) |
| Role Functioning-Physical | 96.7 (95.0–98.4) | 88.4 (80.3–96.5) | 88.3 (85.6–91.0) |
| Bodily Pain | 87.8 (85.3–90.3) | 72.4 (63.6–81.2) | 74.4 (71.4–77.3) |
| General Health | 71.8 (69.6–74.0) | 60.3 (55.4–65.2) | 66.4 (64.5–68.3) |
| Psychosocial Components | |||
| Role Functioning-Emotional | 93.6 (91.3–95.8) | 87.6 (79.9–95.3) | 85.9 (83.2–88.6) |
| Role Functioning-Behavioral | 94.1 (91.5–96.6) | 87.6 (79.6–95.6) | 86.5 (83.8–89.3) |
| Behavior | 79.3 (77.4–81.3) | 81.0 (75.4–86.6) | 76.6 (74.7–78.5) |
| Mental Health | 77.8 (75.6–80.1) | 77.2 (70.3–84.1) | 72.7 (70.6–74.8) |
| Self-Esteem | 82.6 (80.3–84.9) | 81.8 (77.3–86.3) | 81.8 (79.8–83.9) |
| Family Related Components | |||
| Family Activities | 81.5 (78.4–84.7) | 79.7 (73.6–85.8) | - |
Family Activities scale was not provided in these samples (10).
Comparison of HRQoL by Selected Characteristics
Summary T-Scores for 5–<10 year olds (PF50)
In 5–<10 year-olds, mean Physical and Psychosocial Summary T-scores were not associated with sex, adoption status, maternal education, symptom count, or APRI (p>0.05, Supplemental Digital Content, Table 3a, Participant characteristics and unadjusted associations with caregiver reported HRQoL (PF50) summary T-scores among children 5–<10 years of age in HBRN). Spearman correlation coefficients for ALT with the Physical and Psychosocial Summary T-scores were −0.08 (p>0.05) and −0.28 (p=0.01), respectively. That is, increasing ALT levels were associated with decreased psychosocial HRQoL.
Subscale Scores for 5–<10 year olds (PF50)
For males, the lowest median scores were observed for general health (72.5) and parent impact-emotional (66.7) subscales. For females, the lowest median scores were observed for general health (71.7) and mental health (80.0). For children living with biological parents, the general health subscales had the lowest median score (69.6), with all other components above 80.0. For adoptees, median scores were below 80.0 for both general health (72.5) and parent impact-emotional (75.0). Among children of mothers who did not have at least a bachelor’s degree, four subscales had median scores below 80.0: general health (70.8), behavior (72.5), mental health (75.0), and parent impact-emotional (66.7). For children of mothers with a bachelor’s degree or higher, only the general health subscale median (72.5) was below 80.0. Very little variability was observed for the physical functioning, role functioning-physical, bodily pain, role functioning-emotional/behavioral and parent impact-time subscales: each had a median score of 100.0 across categories of sex, adoption status and maternal education (Supplemental Digital Content, 3b, Participant characteristics and unadjusted associations with caregiver reported HRQoL (PF-50) domains among children 5–<10 years of age in HBRN). In fact, ≥85% of participants obtained the maximum score of 100 for the physical functioning, role functioning-physical and role functioning-emotional/behavioral subscales, regardless of sex, adoptions status or maternal education.
Despite the association of lower Psychosocial T-score with increasing ALT, none of the PF50 subscale comparisons, including those by symptom count or ALT, were statistically significant.
Subscale Scores for 10–<18 year olds (CF87)
Among youth, median subscale scores ranged from 69.6 to 100.0 and 73.8 to 100.0 for males and females, respectively (Table 4). Across all subscales, comparisons by sex, ALT, and APRI were not statistically significant. Median scores ranged from 67.5 to 100.0 and 75.8 to 100.0 for non-adoptees and adoptees, respectively. Adoption status was statistically significantly associated with physical functioning (p<0.01): 15% of non-adoptees and 5% of adoptees scored <100.0. Adoption status was also associated with general health: lower median scores were observed among non-adoptees (p<0.001). Median scores ranged from 70.8 to 100.0 and 73.3 to 100 for children of mothers with less than versus at least a bachelor’s degree. Maternal education was statistically significantly associated with role/functioning-physical (p=0.02) and role/functioning-emotional scales (p=0.03). More youth of mothers with less than a bachelor’s degree (versus at least a bachelor’s degree) scored below 100.0 on the role/functioning-physical (20% versus 3%) and -emotional subscales (33% versus 13%).
Table 4.
Participant characteristics and unadjusted associations with child reported HRQoL (CF-87) domains among children 10–<18 years of age in HBRN
| Variables | Physical Components | Psychosocial Components | Family Components | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| N | Physical Functioning (PF) | Role Functioning-Physical (RP) | Bodily Pain (BP) | General Health (GH)d | Role Functioning-Emotional (RE) | Role Functioning-Behavioral (RB) | Behavior (BE) | Mental Health (MH) | Self-Esteem (SE)d | Family Activities (FA)d | ||
| Sex a | 161 | |||||||||||
| Male | Median (25th, 75th) | 74 | 100.0 (87.5, 100.0) | 100.0 (100.0, 100.0) | 100.0 (80.0, 100.0) | 69.6 (59.2, 77.9) | 100.0 (88.9, 100.0) | 100.0 (88.9, 100.0) | 79.3 (69.1, 89.7) | 81.3 (70.3, 89.1) | 85.7 (69.6, 92.9) | 83.3 (70.8, 100.0) |
| N (%) < 100 | 34 (46%) | 12 (16%) | 34 (46%) | 71 (97%) | 21 (28%) | 19 (26%) | 73 (99%) | 73 (99%) | 67 (92%) | 50 (68%) | ||
|
| ||||||||||||
| Female | Median (25th, 75th) | 87 | 100.0 (95.8, 100.0) | 100.0 (100.0, 100.0) | 100.0 (80.0, 100.0) | 73.8 (65.4, 83.3) | 100.0 (100.0, 100.0) | 100.0 (100.0, 100.0) | 80.0 (70.6, 92.6) | 79.7 (65.6, 87.5) | 85.7 (75.0, 96.4) | 91.7 (70.8, 100.0) |
| N (%) < 100 | 32 (37%) | 6 (7%) | 36 (41%) | 85 (98%) | 15 (17%) | 10 (11%) | 86 (99%) | 84 (97%) | 77 (89%) | 61 (70%) | ||
|
| ||||||||||||
| Adoption Status a | 161 | ‡ | ‡ | |||||||||
| No | Median (25th, 75th) | 87 | 95.8 (87.5, 100.0) | 100.0 (100.0, 100.0) | 90.0 (80.0, 100.0) | 67.5 (57.1, 77.1) | 100.0 (88.9, 100.0) | 100.0 (100.0, 100.0) | 80.0 (69.7, 90.3) | 79.7 (68.8, 87.5) | 85.7 (69.6, 92.9) | 83.3 (66.7, 100.0) |
| N (%) < 100 | 45 (52%) | 15 (17%) | 44 (51%) | 85 (99%) | 23 (26%) | 19 (22%) | 86 (99%) | 85 (98%) | 78 (91%) | 64 (74%) | ||
|
| ||||||||||||
| Yes | Median (25th, 75th) | 74 | 100.0 (95.8, 100.0) | 100.0 (100.0, 100.0) | 100.0 (80.0, 100.0) | 75.8 (69.6, 85.4) | 100.0 (100.0, 100.0) | 100.0 (100.0, 100.0) | 80.0 (69.7, 89.7) | 81.3 (70.3, 90.6) | 87.5 (76.8, 96.4) | 91.7 (75.0, 100.0) |
| N (%) < 100 | 21 (28%) | 3 (4%) | 26 (35%) | 71 (96%) | 13 (18%) | 10 (14%) | 73 (99%) | 72 (97%) | 66 (89%) | 47 (64%) | ||
|
| ||||||||||||
| Maternal Education a | 146 | |||||||||||
| Bachelor’s degree or higher | e | e | ||||||||||
| No | Median (25th, 75th) | 75 | 100.0 (87.5, 100.0) | 100.0 (100.0, 100.0) | 100.0 (80.0, 100.0) | 70.8 (57.1, 80.0) | 100.0 (77.8, 100.0) | 100.0 (88.9, 100.0) | 80.0 (70.6, 91.2) | 79.7 (68.8, 87.5) | 85.7 (69.6, 92.9) | 87.5 (66.7, 100.0) |
| N (%) < 100 | 33 (44%) | 15(20%) | 35 (47%) | 74 (99%) | 25 (33%) | 19 (25%) | 74 (99%) | 74 (99%) | 70 (93%) | 54 (72%) | ||
|
| ||||||||||||
| Yes | Median (25th, 75th) | 71 | 100.0 (95.8, 100.0) | 100.0 (100.0, 100.0) | 100.0 (80.0, 100.0) | 73.3 (66.7, 84.2) | 100.0 (100.0, 100.0) | 100.0 (100.0, 100.0) | 80.0 (70.6, 92.6) | 81.3 (71.9, 90.6) | 87.5 (75.0, 98.2) | 91.7 (75.0, 100.0) |
| N (%) < 100 | 24 (34%) | 2 (3%) | 26 (37%) | 67 (96%) | 9 (13%) | 9 (13%) | 70 (99%) | 68 (96%) | 59 (84%) | 46 (66%) | ||
|
| ||||||||||||
| ALT (U/L) b | 158 | |||||||||||
| Spearman correlation coefficient | 0.04 | −0.13 | −0.02 | −0.22 | 0.06 | −0.03 | −0.13 | 0.01 | −0.07 | −0.03 | ||
|
| ||||||||||||
| APRI b | 139 | |||||||||||
| Spearman correlation coefficient | 0.18 | 0.08 | 0.00 | −0.02 | 0.07 | 0.10 | −0.06 | 0.01 | 0.04 | 0.05 | ||
|
| ||||||||||||
| Symptom Count a,c | 161 | † | † | † | † | |||||||
| 0 | Median (25th, 75th) | 86 | 100.0 (95.8,100.0) | 100.0 (100.0,100.0) | 100.0 (80.0,100.0) | 73.8 (66.7,82.1) | 100.0 (100.0,100.0) | 100.0 (100.0,100.0) | 83.4 (70.6,93.2) | 82.8 (75.0,92.2) | 89.3 (75.0,96.4) | 91.7 (70.8,100.0) |
| N (%) < 100 | 30 (35%) | 7 (8%) | 31 (36%) | 82 (96%) | 15 (17%) | 10 (12%) | 85 (99%) | 83 (97%) | 73 (86%) | 53 (62%) | ||
|
| ||||||||||||
| 1 | Median (25th, 75th) | 29 | 100.0 (95.8,100.0) | 100.0 (100.0,100.0) | 100.0 (80.0,100.0) | 75.0 (65.4,83.3) | 100.0 (88.9,100.0) | 100.0 (100.0,100.0) | 80.0 (74.1,90.3) | 81.3 (75.0,89.1) | 92.9 (82.1,96.4) | 87.5 (70.8,100.0) |
| N (%) < 100 | 13 (45%) | 5 (17%) | 11 (38%) | 29 (100%) | 8 (28%) | 5 (17%) | 28 (97%) | 29 (100%) | 26 (90%) | 21 (72%) | ||
|
| ||||||||||||
| 2 | Median (25th, 75th) | 18 | 100.0 (95.8,100.0) | 100.0 (100.0,100.0) | 100.0 (80.0,100.0) | 69.2 (59.2,77.9) | 100.0 (100.0,100.0) | 100.0 (100.0,100.0) | 76.8 (70.6,82.9) | 80.5 (68.8,84.4) | 79.5 (73.2,87.5) | 83.3 (75.0,100.0) |
| N (%) < 100 | 7 (39%) | 1 (6%) | 7 (39%) | 18 (100%) | 3 (17%) | 3 (17%) | 18 (100%) | 17 (94%) | 18 (100%) | 13 (72%) | ||
|
| ||||||||||||
| 3+ | Median (25th, 75th) | 28 | 93.8 (85.4,100.0) | 100.0 (100.0,100.0) | 80.0 (65.0,90.0) | 62.3 (56.0,71.9) | 100.0 (83.3,100.0) | 100.0 (83.3,100.0) | 69.7 (62.2,75.6) | 66.4 (50.8,78.9) | 73.2 (63.4,86.6) | 83.3 (60.4,91.7) |
| N (%) < 100 | 16 (57%) | 5 (18%) | 21 (75%) | 27 (96%) | 10 (36%) | 11 (39%) | 28 (100%) | 28 (100%) | 27 (96%) | 24 (86%) | ||
For categorical variables, the first row gives median scores; the second row gives (25th percentile, 75th percentile); the third row gives the frequency (percent) of individuals scoring below 100.0. Kruskal-Wallis non-parametric tests were used to compare the distribution of CHQ scores across categories.
For continuous variables, Spearman correlation was performed to test if HRQoL domain scores were statistically significantly associated with continuous covariates.
Symptoms included fatigue, pain over liver, nausea, poor appetite, weight loss, itching, depression/sadness, irritability, jaundice, and dark urine.
One participant had missing GH, SE, and FA subscale scores. The Bonferonni-Holm adjustment for multiple comparisons (across the 10 subscales) was used to calculate p-values.
Adoption status was statistically significantly associated with better Physical Functioning (p<0.01) and General Health (p<0.001).
Higher maternal education was statistically significantly associated with better Role/Functioning-Physical (p=0.02) and Role/Functioning-Emotional (p=0.03).
Symptom count was statistically significantly associated with 4 of 10 subscales based on the K-W test: Bodily Pain (p<0.01), Behavior (p<0.01), Mental Health (p<0.01), and Self-Esteem (p<0.01).
All other comparisons were statistically non-significant (p>0.05)
As compared to adoptees, non-adoptees were more likely to have mothers with a lower education level. This was true for both the 5–<10 year olds (23% of adoptees v. 45% of non-adoptees had mothers with less than a bachelor’s degree, p = 0.051) but was most striking for youth (23% of adoptees v. 75% of non-adoptees had mothers with less than a bachelor’s degree, p = 0.01)
The number of reported symptoms (regardless of the specific symptom(s)) was significantly associated with 4 of 10 subscales: bodily pain, behavior, mental health, and self-esteem (p<0.01). For behavior, mental health and self-esteem subscales, median scores were lowest for those with 3+ symptoms, and highest for those with no symptoms. For the bodily pain subscale, median scores were 100.0 for each group other than those with 3+ symptoms (median 80.0). The proportion of youth scoring less than 100.0 on the bodily pain subscale was highest for those with the most symptoms (0 symptoms: 36%, 1 symptom: 38%, 2 symptoms: 39%, and 3+symptoms: 75%).
Discussion
This study examines HRQoL in the largest cohort of children with chronic HBV assembled in the US and Canada. Our cohort was generally asymptomatic with a low frequency of co-morbid conditions and overall HRQoL similar to the normal range for healthy children. However, our study showed that clinical factors are associated with HRQoL in children with chronic HBV in several crucial ways. First, among young children, increased ALT levels were negatively associated with children’s parent-reported overall psychosocial functioning, an important association for clinicians to consider when caring for young children with HBV and their families. Our data cannot determine if this results from the direct effect of HBV disease or from the parent’s perception of diminished health as a result of knowing the child’s ALT levels. While liver biopsies were not available to stage disease in our cohort, APRI scores were in a normal range in most patients, suggesting the likely absence of significant hepatic fibrosis or inflammation in this cohort (18). Clinicians should know that caregivers of children with chronic hepatitis B and elevated ALT may be experiencing an emotional impact that they may not reveal in the clinical visit.
Second, older children reported more physical symptoms, and the number of these symptoms was associated with reports of worse HRQoL related to bodily pain, mental health, self-esteem, and behavior. Report of sadness and depression increased in the older age group and 5 of the children reported medication for these conditions. For clinicians, this means that the well-appearing child with few serologic markers of significant liver disease may still be experiencing impairment in facets of HRQoL. Adults with chronic HBV have a high risk of depression and anxiety (19, 20). In adolescents after liver transplantation (predominantly for biliary atresia, autoimmune hepatitis, and hepatitis (type unspecified)), low HRQoL was associated with poor adherence to the prescribed medication regimen (7). Awareness of diminished HRQoL in children with chronic HBV may allow intervention to provide disease-specific education and psychosocial support early in adolescence and young adulthood, to reduce later depression and anxiety, and insure that prescribed anti-viral treatments are as successful as possible. There may be benefit in directly addressing the child’s and caregiver’s fears, which are revealed in the study questionnaires, but rarely openly discussed in the clinical setting. Future studies should explore whether caregivers of children with chronic HBV would benefit from improved education about the disease and its likely course, parent support groups, or clear guidelines regarding any limitations (or lack of limitations) on their child’s future.
Also in youth, adoption status was associated with physical functioning and general health, which were worse for non-adoptees. Youth with mothers with less than a bachelor’s degree reported more limitations in schoolwork or activities with friends. A similar association of lower maternal education and HRQoL was noted in studies of children after liver transplantation (21, 22) and children with kidney disease (22). The majority of our cohort was born outside North America (97%,), children of either adoption or immigrant families (23). For both young children and youth, non-adoptees were more likely to have mothers with lower education level. These observations suggest that reduced HRQoL in these youth may not be directly related to HBV status, but that socioeconomic status, and, perhaps, immigration status, may contribute as well. Providers caring for children with hepatitis B may want to consider the more global health concerns facing this population.
It is tempting to ascribe some reduction in HRQoL in children with chronic HBV to stigma or discrimination perceived by parent or child. Chinese adults with chronic HBV experience more difficulties in interpersonal relationships as a result of stigma—they believe they bring trouble to their families and are less desirable as spouses (24). They also report feeling stigma related to employment and physical relationships. Among Chinese adults living in Canada, stigma associated with chronic HBV infection is a reason for avoiding HBV screening (25). No similar studies have been performed in children.
Our data affirms the usefulness of HRQoL in children with liver disease as a measure of disease impact on the lives of parents and children. A similar study of HRQoL in 114 treatment naive children with chronic hepatitis C virus demonstrated that, relative to normative data, caregivers were more likely to believe their child’s health was poor, reported higher concern about their child’s health status, and these perceptions limited family activities (26). In studies of children with non-alcoholic fatty liver disease (27) and autoimmune hepatitis (28), decreases in caregivers’ quality of life were found, although use of different HRQoL instruments makes direct comparison difficult. Information on HRQoL in children with chronic HBV is limited. Woo, et al., found no association of HRQoL and ALT in adults with hepatitis B, although they used a different HRQoL instrument from ours (29).
Our study has some limitations. Children were enrolled at tertiary medical centers; the population studied may not reflect the US and Canadian population of HBV-infected children. However, children are often referred to tertiary centers for evaluation and management even with asymptomatic HBV. The study was conducted in English because the HRQoL instrument was not translated into some of the languages most common among our non-English speakers. This may impact how well our study reflects the experiences of refugee children with HBV. Despite the large number of children enrolled in this study, our sample of young children with hepatitis B was small, and assessment of a larger cohort may be informative. Although population-normed scores are available for the PF50, there are important socio-demographic differences between the HBRN Pediatric Cohort and our comparison samples, particularly with respect to numbers of immigrant and adopted children, and ethnic and racial composition. Such differences make comparisons between the study and normative populations problematic, and were therefore not undertaken. Information from caregivers of youth was not available for this study, limiting direct comparison of caregivers’ impressions of HRQoL from childhood to adolescence. We were unable to evaluate fibrosis in our population; significant fibrosis is rare in children with HBV infection and is unlikely to be a determinant of HRQoL in this cohort.
Conclusion
Overall, HRQoL is preserved in children with chronic HBV infection. However, some socio-demographic and HBV-related clinical factors were associated with impaired HRQoL in our pediatric cohort study sample at baseline, including elevated ALT in young children and symptom-reporting, maternal education, and adoption status in youth. Measuring HRQoL may alert clinicians to children and families in need of psychological support and education to dispel health-related anxiety and reduce risk of non-adherence to therapy and subsequent behavioral and mood problems. This is crucial, as children with elevated ALT are more likely to need medical therapy for HBV, and early intervention may reduce poor adherence to therapy associated with reduced HRQoL. Future longitudinal studies in this cohort will track changes of HRQoL in children with the evolving clinical state of their chronic HBV.
Supplementary Material
Table 3a. Participant characteristics and unadjusted associations with caregiver reported HRQoL (PF50) summary T-scores among children 5–<10 years of age in HBRN
Table 3b. Participant characteristics and unadjusted associations with caregiver reported HRQoL (PF-50) domains among children 5–<10 years of age in HBRN
Table 5. Maternal education and adoption status
What is known
Chronic hepatitis B (HBV) affects children of international adoption and refugees from countries where HBV is endemic
Adults with HBV have poor health-related quality of life (HRQoL) and are at risk for depression and other psychosocial disorders
What is new
In North American children with chronic HBV some socio-demographic and clinical factors were associated with impaired HRQoL, including elevated alanine-amino-transferase in young children and symptom-reporting, maternal education, and adoption status in youth.
Measuring HRQoL may alert clinicians to children and families needing psychological support and education to dispel anxiety and reduce risk of non-adherence to therapy and behavioral and mood problems.
Acknowledgments
Funding: The Hepatitis B Research Network (HBRN) was funded by a U01 grant from the National Institute of Diabetes and Digestive and Kidney Diseases to the following investigators Kathleen B. Schwarz (U01 DK082916), Steven H. Belle PhD (DK082864), Harry L. A. Janssen, MD, PhD (DK082874), Norah A. Terrault, MD, MPH (U01 DK082944), Lewis R. Roberts, MB, ChB, PhD (DK 082843), Adrian M. Di Bisceglie, MD (DK082871) an interagency agreement with NIDDK: Lilia Milkova Ganova-Raeva, PhD (A-DK-3002-001). Additional support was provided by Roche Molecular Systems via a CRADA through the NIDDK.
In addition to the authors, the HBRN would like to acknowledge the contributions of the following: Johns Hopkins University Hongxia Li, MBBS, MS, Douglas Mogul, MD, Robert Anders, MD, PhD, Kim Kafka, RN. (Department of Pediatrics, Johns Hopkins Medical Institutions, Baltimore, MD). Minnesota Alliance for Research in Chronic Hepatitis B Consortium Shannon M. Riggs, LPN, AS (Department of Pediatrics, University of Minnesota, Minneapolis, MN). Midwest Hepatitis B Consortium: Rosemary Nagy, RDN, LD, MBA, Jacki Cerkoski, RN MSN (Saint Louis University, St. Louis, MO). University of Toronto Consortium: Athena Hau, BSc, Caitlin Yuan, BSc, MSc, Rosemary Swan, BN, Constance O’Connor, MN (Department of Paediatrics, The Hospital for Sick Children, University of Toronto, Toronto, Ontario). HBV CRN North Texas Consortium: Laurie A. Rodgers-Augustyniak, RN, Shirley Montanye, RN (Department of Pediatrics, UTSW, Dallas, TX). San Francisco Hepatitis B Research Group Consortium: Shannon Fleck, BS, Camille Langlois, MS (Department of Pediatrics, UCSF, San Francisco, CA), PNW/Alaska Clinical Center Consortium: Kara L. Cooper (Department of Pediatrics, University of Washington, Seattle, WA). Liver Disease Research Branch, NIDDK, NIH: Jay H. Hoofnagle, MD, Averell H. Sherker, MD, Edward Doo, MD, Rebecca J. Torrance, RN, MS, Sherry R. Hall, MS. Data Coordinating Center: Michelle Danielson, PhD, Tamara Haller, Geoffrey Johnson, MS, Stephanie Kelley, MS, Sharon Lawlor, MBA, Ruosha Li, PhD, Manuel Lombardero, MS, Joan M. MacGregor, MS, Andrew Pelesko, BS, Donna Stoliker, Barbara Walters, Ella Zadorozny, MS (Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA).
Footnotes
Conflict of Interest
Potential conflicts of interest: The authors have no conflicts of interest relevant to this article to disclose.
Author Roles
Dr. Schwarzenberg M.D. conceived this project and designed it with contributions from Drs. Ling, Cloonan, Murray, Rodriguez-Baez, Rosenthal, Teckman, and Schwartz. Dr. Cloonan and Ms Lin performed statistical analysis, with Dr. Evon providing additional assistance and interpretation. Dr. Schwarzenberg drafted the manuscript along with Dr. Cloonan and Ms Lin, and all authors edited the manuscript and reviewed it. All authors agree to be accountable for all aspects of the work
Financial disclosures
Sarah Jane Schwarzenberg receives supplies and support from Bristol Myers Squibb, Roche Therapeutics, and Roche Molecular Diagnostic via a CRADA through NIDDK
Simon C. Ling receives supplies and support from Bristol Myers Squibb, Roche Therapeutics, and Roche Molecular Diagnostic via a CRADA through NIDDK.
Yona Keich Cloonan has no financial interest relevant to this article to disclose.
Hsing-Hua Sylvia Lin has no financial interest relevant to this article to disclose.
Donna M. Evon is a consultant for Gilead
Karen F. Murray receives supplies and support from Bristol Myers Squibb, Roche Therapeutics, and Roche Molecular Diagnostic via a CRADA through NIDDK; she receives funding from Gilead and Shire for studies related to hepatitis.
Norberto Rodriguez-Baez receives supplies and support from Bristol Myers Squibb, Roche Therapeutics, and Roche Molecular Diagnostic via a CRADA through NIDDK; he receives funding from Gilead for studies related to hepatitis.
Philip Rosenthal receives supplies and support from Bristol Myers Squibb, Roche Therapeutics, and Roche Molecular Diagnostic via a CRADA through NIDDK; he receives funding from Gilead, Vertex, BMS, and Roche/Genetech for studies related to hepatitis.
Jeffrey Teckman receives supplies and support from Bristol Myers Squibb, Roche Therapeutics, and Roche Molecular Diagnostic via a CRADA through NIDDK; he receives funding from Gilead, Alnylam, Arrowhead Research, GLG Pharma, Genkyotex, Dicerna, RxCelerate.
Kathleen B. Schwarz receives supplies and support from Bristol Myers Squibb, Roche Therapeutics, and Roche Molecular Diagnostic via a CRADA through NIDDK; she also receives funding from Gilead, Bristol Myers Squibb and Roche Therapeutics for clinical trials in hepatitis and consulting from Gilead and Roche Therapeutics relating to hepatitis.
References
- 1.Bortolotti F, Calzia R, Cadrobbi P, Giacchini R, Ciravegna B, Armigliato M, et al. Liver cirrhosis associated with chronic hepatitis B virus infection in childhood. J Pediatr. 1986;108(2):224–7. doi: 10.1016/s0022-3476(86)80987-8. [DOI] [PubMed] [Google Scholar]
- 2.Hsu HC, Wu MZ, Chang MH, Su IJ, Chen DS. Childhood hepatocellular carcinoma develops exclusively in hepatitis B surface antigen carriers in three decades in Taiwan. Report of 51 cases strongly associated with rapid development of liver cirrhosis. J Hepatol. 1987;5(3):260–7. doi: 10.1016/s0168-8278(87)80030-2. [DOI] [PubMed] [Google Scholar]
- 3.Stadler LP, Mezoff AG, Staat MA. Hepatitis B virus screening for internationally adopted children. Pediatrics. 2008;122(6):1223–8. doi: 10.1542/peds.2007-2559. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell T, Armstrong GL, Hu DJ, Wasley A, Painter JA. The increasing burden of imported chronic hepatitis B--United States, 1974–2008. PLoS One. 2011;6(12):e27717. doi: 10.1371/journal.pone.0027717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwarz KB, Cloonan YK, Ling SC, Murray KF, Rodriguez-Baez N, Schwarzenberg SJ, et al. Children with Chronic Hepatitis B in the United States and Canada. J Pediatr. 2015;167(6):1287–94. e2. doi: 10.1016/j.jpeds.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhuang G, Zhang M, Liu Y, Guo Y, Wu Q, Zhou K, et al. Significant impairment of health-related quality of life in mainland Chinese patients with chronic hepatitis B: a cross-sectional survey with pair-matched healthy controls. Health Qual Life Outcomes. 2014;12:101. doi: 10.1186/1477-7525-12-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredericks EM, Magee JC, Opipari-Arrigan L, Shieck V, Well A, Lopez MJ. Adherence and health-related quality of life in adolescent liver transplant recipients. Pediatr Transplant. 2008;12(3):289–99. doi: 10.1111/j.1399-3046.2008.00901.x. [DOI] [PubMed] [Google Scholar]
- 8.Uzark K, King E, Spicer R, Beekman R, Kimball T, Varni JW. The clinical utility of health-related quality of life assessment in pediatric cardiology outpatient practice. Congenit Heart Dis. 2013;8(3):211–8. doi: 10.1111/chd.12002. [DOI] [PubMed] [Google Scholar]
- 9.Ghany MG, Perrillo R, Li R, Belle SH, Janssen HLA, Terrault NA, et al. Characteristics of adults in the hepatitis B research network in North America reflect their country of origin and hepatitis B virus genotype. Clin Gastroenterol Hepatol. 2015;13(1):183–92. doi: 10.1016/j.cgh.2014.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landgraf JM, Abetz L, Ware JE. The Health Institute NEMC, editor. The CHQ User’s Manual. Boston: 1996. [Google Scholar]
- 11.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 12.Jin W, Lin Z, Xin Y, Jiang X, Dong Q, Xuan S. Diagnostic accuracy of the aspartate aminotransferase-to-platelet ratio index for the prediction of hepatitis B-related fibrosis: a leading meta-analysis. BMC Gastroenterol. 2012;12:14. doi: 10.1186/1471-230X-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGoogan KE, Smith PB, Choi SS, Berman W, Jhaveri R. Performance of the AST-to-platelet ratio index as a noninvasive marker of fibrosis in pediatric patients with chronic viral hepatitis. J Pediatr Gastroenterol Nutr. 2010;50(3):344–6. doi: 10.1097/MPG.0b013e3181aed725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.HealthActChQ. HealthActChQ. The Child Health Questionnaire Scoring and Interpretation Manual. Cambridge, MA: HealthActCHQ; 2008. [Google Scholar]
- 15.landgraf JM, Abetz LN. Functional status and well-being of children representing three cultural groups: initial self-reports using the CHQ-CF87. Psychol Health. 1997;12:839–54. [Google Scholar]
- 16.Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, et al. Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem. 2012;58(5):854–68. doi: 10.1373/clinchem.2011.177741. [DOI] [PubMed] [Google Scholar]
- 17.Schwimmer JB, Dunn W, Norman GJ, Pardee PE, Middleton MS, Kerkar N, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138(4):1357–64. 64.e1–2. doi: 10.1053/j.gastro.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhijian Y, Hui L, Weiming Y, Zhanzhou L, Zhong C, Jinxin Z, et al. Role of the Aspartate Transaminase and Platelet Ratio Index in Assessing Hepatic Fibrosis and Liver Inflammation in Adolescent Patients with HBeAg-Positive Chronic Hepatitis B. Gastroenterol Res Pract. 2015;2015:906026. doi: 10.1155/2015/906026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Modabbernia A, Ashrafi M, Malekzadeh R, Poustchi H. A review of psychosocial issues in patients with chronic hepatitis B. Arch Iran Med. 2013;16(2):114–22. [PubMed] [Google Scholar]
- 20.Karaivazoglou K, Iconomou G, Triantos C, Hyphantis T, Thomopoulos K, Lagadinou M, et al. Fatigue and depressive symptoms associated with chronic viral hepatitis patients. health-related quality of life (HRQOL) Ann Hepatol. 2010;9(4):419–27. [PubMed] [Google Scholar]
- 21.Bucuvalas JC, Britto M, Krug S, Ryckman FC, Atherton H, Alonso MP, et al. Health-related quality of life in pediatric liver transplant recipients: A single-center study. Liver Transplantation. 2003;9(1):62–71. doi: 10.1053/jlts.2003.50012. [DOI] [PubMed] [Google Scholar]
- 22.Gerson AC, Wentz A, Abraham AG, Mendley SR, Hooper SR, Butler RW, et al. Health-related quality of life of children with mild to moderate chronic kidney disease. Pediatrics. 2010;125(2):e349–57. doi: 10.1542/peds.2009-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwarz KB, Cloonan YK, Ling SC, Murray KF, Rodriguez-Baez N, Schwarzenberg SJ, et al. Children with Chronic Hepatitis B in the United States and Canada. J Pediatr. 2015;167(6):1287–94. e2. doi: 10.1016/j.jpeds.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Guan ML, Balch J, Wu E, Rao H, Lin A, et al. Survey of Hepatitis B Knowledge and Stigma among Chronically Infected Patients and Uninfected Persons in Beijing, China. Liver Int. 2016 doi: 10.1111/liv.13168. [DOI] [PubMed] [Google Scholar]
- 25.Li D, Tang T, Patterson M, Ho M, Heathcote J, Shah H. The impact of hepatitis B knowledge and stigma on screening in Canadian Chinese persons. Can J Gastroenterol. 2012;26(9):597–602. doi: 10.1155/2012/705094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, et al. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr. 2009;48(3):341–7. doi: 10.1097/MPG.0b013e318185998f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mazzone L, Postorino V, De Peppo L, Della Corte C, Lofino G, Vassena L, et al. Paediatric non-alcoholic Fatty liver disease: impact on patients and mothers’ quality of life. Hepat Mon. 2013;13(3):e7871. doi: 10.5812/hepatmon.7871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulati R, Radhakrishnan KR, Hupertz V, Wyllie R, Alkhouri N, Worley S, et al. Health-related quality of life in children with autoimmune liver disease. J Pediatr Gastroenterol Nutr. 2013;57(4):444–50. doi: 10.1097/MPG.0b013e31829ef82c. [DOI] [PubMed] [Google Scholar]
- 29.Woo G, Tomlinson G, Yim C, Lilly L, Therapondos G, Wong DKH, et al. Health state utilities and quality of life in patients with hepatitis B. Can J Gastroenterol. 2012;26(7):445–51. doi: 10.1155/2012/736452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 3a. Participant characteristics and unadjusted associations with caregiver reported HRQoL (PF50) summary T-scores among children 5–<10 years of age in HBRN
Table 3b. Participant characteristics and unadjusted associations with caregiver reported HRQoL (PF-50) domains among children 5–<10 years of age in HBRN
Table 5. Maternal education and adoption status